重要育种亲本川麦44对衍生品种的遗传贡献
2021-10-29罗江陶郑建敏邓清燕刘培勋蒲宗君
罗江陶,郑建敏,邓清燕,刘培勋,蒲宗君
重要育种亲本川麦44对衍生品种的遗传贡献
罗江陶,郑建敏,邓清燕,刘培勋,蒲宗君*
四川省农业科学院作物研究所/农业农村部西南地区小麦生物学与遗传育种重点实验室,成都 610066
【目的】小麦品种川麦44不仅本身具有高产、稳产、广适等特性,而且以其为亲本已选育审定新品种11个,是小麦育种的一个重要亲本。明确川麦44的遗传特性,鉴定其含有的重要基因或QTL位点,为更好地利用川麦44选育新品种提供理论支撑。【方法】利用荧光原位杂交明确小麦-外源易位对川麦44及其衍生品种的影响以及川麦44及其衍生品种在染色体层面的遗传规律。利用660K SNP芯片数据分析川麦44对其衍生品种的遗传贡献,明确衍生品种中来源于川麦44的高传递率区段。利用已知的小麦基因功能标记及QTL连锁标记,对川麦44中有利于育种的重要基因位点进行鉴定。【结果】细胞学鉴定表明川麦44不含四川小麦品种中常见的2条易位染色体6VS/6AL和1RS/1BL。其衍生品种中,仅昌麦32和昌麦34含1对1RS/1BL易位染色体,其余品种不含有小麦-外源易位染色体。系谱分析表明,昌麦32和昌麦34的易位染色体遗传自另外一个杂交亲本——昌麦19。1RS/1BL易位的导入可能是昌麦32和昌麦34表现为弱筋的原因之一。除了小麦-外源易位染色体,多个染色体的核型在川麦44及其10个衍生品种中表现出多态性。其中,4A染色体有2种类型,80%的衍生品种与川麦44相同核型相同;5A染色体有4种类型,与川麦44相同的频率为40%;6B染色体有2种类型,与川麦44相同的频率为40%,7B染色体有2种类型,与川麦44相同的频率为40%。660K SNP芯片分析共鉴定到1 106个分布于川麦44所有染色体上的高遗传率区段,平均长度为1.57 Mb。从基因组层面来看,B基因组的区段总长度和总数均最大。从不同染色体来看,区段最长的3条为别为4A、2B和5B,区段数最多的3条染色体分别为4A、2B和3B。利用61个已知的小麦基因功能标记及13个产量相关QTL连锁SNP标记分析川麦44及其衍生品种,再与之前获得的川麦44高传递率区段对比,发现有9个基因的标记和3个QTL位点标记锚定在川麦44高传递率区段内,这些基因被认为是潜在的川麦44高被选择基因。依据功能标记或连锁标记的等位类型推断,其中2个功能基因、和3个QTL位点、、可能是川麦44携带的重要优势等位基因或位点,在培育衍生品种过程中被优先选择保留。5个基因或QTL位点分别对穗发芽、有效分蘖数、千粒重和穗长4个性状具有正向效应。【结论】重要育种亲本川麦44基因组片段在衍生品种中的长度短,具有较高的遗传配合力,易于与不同的同源染色体重组,不易导致连锁累赘问题。、、、和是利用川麦44育种的5个重要靶基因位点,可加强对其在分子标记辅助育种中应用。
川麦44;遗传贡献;有益基因;QTL;高传递率
0 引言
【研究意义】小麦是中国最重要的粮食作物之一,其生产发展离不开优异种质资源的创新与利用。在新品种选育过程中,一些品种(系)表现出优异的丰产性、抗病性和高配合力等优点,从而被育种家广泛应用,进而培育出较多的衍生品种,这类材料被育种家称为骨干亲本[1]。骨干亲本的利用对提高品种产量、促进品种更新换代具有重要意义。【前人研究进展】前人对于小麦骨干亲本在衍生品种中遗传贡献的研究,主要是运用农艺性状分析、品质分析[2]、抗性鉴定、SSR分子标记检测[3]及通量更高的SNP全基因组扫描[4-6]等方法估算骨干亲本的遗传贡献。这些研究仅从表型或基因组水平上进行,而未从基因水平上估算骨干亲本对衍生品种的遗传贡献。对育种家而言,充分解析骨干亲本中具体哪些优异性状基因直接传递到现有的衍生品种,对更好地利用骨干亲本进行新品种选育,提高育种效率,培育高产、抗病新品种具有重要意义。随着SNP检测技术的发展,已开发出基于KASP技术的小麦功能基因标记,涉及矮秆基因[7]、产量性状相关基因[8-17]、春化基因[18-21]、光周期基因[22-24]、抗病基因[25-29]、抗倒伏基因[30]、抗旱基因[31]、抗逆基因[32]、抗穗发芽基因[33-36]、品质相关基因[37-46]、开花基因[47],这些基因功能标记将为研究骨干亲本中的基因传递提供技术支持。【本研究切入点】川麦44是由四川省农业科学院作物研究所选育的中强筋品种,具有矮秆、高产、广适等特性。截至目前,利用川麦44作为直接亲本已培育出新品种11个。郑建敏等[48]于2018年利用系谱分析方法,初步分析了川麦44的核质贡献。次年,利用小麦660K SNP芯片从DNA水平上分析了川麦44对其6个衍生品种的遗传贡献[49]。然而,相关研究还不够深入,并且还未从功能基因或QTL位点层面解析川麦44的育种贡献。【拟解决的关键问题】本研究利用荧光原位杂交对川麦44及其10个衍生品种进行分析,以明确是否存在外缘染色体的影响以及川麦44及其衍生品种在染色体层面的遗传规律;利用目前已知的部分小麦基因功能标记及Ye等[50]鉴定出的四川小麦产量性状QTL连锁分子标记对川麦44及其10个衍生品种进行分析,并结合660K SNP数据,筛选川麦44中可能的重要基因,为进一步利用川麦44进行新品种选育提供参考。
1 材料与方法
1.1 材料
材料包括10个川麦44的衍生品种,以及川麦44和另外6个参与杂交组配的亲本材料(西昌19、川麦42、川麦36、贵农21、川麦30和川农23)。10个衍生品种中,川麦63、川麦1131、川麦1145、川麦1826均来源于杂交组合川麦44/川农23;川麦66和川麦68均来源于川麦42/98-266//川麦44;川麦67的杂交组合为川麦42/川麦36//川麦44;川麦601的杂交组合为贵农21/川麦30//川麦42/川麦44;昌麦32和昌麦34的杂交组合为川麦44/昌麦19。
1.2 原位杂交分析
随机选取川麦44及其衍生品种和亲本的种子各5粒,在垫有湿润滤纸的培养皿中发芽。待根尖长至3—4 cm时,剪取根尖。参考Luo等[51]方法进行根尖的处理和体细胞制片。原位杂交探针包括2个寡核苷酸序列探针Oligo-pSc119.2-1和Oligo-pTa535[51],由成都擎科生物科技有限公司合成(中国,成都)。使用配备CCD镜头的奥林巴斯BX63荧光显微镜进行杂交信号检测并采集图像。
1.3 衍生品种中川麦44基因组区段分析
所用的660K SNP芯片分型数据来源于郑建敏等[49]研究,包括川麦44及其8个衍生品种(川麦63、川麦1131、川麦1145、川麦66、川麦68、川麦67、川麦601和昌麦32)。参照Hao等[52]方法,以中国春参考基因组v1.0为参考,将所有21条染色体分割成1 Mb大小的连续区间,统计每1 Mb区间范围内,衍生品种与其亲本在该区间内相同标记比例。如果衍生品种一个区间内标记的分析结果与某个亲本相同的比例高于其他亲本,且相同标记的比例大于0.50,则判断该区段来源于此亲本,否则判断为无法识别。统计仅能够识别来源的区段用于计算川麦44对其衍生品种的遗传贡献,计算公式为:遗传贡献率=川麦44来源区段数/总共识别出的区段数×100%。如果一个来源于川麦44的区段在8个衍生品种中的频率大于50%,则将其定义为高传递频率区段。高传递频率区段在染色体上的分布画图采用R包ggplot2(v.2.2.1)绘制。
1.4 基因功能标记和四川小麦品种产量相关性状QTL位点分析
61个涉及生育期、穗发芽、产量性状、品质、抗逆性、株高基因和抗病性的小麦基因功能基因标记[7-47]由北京中玉金标记有限公司完成。功能标记的检测平台为KASP,分析的材料包括川麦44及其衍生的10个普通小麦品种。对应基因在染色体上的位置通过BLAST中国春参考基因组v1.0确定(http://202.194.139.32/blast/viroblast.php,比对参数为默认值)。四川小麦品种与产量相关的QTL及其连锁分子标记信息从Ye等[50]中获得。
2 结果
2.1 川麦44及其衍生品种染色体核型分析
荧光原位杂交分析表明,川麦44不含有四川小麦品种中常见的2条易位染色体6VS/6AL和1RS/1BL。其衍生的10个品种中,昌麦32和昌麦34为1RS/1BL易位系(图1)。川麦44及其10个衍生品种的核型在部分染色体上呈现多态型。其中,4A染色体有2种多态型,衍生品种中与川麦44相同的频率为80%;5A染色体有4种多态型,与川麦44相同的频率为40%;6B染色体有2种多态型,与川麦44相同的频率为40%,7B染色体有2种多态型,与川麦44相同的频率为40%。
2.2 川麦44选择优势基因组区段及潜在的功能基因
由于没有98—266的660K芯片数据,仅来源于川麦44/川农23杂交组合的川麦63、川麦1131、川麦1145;川麦42/川麦36//川麦44杂交组合的川麦67;贵农21/川麦30//川麦42/川麦44杂交组合的川麦601和川麦44/昌麦19杂交组合昌麦32用于分析川麦44的遗传贡献情况。
川麦44对川麦63、川麦1131和川麦1145的遗传贡献为分别为67.0%(5 827/8 692)、72.8%(6 391/8 774)和71.3%(6 300/8 836),略高于理论值50%;对川麦67的遗传贡献为22.4%(1 994/8 684),显著低于理论值50%;对川麦601的遗传贡献为16.3%(1 189/7 275),略低于理论值25%;对昌麦32的遗传贡献为21.8%(2 237/10 285),显著低于理论值50%。
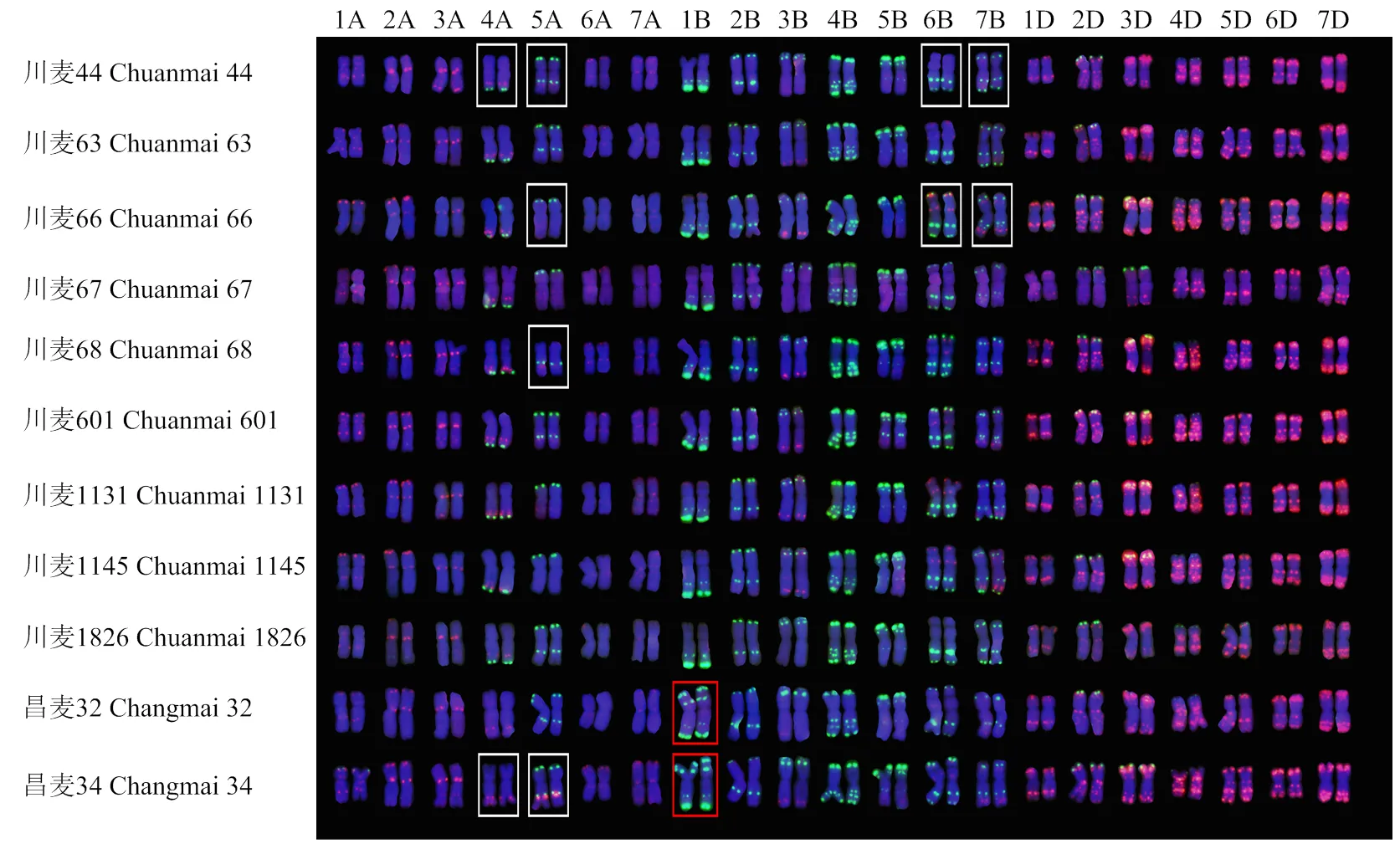
红色方框表示1RS/1BL易位系;白色方框表示染色体多态性类型
以区段在6个后代中的传递频率大于50%为标准,筛选到1 721个高传递频率片段。将相邻的连续片段进行整合后,得到1 106个染色体区段,片段平均长度为1.57 Mb(1—15 Mb),分布在所有21条染色体上(图2)。从基因组层面来看,B基因组的区段总长度和总数均最大(表1)。从不同染色体来看,区段最长的3条分别为4A(165 Mb)、2B(161 Mb)和5B(125 Mb),区段数最多的3条染色体分别为4A(100个)、2B(78个)和3B(72个)。
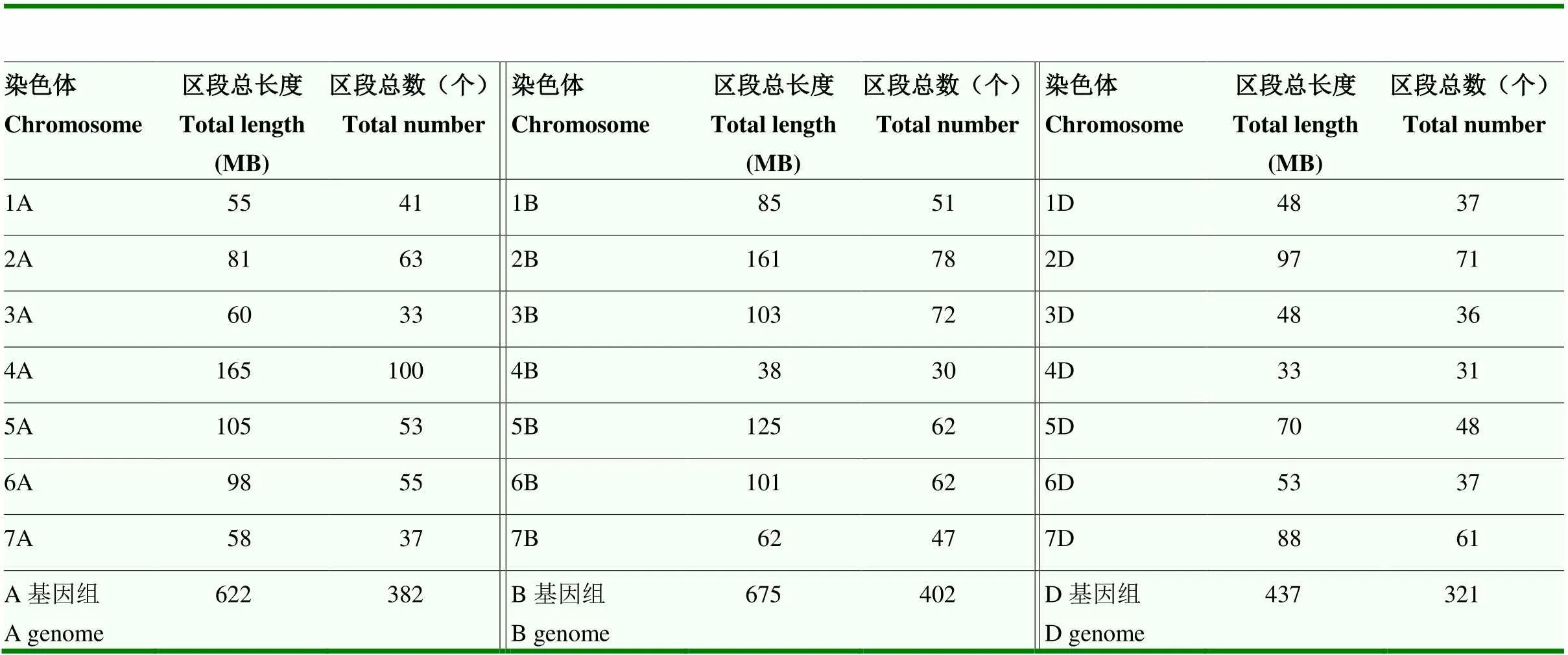
表1 川麦44传递频率大于50%的染色体区段统计情况
2.3 基因功能分子标记检测
为了寻找可能支撑川麦44作为骨干亲本的重要功能基因,利用北京中玉金标记公司开发的61个功能标记检测川麦44及其10个衍生品种。这些功能标记来自于17条染色体上的52个基因,包括产量相关基因标记16个、光周期及春化相关基因标记10个、抗病相关基因标记8个、抗穗发芽相关基因标记6个、品质相关基因标记17个、抗倒伏基因标记1个、抗逆基因标记2个和矮秆基因标记1个(电子附表1)。整体来看,这61个标记在10个衍生品种中的多样性非常低,平均一致频率为0.91(0.50—1.00)。将61个标记在染色体上的物理位置与前面660K芯片分析得到的高传递频率区段进行比较,有9个基因(9/52=17.31%)的标记(12个功能标记)落在了高传递频率区段内,这些基因被认为是潜在的川麦44高被选择基因(表2),其中包括产量相关基因2个、抗病相关基因1个、品质相关基因4个、抗穗发芽相关基因2个。然而,从基因等位类型的功能注释来看,9个基因中,仅2个在川麦44中为有利等位类型(和),分别对应性状为小麦品质和抗穗发芽。结合2个SNP标记可以判断,川麦44的基因等位类型为d;而衍生的10个品种中,除川麦68为b等位类型外,其余9份材料与川麦44相同。
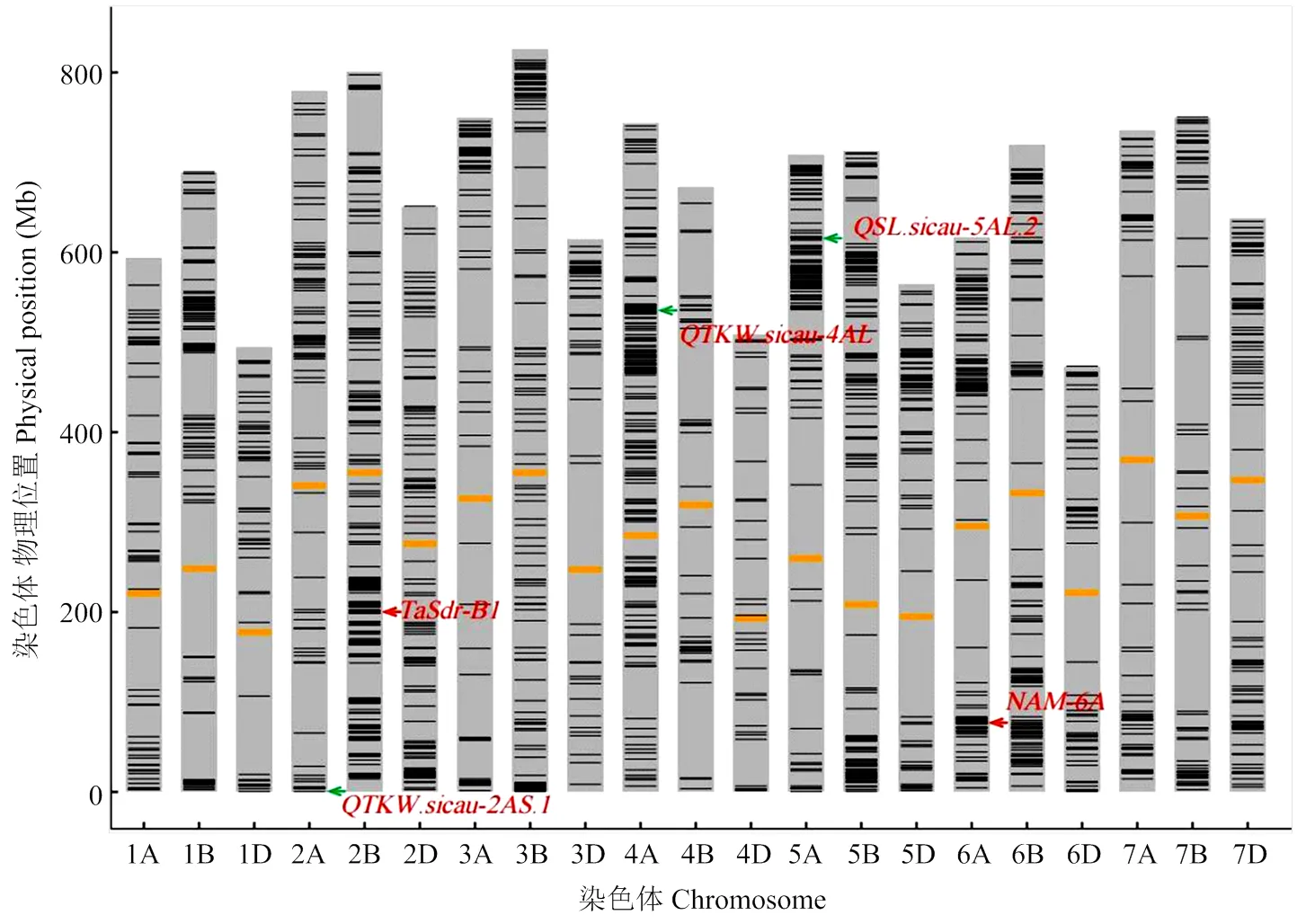
红色箭头表示川麦44的有益基因;绿色箭头表示川麦44有益QTL位点
2.4 川麦44潜在的选择优势QTL位点
Ye等[50]利用165份四川小麦品种(系)进行了产量性状的关联分析,得到13个与产量性状相关的QTL位点,与18个SNP标记连锁。为进一步寻找川麦44中潜在的重要基因位点,对川麦44中这些QTL位点(连锁SNP标记)的等位类型进行了分析。结果表明,69%(9/13)的QTL在川麦44中均为有利等位类型,连锁12个SNP标记。衍生品种与川麦44相同,且位点频率≥50%的QTL有9个(9/13=69.23%),其中3个锚定在川麦44高传递率区段内,包括、和(表3)。这3个QTL可能是川麦44的优势位点,其中,位于2AS和4AL染色体臂的这两个QTL位点对千粒重具有正向效应,而位于5AL的QTL位点对穗长具有正向效应。
3 讨论
中强筋小麦川麦44来源于杂交组合96夏440/贵农21,具有高产、分蘖力强、成穗率高、矮秆、早熟等优异特点。谷蛋白分析表明其含优质亚基5+10[53],可能是其表现为中强筋的支撑基因之一。本研究利用基因功能标记发现,川麦44含有,并且该基因在其衍生品种中的频率高达90%,川麦68是唯一与川麦44不同的品种(等位类型为)。研究表明与有效分蘖和千粒重显著关联,并且在的4种单倍型中(A1a—A1d),A1d对千粒重的贡献最大[54-55]。因此,可能是川麦44强分蘖力和高产性状的一个重要支撑基因。虽然,基因功能标记分析发现,川麦44含有抗穗发芽基因的有利等位位点且在衍生品种中的传递频率也较高(60%),但是其本身并对穗发芽的抗性并不好。穗发芽性状受复杂的基因网络控制,并且其发生受天气环境的影响非常大[56]。因此,该位点可能在育种过程中并没有受到显著的选择压(60% vs 50%的随机选择频率)。另外,本研究利用Ye等[50]发现的四川小麦品种产量性状关联的SNP标记,鉴定到3个可能对川麦44作为重要育种亲本非常重要的QTL位点、和。其中,影响穗长的位点可能具有重要利用价值,因为其在衍生品种中的传递频率为100%,具有明显的选择优势
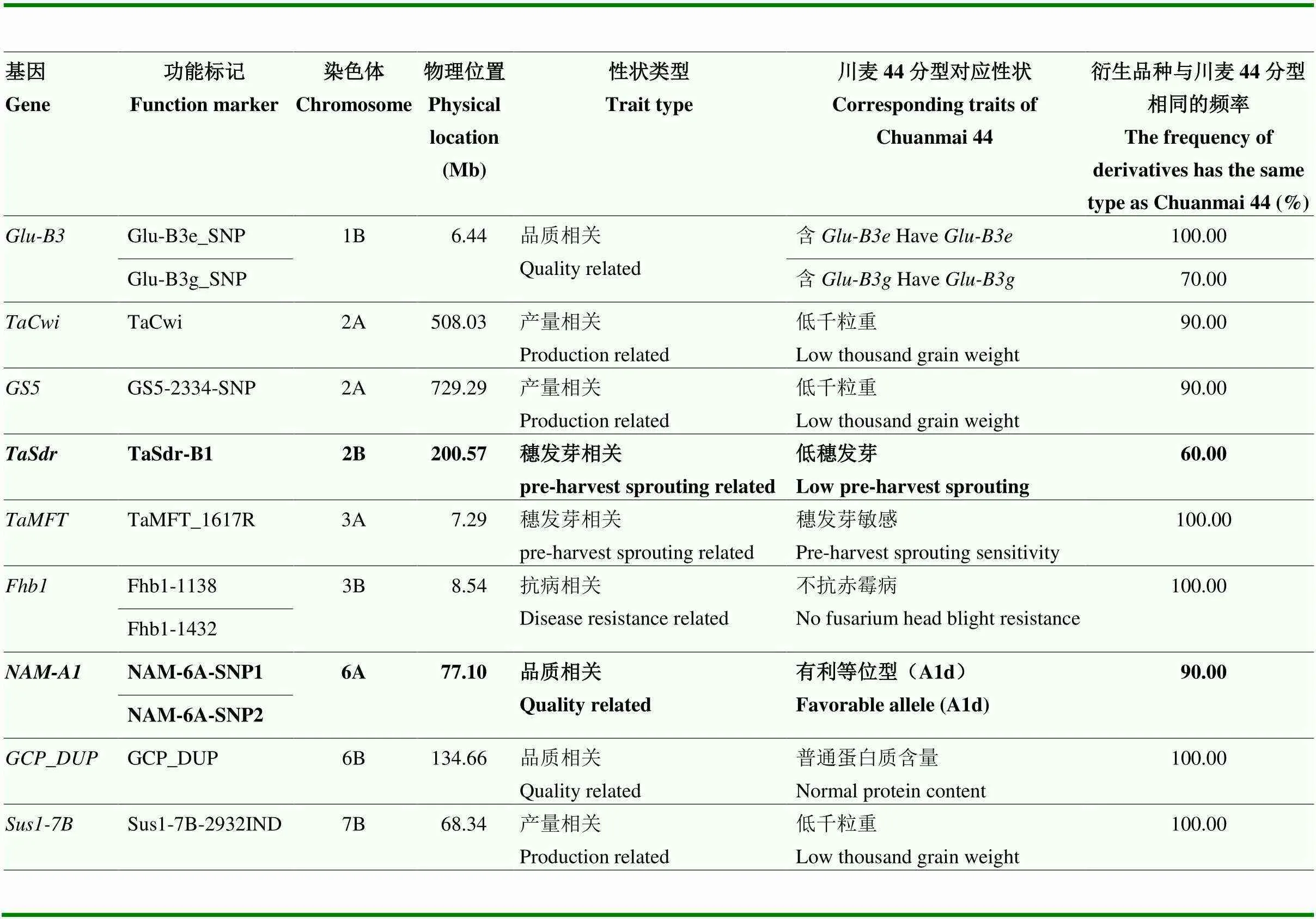
表2 川麦44高传递率基因
加粗字体表示川麦44中高传递率有利基因
The bold indicate the favorable genes with high transmission rate in Chuanmai 44
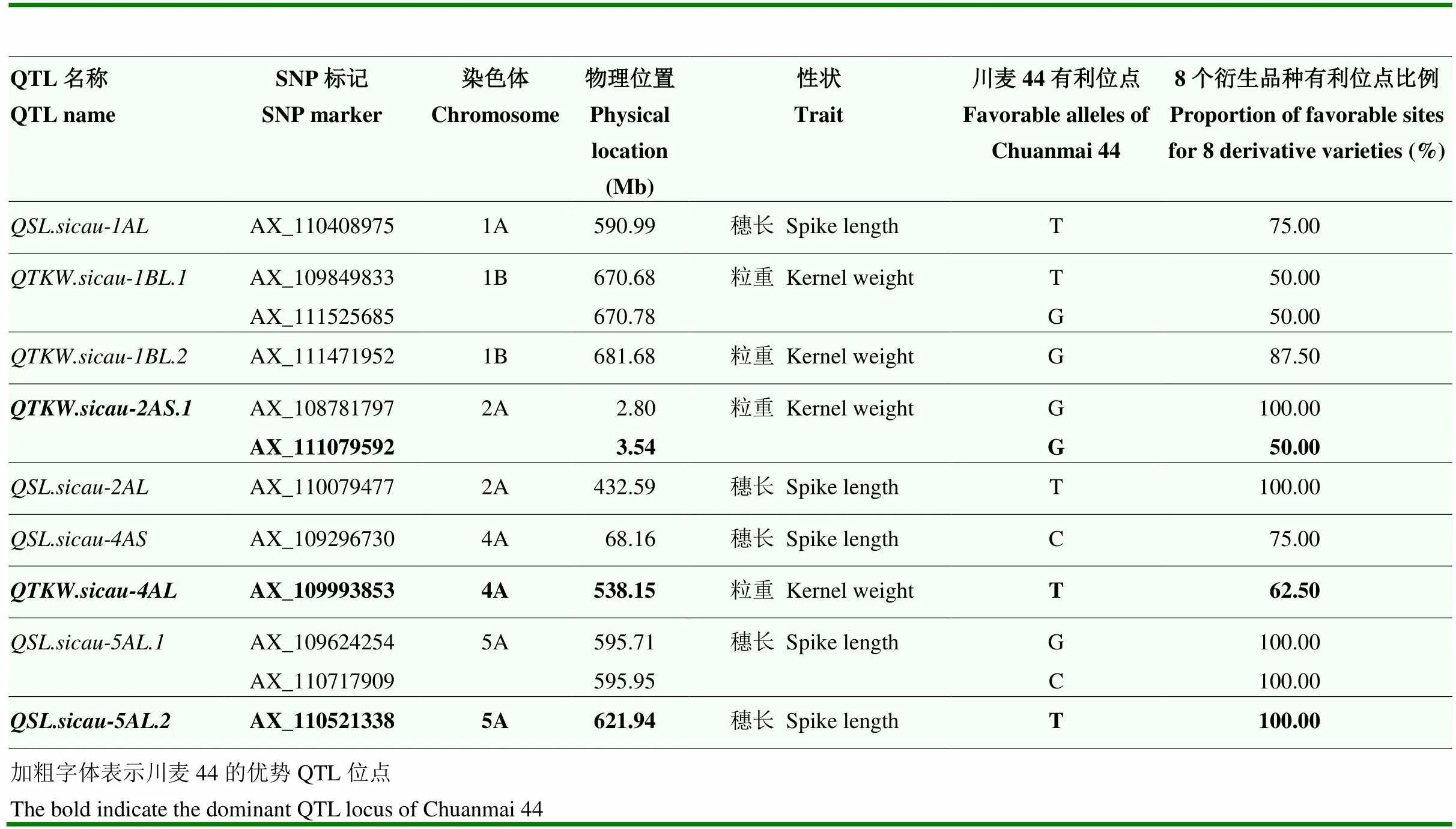
表3 衍生品种与川麦44位点相同且频率≥50%产量相关QTL
本研究分析的10个川麦44衍生品种中,有4个为弱筋,其余都为中筋。细胞学分析表明,弱筋品种中的昌麦32和昌麦34均含有1对1RS/1BL易位染色体,易位染色体来自另外一个杂交亲本-昌麦19。1RS/1BL易位染色体在给小麦带来抗病、抗逆和丰产性的同时,也使得其加工品质变差,主要表现在面团黏性增加,面筋强度减弱,面团形成时间变短,面包体积减小等[57]。因此,1RS/1BL易位染色体可能是昌麦32和昌麦34表现为弱筋的原因之一。
4 结论
在川麦44中共检测到2个高传递率的有利基因:抗穗发芽基因与有效分蘖和千粒基因;3个对产量性状具有正向效应且高传递率频率的QTL位点,其中,2个增加千粒重(和),1个增加穗长()。穗长有利位点在衍生品种中的传递频率为100%,可能具有重要利用价值。
[1] 庄巧生.中国小麦品种改良及系谱分析.北京: 中国农业出版社, 2003: 6.
ZHUANG Q S.Chinese wheat variety improvement and pedigree analysis.Beijing: China Agriculture Press, 2003: 6.(in Chinese)
[2] 唐建卫, 殷贵鸿, 高艳, 王丽娜, 韩玉林, 黄峰, 于海飞, 杨光宇, 李新平, 肖永贵, 张艳, 阎俊.小麦骨干亲本周8435B及其衍生品种(系)的农艺性状和加工品种综合分析.麦类作物学报, 2015, 35(6): 777-784.
TANG J W, YIN G H, GAO Y, WANG L N, HAN Y L, HUANG F, YU H F, YANG G Y, LI X P, XIAO Y G, ZHANG Y, YAN J.Comprehensive analysis on agronomic traits and processing quality of core parent Zhou 8425B and derivatives.Journal Tririceae Crops, 2015, 35(6): 777-784.(in Chinese)
[3] 陈国跃, 刘伟, 何员江, 苟璐璐, 余马, 陈时盛, 魏育明, 郑有良.小麦贵干亲本繁6条锈病成株抗性特异位点及其在衍生品种中的遗传解析.作物学报, 2013, 39(5): 827-836.
CHEN G Y, LIU W, HE Y J, GOU L L, YU M, CHEN S S, WEI Y M, ZHENG Y L.Specific loci for adult-plant resistance to stripe rust in wheat founder parent fan 6 and their genetic dissection in its derivatives.Acta Agronomica Sinica, 2013, 39(5): 827-836.(in Chinese)
[4] 张德强, 宋晓朋, 冯洁, 连俊芳, 孙道杰.小麦周8425B及其衍生品种与黄淮麦区主栽品种的遗传解析.麦类作物学报, 2016, 36(10): 1328-1334.
ZHANG D Q, SONG X P, FENG J, LIAN J F, SUN D J.Genetic dissection on the derived lines from wheat cultivar Zhou 8425B and widely grown cultivars in Huang-huai region.Journal Tririceae Crops, 2016, 36(10): 1328-1334.(in Chinese)
[5] 高艳, 唐建卫, 邹少奎, 胡润雨, 张根源, 孙玉霞, 王磊, 殷贵鸿.小麦周麦22及其衍生品种的遗传多样性分析.植物遗传资源学报, 2021, 22(1): 38-49.
GAO Y, TANG J W, ZOU S K, HU R Y, ZHANG G Y, SUN Y X, WANG L, YIN G H.Genetic diversity analysis of wheat cultivars/lines derived from wheat cultivar Zhoumai 22.Journal of Plant Genetic Resources, 2020, 22(1): 38-49.(in Chinese)
[6] 孙子明, 宋晓朋.小麦品种周麦16的遗传构成分析.种子, 2020, 39(9): 117-119.
SUN Z M, SONG X P.Genetic composition analysis of wheat variety Zhoumai 16.Seed, 2020, 39(9): 117-119.(in Chinese)
[7] ELLIS M, SPIELMEYER W, GALE K, REBETZKE G, RICHARDS R."Perfect" markers for theanddwarfing genes in wheat.Theoretical and Applied Genetics, 2002, 105: 1038-1042.
[8] JIANG Y M, JIANG Q Y, HAO C Y, HOU J, WANG L F, ZHANG H N, ZHANG S N, CHEN X H, ZHANG X Y.A yield-associated gene, in wheat: its function, selection and evolution in global breeding revealed by haplotype analysis.Theoretical and Applied Genetics, 2015, 128: 131-143.
[9] LI X P, ZHAO X Q, HE X, ZHAO G Y, LI B, LIU D C, ZHANG A M, ZHANG X Y, TONG Y P, LI Z S.Haplotype analysis of the genes encoding glutamine synthetase plastic isoforms and their association with nitrogen-use-and yield-related traits in bread wheat.New Phytologist, 2011, 189: 449-458.
[10] ZHANG Y, LI D, ZHANG D B, ZHAO X G, GAO X M, DONG L L, LIU J X, CHEN K L, ZHANG H W, GAO C X, WANG D W.Analysis of the functions ofhomoeologs in wheat grain weight and protein content traits.The Plant Journal, 2018, 94: 857-866.
[11] HOU J, JIANG Q Y, HAO C Y, WANG Y Q, ZHANG H N, ZHANG X Y.Global selection on sucrose synthase haplotypes during a century of wheat breeding.Plant Physiology, 2014, 164: 1918-1929.
[12] JIANG Q Y, HOU J, HAO C Y, WANG L F, GE H M, DONG Y S, ZHANG X Y.The wheat () sucrose synthase 2 gene () active in endosperm development is associated with yield traits.Functional & Integrative Genomics, 2011, 11: 49-61.
[13] MA D Y, YAN J, HE Z H, WU L, XIA X C.Characterization of a cell wall invertase geneon common wheat chromosome 2A and development of functional markers.Molecular Breeding, 2012, 29: 43-52.
[14] ZHANG Y J, LIU J D, XIA X C, HE Z H., an ortholog of rice, is associated with grain weight and grain length in common wheat.Molecular Breeding, 2014, 34: 1097-1107.
[15] ZHANG B, LIU X, XU W N, CHANG J Z, LI A, MAO X G, ZHANG X Y, JING R L.Novel function of a putativeortholog associated with spikelet number per spike in common wheat.Scientific Reports, 2015, 5: 12211.
[16] HANIF M, GAO F M, LIU J D, WEN W E, ZHANG Y J, RASHEED A, XIA X C, HE Z H, CAO S H., an ortholog of rice, is associated with grain weight and yield in bread wheat.Molecular Breeding, 2016, 36: 1.
[17] HU M J, ZHANG H P, LIU K, CAO J J, WANG S X, JIANG H, WU Z Y, LU J, ZHU X F, XIA X C, SUN G L, MA C X, CHANG C.Cloning and characterization ofgene associated with grain weight in wheat via SLAF-seq-BSA.Frontiers in Plant Science, 2016, 7: 1902.
[18] MILEC Z, TOMKOVA L, SUMIKOVA T, PANKOVA K.A new multiplex PCR test for the determination ofalleles in bread wheat (L.).Molecular Breeding, 2012, 30: 317-323.
[19] DIAZ A, ZIKHALI M, TURNER A S, ISAAC P, LAURIE D A.Copy number variation affecting theandgenes is associated with altered flowering time in wheat ().Plos One, 2012, 7(3): e33234.
[20] YAN L, HELGUERA M, KATO K, FUKUYAMA S, SHERMAN J, DUBCOVSKY J.Allelic variation at thepromoter region in polyploid wheat.Theoretical and Applied Genetics, 2004, 109: 1677-1686.
[21] FU D L, SZUCS P, YAN L, HELGUERA M, SKINNER J S, ZITZEWITZ J V, HAYES P M, DUBCOVSKY P M.Large deletions within the first intron inare associated with spring growth habit in barley and wheat.Molecular Genetics and Genomics, 2005, 274: 442-443.
[22] BEALES J, TURNER A, GRIFFITHS S, SNAPE J W, LAURIE D A.A pseudo-response regulator is mis expressed in the photoperiod insensitivemutant of wheat (L.).Theoretical and Applied Genetics, 2007, 115: 721-733.
[23] WILHELM E P, TURNER A S, LAURIE D A.Photoperiod insensitivemutations in tetraploid wheat (Desf.).Theoretical and Applied Genetics, 2009, 118: 285-294.
[24] NISHIDA H, YOSHIDA T, KAWAKAMI K, FUJITA M, BO L, AKASHI Y, LAURIE D A, KATO K.Structural variation in the 5’ upstream region of photoperiod-insensitive allelesandidentified in hexaploid wheat (L.), and their effect on heading time.Molecular Breeding, 2013, 31(1): 27-37.
[25] LAGUDAH E S, KRATTINGER S G, HERRERA-FOESSEL S, SINGH R P, HUERTA-ESPINO J, SPIELMEYER W, BROWN- GUEDURA G, SELTER L L, KELLER B.Gene-specific markers for the wheat genewhich confers resistance to multiple fungal pathogens.Theoretical and Applied Genetics, 2009, 119: 889-898.
[26] KRATTINGER S G, LAGUDAH E S, SPIELMEYER W, SINGH R P, HUERTA-ESPINO J, MCFADDEN H, BOSSOLINI E, SELTER L L, KELLER B.A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat.Science, 2009, 323(5919): 1360-1363.
[27] PURNHAUSER L, BONA L, LANG L.Occurrence of 1BL.1RS wheat-rye chromosome translocation and ofresistance gene cluster in wheat cultivars registered in Hungary.Euphytica, 2011, 179: 287-295.
[28] LI G Q, ZHOU J Y, JIA H Y, GAO Z X, FAN M, LUO Y J, ZHAO P T, XUE S L, LI N, YUAN Y, MA S W, KONG Z X, JIA L, AN X, JIANG G, LIU W X, CAO W J, ZHANG R R, FAN J C, XU X W, LIU Y F, KONG Q Q, ZHENG S H, WANG Y, QIN B, CAO S Y, DING Y X, SHI J X, YAN H S, WANG X, RAN C F, MA Z Q.Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight.Nature Genetics, 2019, 51: 1106-1112.
[29] XUE S L, XU F, TANG M Z, ZHOU Y, LI G Q, AN X, LIN F, XU H B, JIA H Y, ZHANG L X, KONG Z X, MA Z Q.Precise mapping, a major QTL conditioning resistance toinfection in bread wheat (L.).Theoretical and Applied Genetics, 2011, 123: 1055-1063.
[30] 付路平.小麦茎秆木质素含量相关基因克隆、功能标记开发和关联分析[D].北京: 中国农业科学院, 2016.
FU L P.Cloning, functional marker development and association analysis of TaCOMT, a gene related to lignin content in wheat stems [D].Beijing: Chinese Academy of Agricultural Sciences, 2016.(in Chinese)
[31] ZHANG J J, XU Y J, CHEN W, DELL B, VERGAUWEN R, BIDDULPH B, KHAN N, LUO H, APPELS R, DEN ENDE W V.A wheatvariant underlies enzyme activity for stem WSC remobilization to grain under drought.New Phytologist, 2015, 205: 293-305.
[32] WEI B, JING R L, WANG C S, CHEN J B, MAO X G, CHANG X P, JIA J Z.genes in wheat (L.): development of functional markers and gene mapping based on SNPs.Molecular Breeding, 2009, 23: 13-22.
[33] NAKAMURA S, ABE F, KAWAHIGASHI H, NAKAZONOK K, TAGIRI A, MATSUMOTO T, UTSUGI S, OGAWA T, HANDA H, ISHIDA H, MORI M, KAWAURA K, OGIHARA Y, MIURA H.A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination.The Plant Cell, 2011, 23: 3215-3229.
[34] MACKAY I J, BANSEPT-BASLER P, BARBER T, BENTLEY A R, COCKRAM J, GOSMAN N, GREENLAND A J, HORSNELLl R, HOWELLS R, OSULLIVAN D M, ROSE G A, HOWELL P J.An eight-parent multiparent advanced generation inter-cross population for winter-sown wheat: creation, properties, and validation.Genes Genomes Genetics, 2014, 4(9): 1603-1610.
[35] ZHANG Y J, MIAO X L, XIA X C, HE Z H.Cloning of seed dormancy genes () associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker.Theoretical and Applied Genetics, 2014, 127: 855-866.
[36] YANG Y, MA Y Z, XU Z S, CHEN X M, HE Z H, YU Z, WILKINSON M, JINES H D, SHEWRY P R, XIA L Q.Isolation and characterization ofgenes in wheat cultivars with distinct ABA sensitivity and pre-harvest sprouting tolerance.Journal of Experimental Botany, 2007, 58(11): 2863-2871.
[37] RODRIGUEZ-SUAREZ C, ATIENZA S G.Hordeum chilense genome, a useful tool to investigate the endosperm yellow pigment content in the.BMC Plant Biology, 2012, 12: 200.
[38] CHEN X Y, CAO X Y, ZHANG Y J, ISLAM S, ZHANG J J, YANG R C, LIU J J, LI G Y, APPELS R, KEEBLE-GAGNERE G, JI W Q, HE Z H, MA W J.Genetic characterization of cysteine-rich type-b avenin-like protein coding genes in common wheat.Scientific Reports, 2016, 6: 30692.
[39] UAUY C, DISTELFELD A, FAHIMA T, BLECHL A, DUBCOVSKY J.A NAC Gene regulating senescence improves grain protein, zinc, and iron content in wheat.Science, 2006, 314(5803): 1298-1301.
[40] SI H Q, ZHAO M L, ZHANG X, YAO G L, SUN G L, MA C X.Cloning and characterization of low-molecular-weight glutenin subunit alleles from Chinese wheat landraces (L.).The Scientific World Journal, 2014, 2014: 371045.
[41] WANG L, ZHAO X, He Z, XIA X.Characterization of low- molecular-weight glutenin subunit genes atandloci and development of functional markers in common wheat//Proceedings of the 11th International Wheat Genetics Symposium.Sydney: Sydney University Press, 2008.
[42] CORMIER F, THROUDE M, RAVEL C, GOUIS J C, LEVEUGLE M, LAFARGE S, EXBRAYAT F, DURANTON N, PRAUD S.Detection of NAM-A1 natural variants in bread wheat reveals differences in haplotype distribution between a worldwide core collection and European elite germplasm.Agronomy, 2015, 5: 143-151.
[43] HE X Y, HE Z H, ZHANG L P, SUN D J, MORRIS C F, FUERST E P, XIA X C.Allelic variation of polyphenol oxidase () genes located on chromosomes 2A and 2D and development of functional markers for thegenes in common wheat.Theoretical and Applied Genetics, 2007, 115: 47-58.
[44] HE X Y, ZHANG Y L, HE Z H, WU Y P, XIA Y G, MA C X, XIA X C.Characterization of phytoene synthase 1 gene () located on common wheat chromosome 7A and development of a functional marker.Theoretical and Applied Genetics, 2008, 116: 213-221.
[45] HIMI E, NODA K.Red grain colour gene () of wheat is a Myb-type transcription factor.Euphytica, 2005, 143: 239-242.
[46] HIMI E, MAEKAWA M, MIURA H, NODA K.Development of PCR markers forrelated to R-1, red grain color gene in wheat.Theoretical and Applied Genetics, 2011, 122: 1561-1576.
[47] ZIKHALI M, WINGEN L U, GRIFFITHS S.Delimitation of the() flowering gene to a subtelomeric chromosomal deletion in bread wheat ().Journal of Experimental Botany, 2016, 67(1): 287-299.
[48] 郑建敏, 罗江陶, 万洪深, 李式昭, 杨漫宇, 李俊, 杨恩年, 刘于斌, 蒲宗君.川麦44及其9个衍生品种比较分析.西南农业学报, 2018, 31(12): 2472-2477.
ZHENG J M, LUO J T, WAN H S, LI S Z, YANG M Y, LI J, YANG E N, LIU Y B, PU Z J.Chinese wheat variety improvement and pedigree analysis, Chuanmai 44 and its 9 derivative varieties comparative analysis.Southwest Agricultural Journal, 2018, 31(12): 2472-2477.(in Chinese)
[49] 郑建敏, 罗江陶, 万洪深, 李式昭, 杨漫宇, 李俊, 刘于斌, 蒲宗君.利用小麦660K SNP芯片分析川麦44在其衍生后代中的遗传贡献.麦类作物学报, 2019, 39(11): 1293-1300.
ZHENG J M, LUO J T, WAN H S, LI S Z, YANG M Y, LI J, LIU Y B, PU Z J.Using wheat 660K SNP chip to analyze the genetic contribution of Chuanmai 44 in its derived progeny.Journal of Triticeae Crops, 2019, 39(11): 1293-1300.(in Chinese)
[50] YE X L, LI J, CHENG Y K, YAO F J, LONG L, WANG Y Q, WU Y, LI J, WANG J R, JIANG Q T, KANG H Y, LI W, QI P F, LAN X J, MA JIAN, LIU Y X, JIANG Y F, WEI Y M, CHEN X M, LIU C J, ZHENG Y L, CHEN G Y.Genome-wide association study reveals new loci for yield-related traits in Sichuan wheat germplasm under stripe rust stress.BMC Genomics, 2019, 20: 640.
[51] LUO J T, ZHAO L B, ZHENG J M, LI Y Z, ZANG L Q, LIU D C, PU Z J, HAO M.Karyotype mosaicism in early generation synthetic hexaploid wheats.Genome, 2020, 63(7): 329-336.
[52] HAO M, ZHANG L Q, ZHAO L B, DAI S F, LI A L, YANG W Y, XIE D, LI Q C, NING S Z, YAN Z H, WU B H, LAN X J, YUAN Z W, HUANG L, WANG J R, ZHENG K, CHENG W S, YU M, CHEN X J, CHEN M P, WEI Y M, ZHANG H G, KISHII M, HAWKESFORD M J, MAO L, ZHENG Y L, LIU D C.A breeding strategy targeting the secondary gene pool of bread wheat: introgression from a synthetic hexaploid wheat.Theoretical and Applied Genetics, 2019, 132: 2285-2294.
[53] 蒲宗君, 饶世达, 杨武云, 张增艳, 蒲至恩.优质高产小麦新品种川麦44的选育研究.中国农学通报, 2006, 22(1): 120-123.
PU Z J, RAO S D, YANG W Y, ZHANG Z Y, PU Z E.Breeding of a new wheat variety Chuanmai 44 with high quality and high yield.Chinese Agricultural Science Bulletin, 2006, 22(1): 120-123.(in Chinese)
[54] ALHABBAR Z, YANG R, JUHASZ A, XIN H, SHE M, ANWAR M, SULTANA N, DIEPEVEEN D, MA W, ISLAM S.gene allelic composition and its relation to grain-filling duration and nitrogen utilisation efficiency of Australian wheat.PLoS One, 2018, 13(10): e0205448.
[55] ORLOVSKAYA O A, VAKULA S I, KHOTYLEVA L V, KILCHEVSKY A V.Estimation ofhaplotypes effect on the level of quantitative traits and grain protein content in wheat.Faktori Eksperimental Noi Evolucii Organizmiv, 2020, 26: 114-119.
[56] MOLDESTAD A, FERGESTAD E M, HOEL B, SKJELVAG A O, UHLEN A K.Effect of temperature variation during grain filling on wheat gluten resistance.Journal of Cereal Science, 2011, 53(3): 347-354.
[57] WIESER H, KIEFFER R, LELLEY T.The influence of 1B/1R chromosome translocation on gluten protein composition and technological properties of bread wheat.Journal of the Science of Food and Agricultural, 2000, 80: 1646.
The genetic contribution of the important breeding parent Chuanmai 44 to its derivatives
LUO JiangTao, ZHENG JianMin, Deng QingYan, LIU PeiXun, PU ZongJun*
Crop Research Institute of Sichuan Academic of Agricultural Sciences/Key Laboratory of Wheat Biology and Genetic Improvement on Southwestern China, Ministry of Agriculture and Rural Areas, Chengdu 610066
【Objective】Common wheat variety Chuanmai 44 has the characteristics of high yield, stable yield and wide adaptability.Ten new varieties have been selected and approved in breeding program using Chuanmai 44 as parent.It indicates Chuanmai 44 is an important breeding parent.To clarify the genetic base of Chuanmai 44 as a vital parent in breeding exercise and identify important genes or QTL within it will be helpful in breeding new elite varieties using Chuanmai 44.【Method】Fluorescencehybridization was applied to Chuanmai 44 and its ten derived varieties to identify whether there were wheat-alien translocations, and to analyze the chromosome diversity among them.The 660K SNP array data of Chuanmai 44 and its derived varieties were used to calculate the genetic contribution of Chuanmai 44 to its derived varieties and clarify the high transmission genomic segments.Functional molecular markers within cloned genes and linked molecular markers for yield-related traits were used to identify important genes or QTL in Chuanmai 44 for breeding.【Result】Chuanmai 44 did not harbor the 6VS/6AL and 1RS/1BL translocation chromosomes which both frequently existed in wheat varieties in Sichuan.Only two out of its ten derivatives, Changmai 32 and Changmai 34, contained 1RS/1BL translocation, which is inherited from another parent Changmai 19.The existence of 1RS/1BL translocation in the two varieties may explain their weak gluten phenotype.Except wheat-relative translocation, the karyotypes of Chuanmai 44 and its 10 derivative varieties also showed polymorphisms on some chromosomes.For instance, there were two types of chromosome 4A among derivatives, and 80% of them showed the same as Chuanmai 44.Chromosomes 5A, 6B and 7B had 4, 2 and 2 karyotypes, respectively.These three chromosomes in the derivative population of Chuanmai 44 showed the same karyotype with Chuanmai 44 in a frequency of 40%.660K SNP chip analysis identified 1127 genomic segments with high transmission frequency (>50%) within its derived varieties.These genomic segments located on all 21 chromosomes and their mean length was 1.57 Mb.B genome owned the most number and the largest length of the high transmission frequency segments.Chromosomes 4A, 2B and 5B were the three chromosomes with the longest high transmission frequency segments.Chromosomes 4A, 2B and 3B were the three chromosomes with the most number of high transmission frequency segments.Combing the genotype data of 61 functional markers of cloned wheat gene and 13 SNP markers linked with yield-related QTL and the distribution of Chuanmai 44 high transmission genomic regions, we discovered that there are 9 genes markers and 3 QTL markers are anchored in the high transmission rate section of Chuanmai 44.The twelve markers responding to two favorable alleles and three QTL, including,,,,, which exhibited positive effect on preharvest sprouting resistance, effective tiller number, thousand grain weight and spike length, respectively.【Conclusion】The length of genomic segments retained within its derived varieties was short.It suggested that Chuanmai 44 as a breeding parent had high genetic combining ability, and its chromosomes were easy to recombine with different homologous chromosomes in resulting hybrids, which is beneficial to reduce linkage drag.Therefore, it plays an important role as a skeleton parent in breeding excercise.,,,andwere the important loci in Chuanmai 44, which should be widely used in further breeding program under molecular marker assisted selecting.
Chuanmai 44; genetic contribution; beneficial gene; QTL; high transmission rate
2021-02-03;
2021-04-06
国家重点研发计划(2016YFD0101600,2017YFD0100905)、四川省科技计划(2018JY0627)、四川省财政创新能力提升工程项目(2016ZYPZ-015)、四川省育种攻关项目(2021YFYZ0002)
罗江陶,E-mail:jtluohao@163.com。通信作者蒲宗君,E-mail:pzjun68@163.com
(责任编辑 李莉)
