lncreased ammonification,nitrogenase,soil respiration and microbial biomass N in the rhizosphere of rice plants inoculated with rhizobacteria
2021-10-22ZHANGJunhuaHUANGJingSajidHUSSAlNZHULianfengCAOXiaochuangZHUChunquanJlNQianyuZHANGHui
ZHANG Jun-hua,HUANG Jing,Sajid HUSSAlN,ZHU Lian-feng,CAO Xiao-chuang,ZHU Chunquan,JlN Qian-yu,ZHANG Hui
1 State Key Laboratory of Rice Biology,China National Rice Research Institute,Hangzhou 310006,P.R.China
2 Agricultural Resources and Environment Institute,Jiangsu Academy of Agricultural Sciences,Nanjing 210014,P.R.China
Abstract Azospirillum brasilense and Pseudomonas fluorescens are well-known plant growth promoting rhizobacteria. However,the effects of A. brasilense and P. fluorescens on the N cycles in the paddy field and rice plant growth are little known. This study investigated whether and how A. brasilense and P. fluorescens contribute to the N transformations and N supply capacities in the rhizosphere,and clarified the effects of A. brasilense and P. fluorescens on the N application rate in rice cultivation. Inoculations with A. brasilense and P. fluorescens coupled with N application rate trials were conducted in the paddy field in 2016 and 2017. The inoculations of rice seedlings included four treatments:sterile saline solution (M0),A. brasilense (Mb),P. fluorescens (Mp),and co-inoculation with a mixture of A. brasilense and P. fluorescens (Mbp). The N application rate included four levels:0 kg N ha-1 (N0),90 kg N ha-1 (N90),180 kg N ha-1 (N180),and 270 kg N ha-1 (N270). The results indicated that the Mbp and Mp treatments significantly enhanced the ammonification activities in the rhizosphere compared with the M0 treatment,especially for higher N applications,while the Mbp and Mb treatments greatly enhanced the nitrogenase activities in the rhizosphere compared with the M0 treatments,especially for lower N applications. Azospirillum brasilense and P. fluorescens did not participate in the nitrification processes or the denitrification processes in the soil. The soil respiration rate and microbial biomass N were greatly affected by the interactions between the rhizobacteria inoculations and the N fertilizer applications. In the Mbp treatment,N supply capacities and rice grain yields showed no significant differences among the N90,N180,and N270 applications. The N application rate in the study region can be reduced to 90 kg N ha-1 for rice seedlings co-inoculated with a mixture of A. brasilense and P. fluorescens.
Keywords:N transformation,paddy soil,plant growth promoting rhizobacteria,rice productivity
1.lntroduction
Rice (OryzasativaL.) is a major cereal crop contributing to food security and income in Asia (Louet al.2012). Yield from 1 ha of rice could support 5.7 people for 1 year (Yuet al.2018). With an ever-increasing population,rice grain yield needs to increase by more than 60% in the coming three decades to fulfill the demands. N fertilization is one of the important agricultural practices for increasing rice grain yield in modern farming (Souzaet al.2013). During 1960-2014,the amount of global N application increased from 11.6×106to 108.9×106t,an average increase of up to 9.4 times. In China,the N application amount increased by 45.9 times. About 20% of the world’s rice fields are in China,and the annual consumption of N fertilizers in the paddy fields accounts for 37% of the global consumption (Yuet al.2018). However,about 40% of the applied N fertilizers are taken up by the rice plants,with the other 60% being lostviadenitrification (15-42%),ammonia volatilization (1-47%),runoff (5%) and leaching (2%) (Inselsbacheret al.2009;Caoet al.2014;Yuet al.2018). A large amount of the applied N fertilizers are lost to the ambient environments,resulting in water eutrophication and increasing emissions of greenhouse gases (Daiet al.2017;Leiteet al.2019).To improve the environmental quality and soil fertility deteriorated by excessive N application,it is essential to identify alternative measures to enhance plant productivity (Mahantyet al.2017).
Plant-microbe interactions in the rhizosphere are positively and closely associated with plant health and soil fertility (Piiet al.2015). Etesami and Alikhani (2016) observed that inoculating rice plants with bacterial isolates reduced the N application rates in paddy soil by approximately 25%. Floreset al.(2010) reported that the biological N2-fixation contribution of theAzospirillumbrasilense(A.brasilense) to the total N in plants was as high as 46%. Thus,inoculations with plant growth promoting rhizobacteria (PGPR) have been implemented in certain agricultural settings as a measure to increase plant productivities and to minimize the use of N fertilizers.
PGPR can directly affect plant growth by solubilizing minerals,fixing atmospheric N2,and producing siderophores and plant growth regulators (Mahantyet al.2017). Present studies mainly focus on the PGPR screening and the functions of PGPR. Few studies have focused on the contributions of PGPR to the N cycles in the paddy field. Soil microorganisms participate in a wide range of N cycle processes in the soil and are critical to the N cycling (Perelomov and Chulin 2014). The N cycles in soil include four major steps:N mineralization (ammonification),nitrification,N2fixation,and denitrification. The N mineralization process is an important source of the available N in soil. The microorganisms involved in the mineralization process mainly includePseudomonasfluorescens,ProteusvulgarisandBacillussubtilis(Craig and Fraterrigo 2017). The nitrification process in the paddy soil can increase the NO3--N level in soil,which can be easily absorbed by the rice plants but also easily lost by leaching and gaseous volatilization. Nitrosospira and Nitrosomonas are the main bacteria involved in the nitrification processes (Singh 2013).The non-symbiotic N2fixation process in the paddy field is very important in improving the N supply capacity of soil. The bacterial species with a high N2fixation potential in the rice rhizosphere includeAzospirillumsp.,Azotobactersp.,B.polymyxa,Beijerinckiasp.,etc.(Singh 2013). The results of Rodrigueset al.(2008) demonstrated that 27% of the N in rice came from the biological N2fixation after inoculation of rice plants withA.brasilense. The gaseous N losses caused by the denitrification processes are an important cause of the low N utilization efficiency in the paddy fields,and the N losses caused by the denitrification processes can reach 46% of the applied N fertilizers (Wang 2017). Most denitrifying bacteria are anaerobic bacteria,includingThiobacillus,Propionobaxterium,andAlcaligenes(Cui 2016). Most of the available N in the soil is derived from the microbial ammonification processes,nitrification processes,and N2fixation processes (Kox and Jetten 2015;Craig and Fraterrigo 2017). The involvements of PGPR in the N transformations and N supply capacities in the paddy fields are important in the utilization of N fertilizers in the rice-soil system.
Azospirillum brasilenseandP.fluorescensare wellknown PGPR that can enhance plant productivities in many environments (Salamoneet al.2010;Rêgoet al.2018).The beneficial stimulatory effects ofA.brasilenseandP.fluorescenson plants have been attributed to a variety of mechanisms,including bio-fertilizations and bio-control properties (Bhardwajet al.2014). It has been previously found thatA.brasilenseandP.fluorescenscould improve the ammonification activities and nitrogenase activities (no N input) and the mineralized N contents in the blank soil (Zhanget al.2018). However,whetherA.brasilenseandP.fluorescensplay a role in the N transformations in the paddy field and plant productivities of the rice plants remains unclear. The purposes of the present study were to investigate whether and howA.brasilenseandP.fluorescenscontribute to the N cycles in the paddy field,to assess the effects ofA.brasilenseandP.fluorescenson N supply capacities in the field soil,and to clarify the performances ofA.brasilenseandP.fluorescensin reducing the N application rate in rice cultivation.
2.Materials and methods
2.1.Site description
Field experiments were carried out at China National Rice Research Institute (39°4´49´´N,119°56´11´´E),Zhejiang Province,China,in 2016 and 2017. The site belongs to the northern subtropical humid climatic zone. During the experimental periods,the annual mean temperature was 16.3°C,and the annual mean rainfall was 1 438.6 mm. The texture of the soil (0-30 cm) was loam clay,in which the clay particles (<0.002 mm),silt particles (0.002-0.05 mm) and sand particles (>0.05 mm) accounted for 24.2,68.0 and 7.8%,respectively. The organic matter content of the soil was 40.4 g kg-1,the total N content was 1.5 g kg-1,the alkalihydrolyzable N content was 151.9 mg kg-1,the exchangeable base cation was 2.79 cmol kg-1,the active acidity (pH) was 6.7,and the potential acidity was 2.41 cmol kg-1.
2.2.Bacteria preparation and inoculation treatments
TheA.brasilense(BNCC139156,serial number in BeNa Culture Collection) andP.fluorescens(BNCC159133,serial number in BeNa Culture Collection) strains used in this trial were isolated from the roots of field-grown rice plants,which were provided by the BeNa Culture Collection.TheA.brasilensestrains were cultured inAzospirillummedium at 30°C for 24 h,and the pure bacterial cultures were centrifuged and diluted to a final concentration of 108CFU mL-1by sterile saline solution (0.85% NaCl). TheAzospirillummedium consisted of mannite (20.0 g L-1),yeast extract (0.5 g L-1),K2HPO4(0.8 g L-1),MgSO4·7H2O (0.2 g L-1),CaSO4·2H2O (0.1 g L-1),FeCl3·6H2O (0.0025 g L-1),and Na2MoO4·2H2O (0.002 g L-1). TheP.fluorescensstrains were cultured in nutrient broth medium at 30°C for 24 h,and the pure bacterial cultures were also centrifuged and diluted to a final concentration of 108CFU mL-1by sterile saline solution. The nutrient broth medium consisted of peptone (5 g L-1),NaCl (5 g L-1),and beef extract (3 g L-1).
2.3.Experimental design
A split plot design was adopted in this study withA.brasilenseandP.fluorescensinoculations in the main plots and different N application rates in the subplots. The inoculations of rice seedlings included four treatments:sterile saline solution (M0),A.brasilense(Mb),P.fluorescens(Mp),and coinoculation with a mixture ofA.brasilenseandP.fluorescens(Mbp). For the Mbptreatment,the inoculums were prepared with a 1:1 ratio (v/v) of theA.brasilenseandP.fluorescensstrains. The final concentration of the inoculum was 108CFU mL-1. The roots of the rice seedlings (1 000 seedlings) were submerged in the bacterial suspensions (3 000 mL) for 3 h,and the treated rice seedlings were then immediately transplanted into the fields. The N application rates also consisted of four treatments:0 kg N ha-1(N0),90 kg N ha-1(N90),180 kg N ha-1(N180),and 270 kg N ha-1(N270).
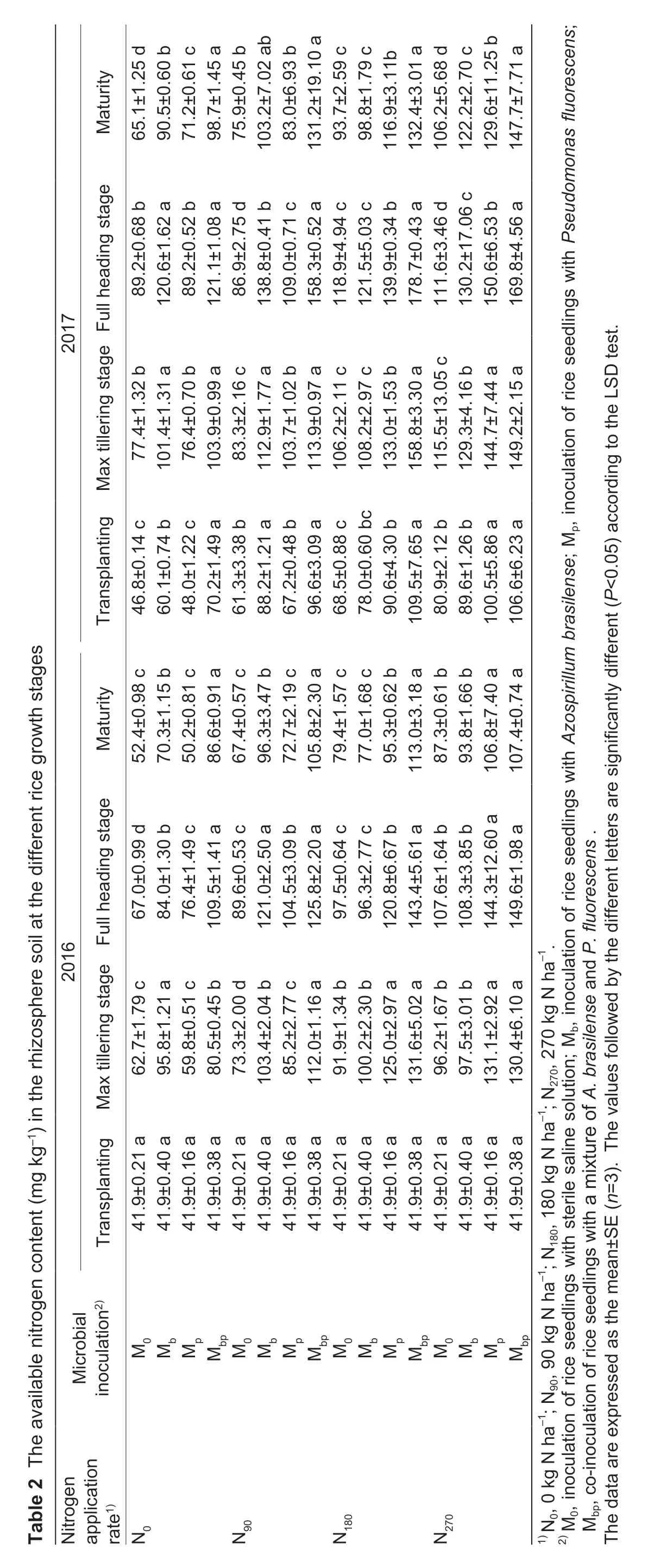
The rice cultivar used in this experiment was Zhongzheyou 8 (indica),and the growth periods ranged from 135 to 145 days from sowing to maturity. The rice seedlings were transplanted at a hill spacing of 17 cm×30 cm with one seedling per hill. The data of the rice growth stages are listed in Table 1.

Table 1 Planting date in 2016 and 2017
The subplots,24.08 m2(5.6 m×4.3 m) each,were replicated 3 times. Urea (46% of N),superphosphate (15% of P2O5),and potassium chloride (60% of K2O) supplied N,P,and K,respectively. A total of 60% of the N fertilizers were basally applied,30% were topdressed at the rice tillering stage,and the remaining 10% were applied at the rice booting stage. The P fertilizers and K fertilizers were applied following local practices.
2.4.Sampling
Rhizosphere soils were collected from the rice roots by shaking off the loosely adhered soils. The soils were sampled at the rice transplanting stage,max tillering stage,full heading stage,and maturity stage to measure the ammonification activities,nitrification activities,nitrogen fixation activities (nitrogenase activities,ARAs),denitrification activities,and available N contents.Rhizosphere soils were also collected at the rice max tillering stage and full heading stage to measure the soil respiration rates and microbial biomass N (MBN) contents. Soils samples were also collected before rice transplanting and after harvesting to measure the total N contents in the soil. The rice grain yield and yield components were also recorded.
2.5.Ammonification activity,nitrification activity,and ARAs
The ammonification activities,nitrification activities,and ARAs in the soils were determined according to Liet al.(2008) with modifications (Zhanget al.2018). The ammonification activities were expressed as mg NH4+-N per kg dry soil per day,the nitrification activities were expressed as the percent reduction in the NO2--N after inoculations,and the ARAs were expressed as nmol ethylene (C2H4)per g dry soil per hour.
2.6.Denitrification activity
The denitrification activities in the soils were measured according to the nitrate loss rate method (Liet al.2008).About 10 g of the soils were inoculated with 100 mL of medium containing NO3--N. The medium was composed of KH2PO4(1.63 g L-1),KNO3(1.82 g L-1),K2HPO4·3H2O (6.39 g L-1),and glucose (0.72 g L-1). The soils were incubated at 25°C in the dark for 2 days to measure the NO3--N contents. The denitrification activities were expressed as the consumption of NO3--N contents per day.
2.7.Soil respiration rate
The soil respiration rates in the rice rhizosphere were measured according to Iovienoet al.(2009) with specific modifications. Soil respirations were measured by CO2evolution rates at 28°C after 24 h of incubations in the darkness,with moisture content adjusted at 60% of water holding capacity. The CO2concentration in the headspace of incubation vials was measured by a gas chromatograph equipped with an electron capture detector (Agilent 7890A,Agilent Technologies Inc.,USA). The soil respiration rates were expressed as mg CO2per kg dry soil per day.
2.8.Microbial biomass N
The MBN contents were determined by a fumigationextraction method as previously described (Kanerva and Smolande 2007). Soil samples were fumigated with ethanolfree chloroform vapor at 28°C for 24 h,and the N flushes from the microbial biomass were calculated by subtracting the K2SO4-extractable N in the unfumigated control samples from those in the fumigated samples. The N flushes were converted to MBN according to the formulas of Martikainen and Palojärvi (1990).
2.9.Available N and Total N
Available N (inorganic N,NH4+-N and NO3--N) contents in the soils were measured by the extraction method as described by Lu (1999). Fresh soil samples were extracted with 2 mol L-1of KCl solution,and then filtered through a 0.45-μm membrane to measure the NH4+-N and NO3--N with a continuous flow analyser (Skalar Co.,the Netherlands). The total N contents in the soils were measured by the Kjeldahl digestion method using a Kjeltec analyser (Kjeltec TM8400 Analyser,Foss,Sweden) (Lu 1999).
2.10.Statistical analysis
Statistical analyses of the data were accomplished by standard analysis of variance (ANOVA),and pairs of mean values were compared based on the least significant difference (LSD) at the 5% level using the SPSS 17.0.Correlation analyses were conducted by the Pearson correlation coefficient using SPSS 17.0. The evaluations of rice grain yield and yield components due to the interactions among year,N application rate,and microbial inoculation were performed with general linear models using SPSS 17.0.
3.Results
3.1.N transformations in the rice rhizosphere
Ammonification activityThe ammonification activities in the rhizosphere increased with the increasing N application rates (r=0.893,P<0.01) (Fig.1). Compared with N0,the ammonification activities in the rhizosphere in N90,N180,and N270increased by 60.6-63.1%,148.1-161.4%,and 267.0-309.0%,respectively. The Mbpand Mptreatments played positive roles in improving the ammonification activities in the rhizosphere. The ammonification activities in Mbpwere 33.7-66.5% higher than those in M0,and those in Mpwere 38.7-73.3% higher than those in M0. The average ammonification activities in Mpwere even higher than those in Mbp,suggesting thatP.fluorescensplayed an important role in promoting the ammonification process in the rhizosphere.
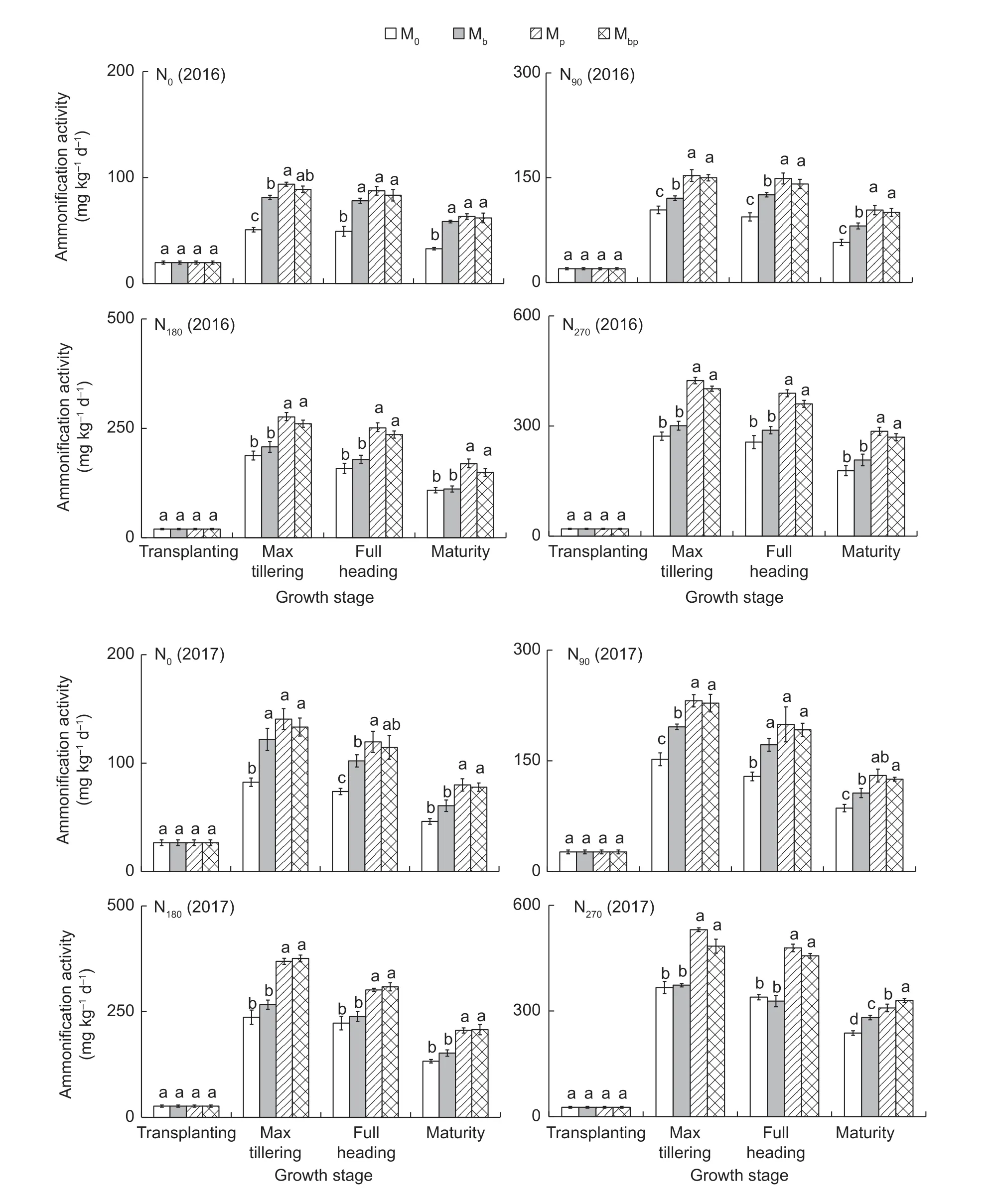
Fig.1 Ammonification activity in the rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
The ammonification activities in the soil were closely associated with different rice ontogenetic stages.Regardless of the magnitude of the N application rates,the ammonification activities were high at the rice max tillering stage and the full heading stage. The higher ammonification activities in the soil suggested higher available N contents in the soil.
Nitrification activityThe nitrification processes can improve the N availabilities in the soil to a certain degree,while the nitrification processes also increase the N losses from the soil to the groundwater through NO3--N leaching. The nitrification activities in the rice rhizosphere were significantly positively related to the N application rates (r=0.941,P<0.01) (Fig.2).No significant differences in nitrification activities were observed between the rhizobacteria treatment and the M0treatment.Azospirillum brasilenseandP.fluorescensmight not participate in the nitrification processes in the soil. The average nitrification activities at the rice maximum tillering,full heading,maturity and transplanting stages were 20.2,16.4,14.7 and 6.1%,respectively. Results suggested that the nitrification activities in the rice rhizosphere were notably affected by the N application rate and rice growth stages.
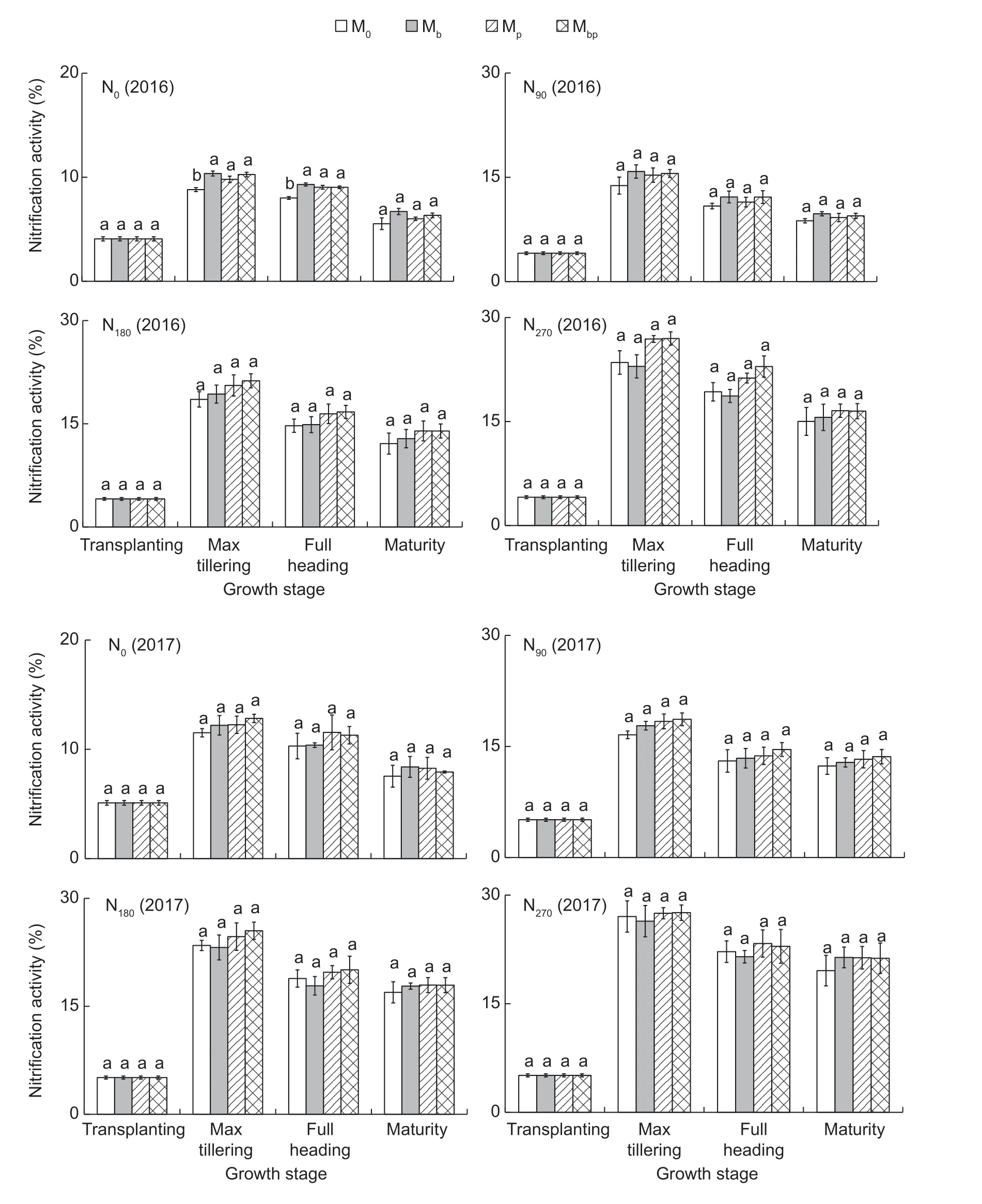
Fig.2 Nitrification activity in the rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
Nitrogen fixation activityLow N application rates (≤90 kg N ha-1) stimulated the ARAs in the rhizosphere,and the ARAs in the soil were seriously inhibited when the N application rate exceeded 90 kg N ha-1(Fig.3). Results of the correlation analysis indicated that the ARAs in the soil were significantly negatively associated with the N application rate (r=-0.606,P=0.01). Compared to the ARAs in N0,those in N90increased by 15.3-16.9%,while those in N180and N270decreased by 19.2-30.1% and 42.4-43.2%,respectively.
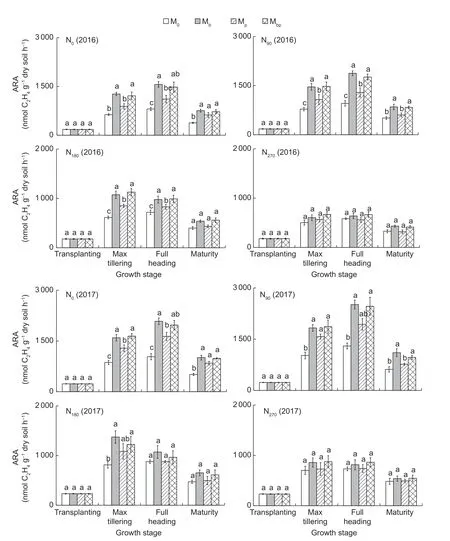
Fig.3 Nitrogenase activity (ARA) in the rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
Both the Mbpand Mbtreatments greatly increased the ARAs in the soil,and the contributions of the Mbpand Mbtreatments to ARAs were significantly higher for lower N than higher N applications. The ARAs in the Mbp,Mb,and Mptreatments under N0were significantly higher than those in the M0treatments,increasing by 79.6-82.8%,88.2-86.0%,and 40.0-51.2%,respectively. However,the ARAs in the Mbp,Mb,and Mptreatments under N270exhibited no significant differences from those in the M0treatment. The contributing effects of the Mbtreatment on the ARAs were even higher than those of the Mbptreatment. The results suggested that theA.brasilensestrain used in this trial played a positive and important role in enhancing the ARAs in the rice rhizosphere. In general,the Mbptreatment contributed the greatest to improving the ammonification activities,nitrification activities,and ARAs in the soil compared to the Mb,Mp,and M0treatments.
Denitrification activityDenitrification processes are highly linked with the N use efficiencies and N losses from the paddy fields. The denitrification activities in the rice rhizosphere were significantly positively related to the N application rates (r=0.976,P<0.01) (Fig.4). The high denitrification activities in the soil resulted from the high N fertilizer inputs accelerated N losses from the soil. There were no significant differences in denitrification activities between the inoculation treatments and the M0treatment.The results illustrated thatA.brasilenseandP.fluorescensdid not participate in the denitrification processes in the soil.
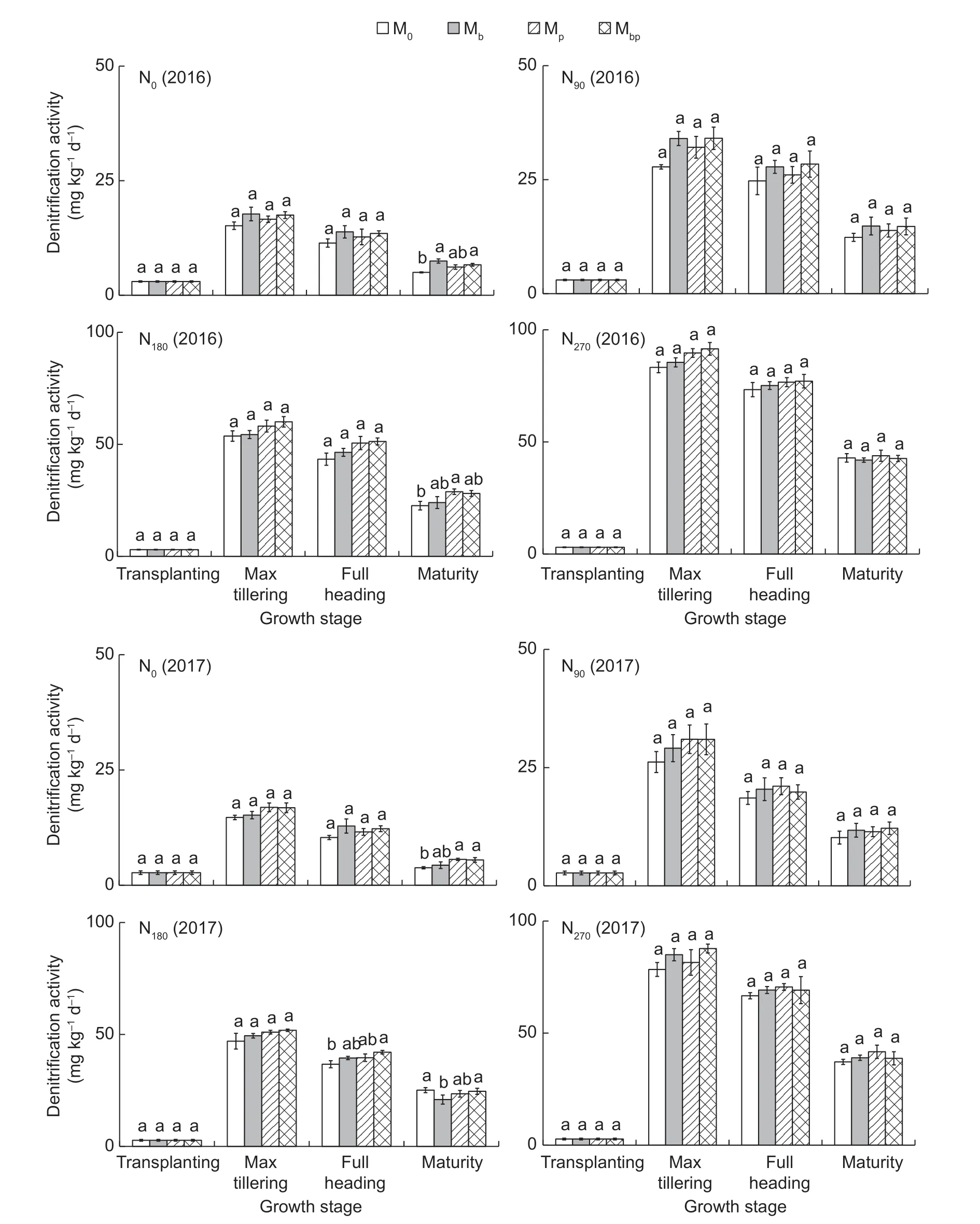
Fig.4 Denitrification activities in the rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
3.2.Microbial activity in the rice rhizosphere
Soil respirationThe soil respiration rates were significantly positively related to the N application rates (r=0.881,P=0.01). The soil respiration rates were also greatly affected by the interactions between rhizobacteria inoculations and the N application rates (Fig.5). The soil respiration rates in the Mbpand Mbtreatments were higher than those in the Mpand M0treatments at low N fertilizer applications,while the soil respiration rates in the Mbpand Mptreatments were higher than those in the Mband M0treatments at high N fertilizer applications. Results suggested that the increased soil respiration rates attributed toA.brasilensewere higher under low N application conditions,while the increased soil respiration rates attributed toP.fluorescenswere higher under high N application conditions.
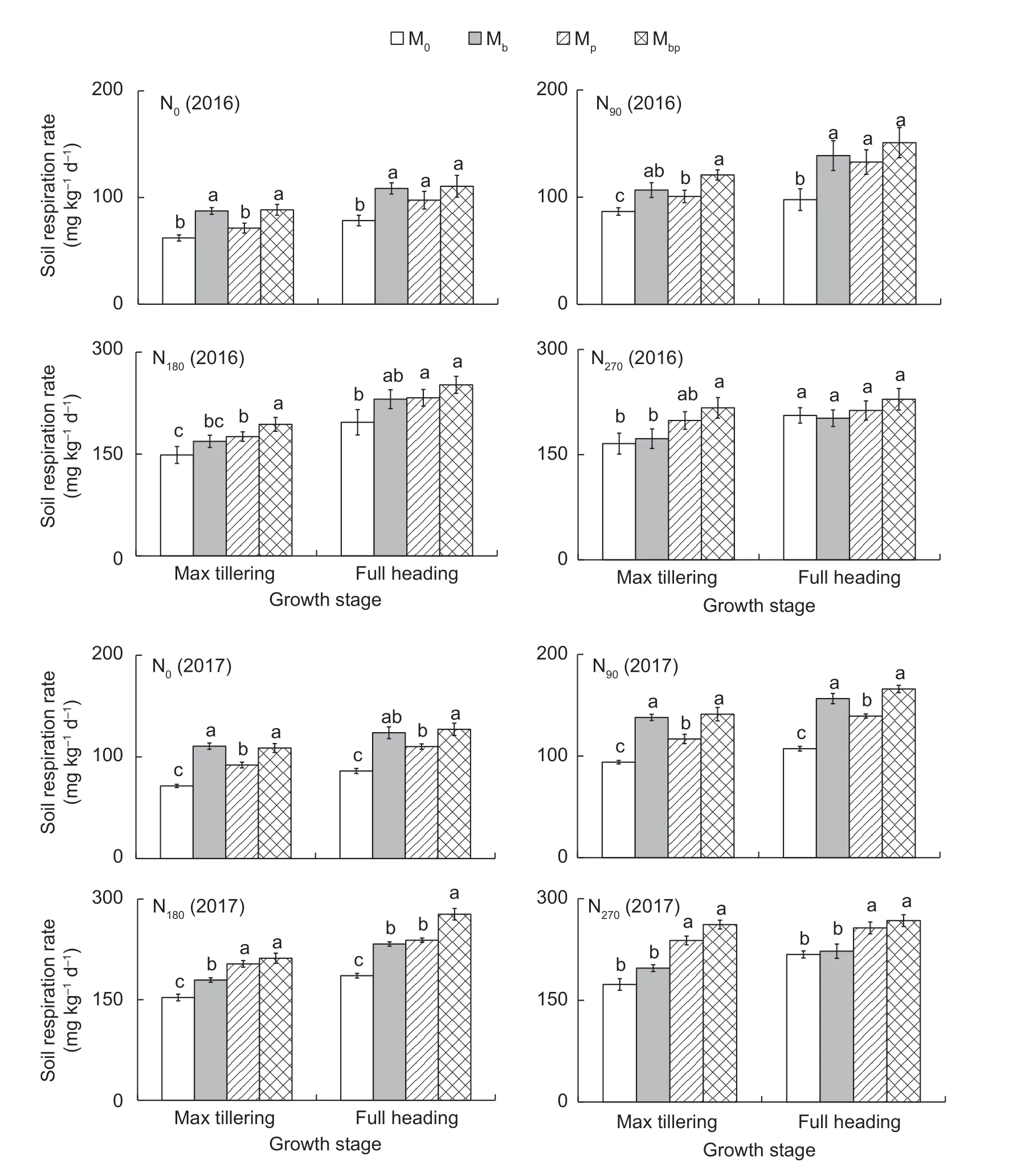
Fig.5 The soil respiration rates in rice rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
Microbial biomass NThe MBN contents in the rhizosphere increased with increasing N application rates when the N application rates were lower than 180 kg N ha-1(r=0.766,P=0.01) (Fig.6). After the N rates exceeded 180 kg N ha-1,applying N fertilizers exerted no positive effects on the MBN contents in the rhizosphere. Consistent with the variations in the soil respiration rates,the MBN contents were also greatly affected by the interactions between the rhizobacteria inoculations and the N application rates. The MBN contents in the Mbpand Mbtreatments were significantly higher than those in the Mpand M0treatments at low N rates (≤90 kg N ha-1),while the MBN contents in the Mbpand Mptreatments were higher than those in the Mband M0treatments at high N rates (>90 kg N ha-1).
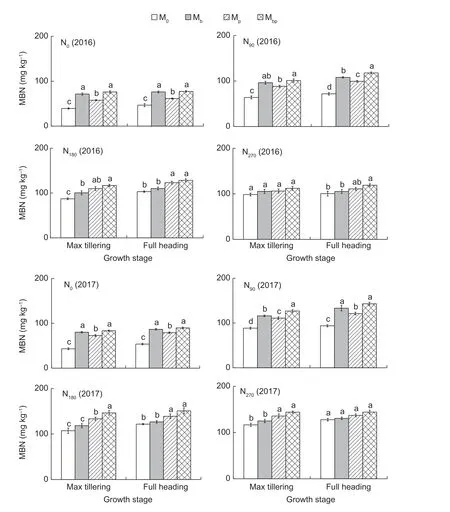
Fig.6 The microbial biomass N (MBN) contents in rhizosphere in 2016 and 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
3.3.N supply capacity
The available N contents in the rhizosphere were significantly positively related to the N application rates (r=0.542,P=0.01) (Table 2). Compared with the available N contents under N0,those under N90,N180,and N270increased by 24.5,40.4,and 50.3%,respectively. Similar to the results of the N transformations and microbial activities in the rhizosphere,A.brasilenseplayed a positive role in increasing available N contents in the rhizosphere for lower N rates,whileP.fluorescensplayed a positive role in increasing available N contents in the rhizosphere for higher N rates.
The available N contents and total N contents in the soil can reflect the N supply capacities of the soil. TheA.brasilenseandP.fluorescensinoculations greatly increased the total N contents in the soil under the same N application rates (Table 3). The total N contents in the rhizosphere in the Mbtreatment were 3.3-16.8% higher than those in the M0treatment,and those in the Mbptreatment were 3.4-16.3% higher than those in the M0treatment,while those in the Mptreatment were (-7.3)-11.1% higher than those in the M0treatment. These results suggested that the Mband Mbptreatments greatly increased the total N contents in the soil,and the contributions were more significant for low N application rates. The N transformations in the rhizosphere (Figs.1-4) suggested that N2-fixation processes improved N supply capacities,and the ARAs in the soil were also higher for low N application rates than for high N application rates. Generally,co-inoculation with a mixture ofA.brasilenseandP.fluorescenspositively contributed the N cycles in the soil,thereby improving the N supply capacities of the soil which were beneficial to rice growth and development.
3.4.Rice grain yield and yield components
There were significant differences in the rice grain yield and yield components (except for the 1 000-grain weight) among the year,nitrogen application rate,and microbial inoculation treatment (P<0.01) (Table 4). No significant interaction effects were observed on the rice grain yield and yield components in this trial.
The Mbor Mbptreatments significantly increased the grain yield under N0. The Mb,Mpand Mbptreatments significantly increased the grain yield under N90. The Mbptreatment significantly increased the grain yield under N180.However,no significant differences were observed between the inoculation treatment and the M0treatment under N270(Fig.7). The Mbptreatment had the greatest contribution to increasing the rice grain yield when the N rates were lower than 180 kg N ha-1. The results were similar to the results for the N transformations,microbial activities,and N supply capacities. In the Mbptreatment,no significant grain yield differences were recorded among the N90,N180,and N270applications.
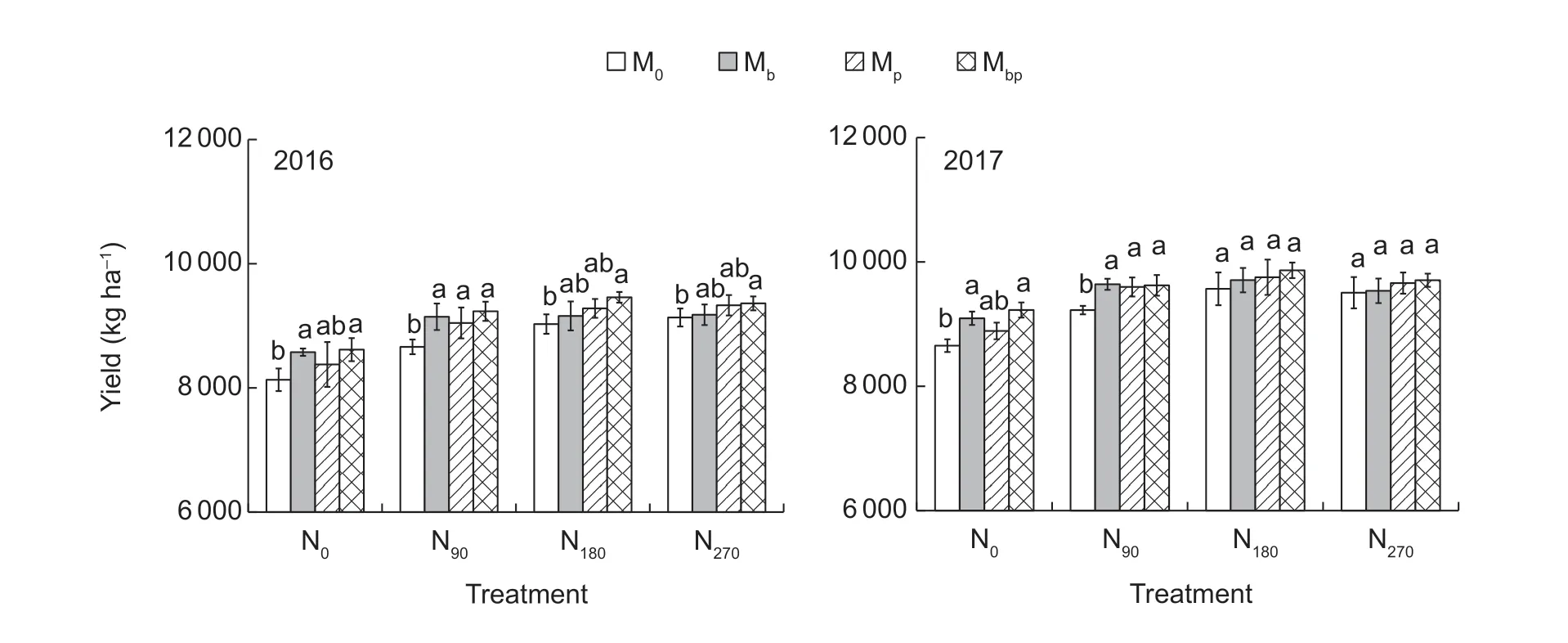
Fig.7 The rice grain yield in 2016 and in 2017. M0,inoculation of rice seedlings with sterile saline solution;Mb,inoculation of rice seedlings with Azospirillum brasilense;Mp,inoculation of rice seedlings with Pseudomonas fluorescens;Mbp,co-inoculation of rice seedlings with a mixture of A. brasilense and P. fluorescens. N0,0 kg N ha-1;N90,90 kg N ha-1;N180,180 kg N ha-1;N270,270 kg N ha-1. Vertical bars are mean±SE (n=3). Bars with the different letters are significantly different at the 0.05 level using LSD values.
The results of the harvest index and spikelet fertility of the rice plants were similar to the results of the rice grain yield (Table 5). The Mbptreatment showed the best results,and the highest values of the harvest index and spikelet fertility were recorded for N rate ranging from 90 to 180 kg N ha-1. The conventional N application rate in this region ranged from 180 to 270 kg N ha-1. Considering the rice grain yield and the economic and environmental benefits of rice planting,the N application rate can be reduced to 90 kg N ha-1for rice seedlings co-inoculated with a mixture ofA.brasilenseandP.fluorescens.
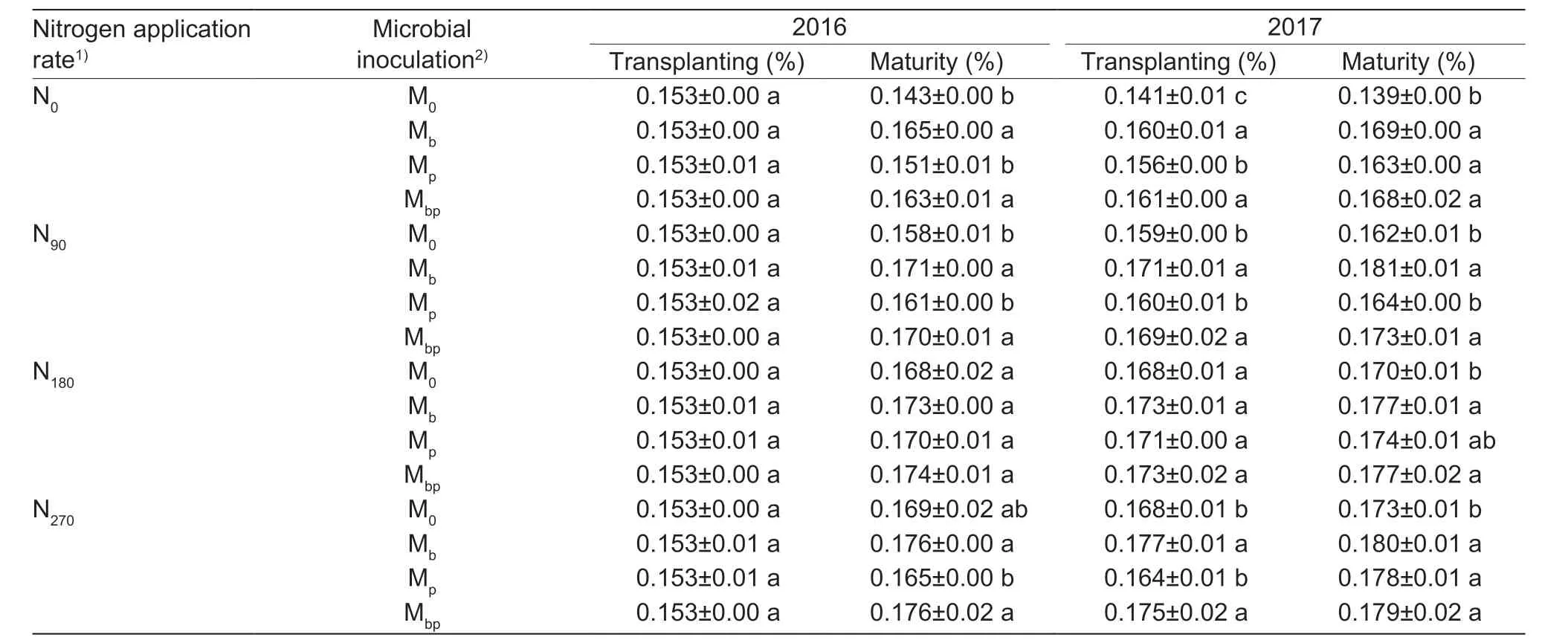
Table 3 The total nitrogen content in the rhizosphere soil before rice transplanting and after rice harvesting
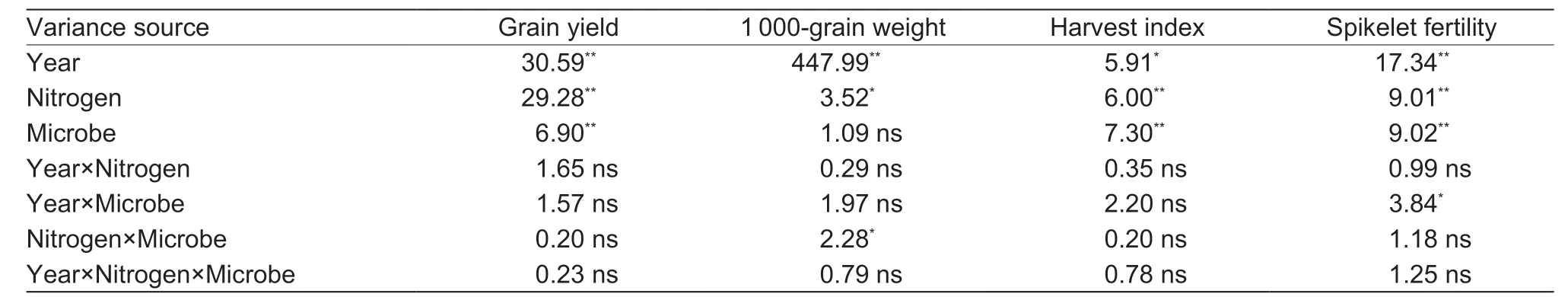
Table 4 Variance analysis of the rice grain yield and yield components under the interactions among the year,nitrogen application rate,and microbial inoculation (F-value)
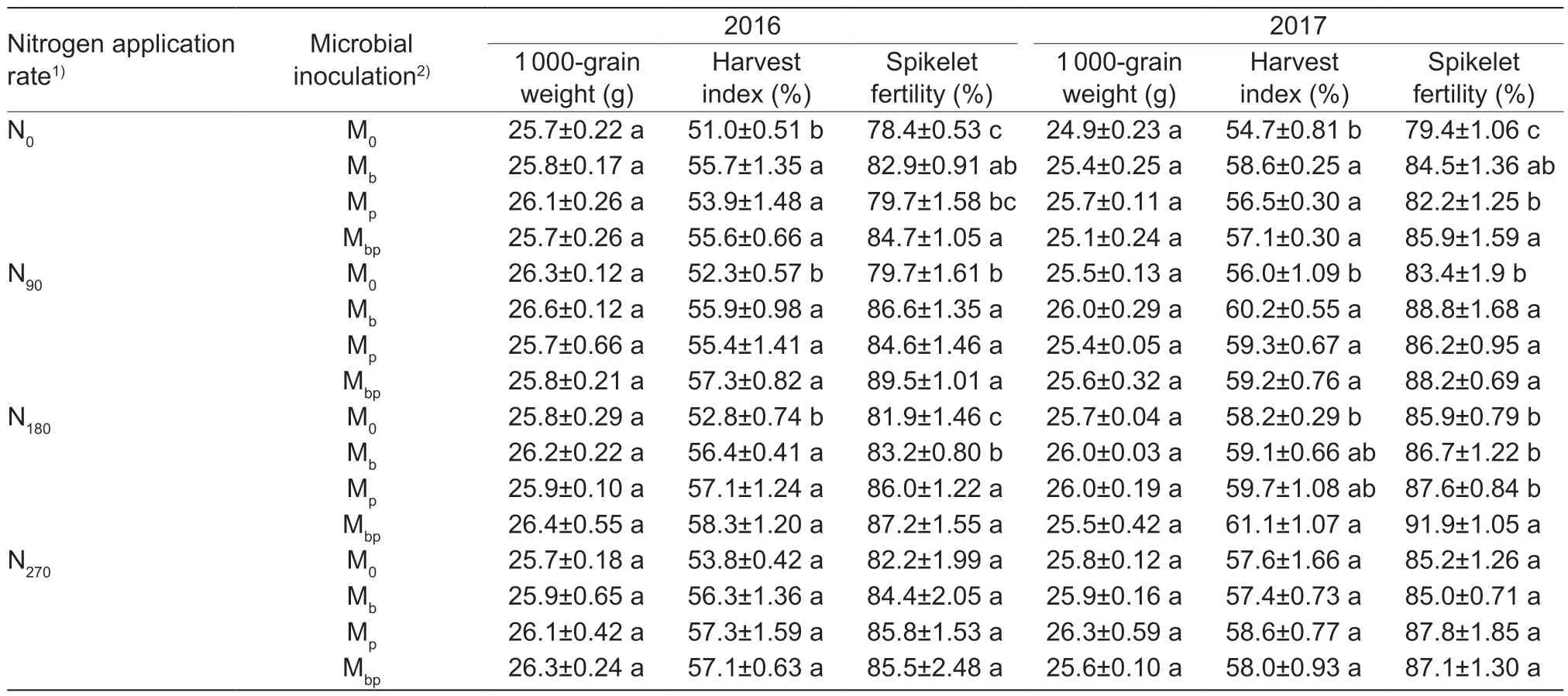
Table 5 The effects of N application rates and inoculating rhizobactetia on the grain yield components of rice
4.Discussion
In the N-limited environment,the N input can act as a stimulator of ammonification by removing microbial N limitations and reducing the C/N ratio of the decomposed substrates,while in the N unlimited environment,the N input can retard N ammonification (Gaoet al.2015). The present study showed that the Mpand Mbptreatments greatly enhanced ammonification activities in the rhizosphere compared with the M0treatment,and the contributing effects were increased with increasing N rates (Fig.1). Etesami and Alikhani (2016) found that theP.fluorescensexhibited high positive root colonization capabilities in rice plants with 75 and 100% fertilizer. Most ofP.fluorescensstrains could improve the biocontrol abilities of the rice plants and increase the P availability in the soil (Prabhukarthikeyanet al.2018). The present results showed thatP.fluorescensstrain used in this study might belong to the ammonifying bacterial community,and played a positive role in promoting the ammonification activities in the rhizosphere.
The nitrification processes could supply available N to plants. However,NO3--N is easily leached from the soil into the surrounding water. The leached NO3--N contents from arable land reached up to 100 kg ha-1in one year due to high rainfall and high N fertilizer rates (Haqueet al.2012). The results of this study illustrated that theA.brasilenseandP.fluorescensstrains used in this trial were not involved in the nitrification processes in the soil,and the nitrification processes in the soil were greatly affected by the N application rates (Fig.2).Considering the N lossesvialeaching from the paddy field,an N application rate lower than 180 kg N ha-1is more suitable.
Approximately 30 kg N ha-1yr-1from biological N2fixation processes enter into the rice planting systems (Herridgeet al.2008),and improving the N2fixation activities can greatly enhance the N supply capacities of the soil. Nitrogenase is the enzyme responsible for N2-fixation. Many studies have observed nitrogenase expression byA.brasilense(Yadavet al.2015). The present results also suggested that theA.brasilensestrain used in this trial possessed higher N2-fixation abilities in the rice rhizosphere,andA.brasilensemight belong to N2-fixing bacteria. Certain N2-fixing bacteria,such asAzospirillumandAzotobacter,showed higher N2-fixing abilities in the N-free or low N input environments (Yadavet al.2015). This study showed that the ARAs in the rice rhizosphere were also high under lower N inputs (Fig.3).
Denitrification may be responsible for more than 20% of the fixed N losses in agriculture (Zhouet al.2001;Gaoet al.2015). The abundance of certain denitrification genes (napA,nirS,nirK,andnosZ) in the soils increased with increasing NH4+concentrations (Levy-Boothet al.2014). Mamaiet al.(2013) found that the losses of N2during denitrification were significant when the nitrifying activities in the soil were high. The present study suggested that the denitrification activities in the rice rhizosphere were significantly and positively related to the N application rate (Fig.4). The high denitrification activities resulting from a high N application rate in the rhizosphere can result in high N losses from the paddy field,which can decrease the environmental quality and increase the cost of rice planting. The inoculations withA.brasilenseorP.fluorescensin this trial did not significantly affect the denitrification activities in the rice rhizosphere,suggesting thatA.brasilenseandP.fluorescensdid not participate in thedenitrification processes in the soil. Many studies suggested thatA.brasilensein the plants rhizosphere possessed N2-fixing properties,andP.fluorescensin the plants rhizosphere possessed bio-control properties (Floreset al.2010;Prabhukarthikeyanet al.2018). The present study showed that theA.brasilensestrain used in this trial played a positive role in promoting the N2-fixing process,and theP.fluorescensstrain used in this trial played a positive role in promoting the N mineralization process (ammonification).
The soil respiration rates and MBN contents represent the microorganism activities in the soil and were greatly affected by the interactions between rhizobacteria inoculations and the N application rate in this trial (Figs.5 and 6). The soil respiration rates and MBN contents contributed by theA.brasilensewere higher for low N application rates,while the soil respiration rates and MBN contents contributed by theP.fluorescenswere higher for high N application rates. The results suggested that colonizing capacity of theA.brasilensewas higher than that of theP.fluorescensfor low N application rates,while colonizing capacity of theP.fluorescenswas higher than that of theA.brasilensefor high N application rates. The results were also consistent with the results of ammonification activities and ARAs in the soil (Figs.1 and 3). Regardless of the magnitude of the N application rate,the Mbptreatment significantly improved the soil respiration rates and MBN contents in the soil. The activities ofA.brasilenseandP.fluorescenswere perhaps stimulated by the co-inoculation environments,and the reasons and mechanisms require further study. The MBN contents in the soil were also closely associated with the N supply capacities of the soil (Zhanget al.2018;Yuanet al.2019).
The available N contents and total N contents in the soil can reflect the N supply capacities of the soil. The coinoculation with a mixture ofA.brasilenseandP.fluorescensin this trial significantly increased the available N contents and total N contents in the soil,thus improving the N supply capacities of the soil,which were beneficial to rice growth and development (Tables 2 and 3). After the two-year experiments,the available N contents and total N contents in the Mbptreatment under N90were higher than those in the M0treatments under N180and N270. Higher ammonification activities were observed under N180,and higher nitrification activities were recorded under N270,while higher ARAs were observed under N90,and higher denitrification activities were observed under N270(Figs.1-4). High nitrification activities and denitrification activities could easily result in high N losses from the paddy soils. Considering the N use efficiencies and N losses in the soil,the optimum N application strategy in the study region consists of an N application rate of 90 kg N ha-1and co-inoculation of rice seedlings with a mixture ofA.brasilenseandP.fluorescens.
The results of Piccininet al.(2013) observed that inoculating rice plants with PGPR reduced the N application rate by 50%. Pedrazaet al.(2009) also indicated that inoculating rice plants withA.brasilensesignificantly improved the total N content and grain yield in rice cultivation. The present study illustrated thatA.brasilenseandP.fluorescenswere positively involved in the N cycles in soil and the rice grain yield. The performances of the N cycles in soil and the rice grain yield suggested that multistrain inoculations can promote plant growth more reliably and effectively than single-strain inoculations.
5.Conclusion
Pseudomonas fluorescenssignificantly improved the ammonification activities in the rhizosphere when the N application rate was higher than 90 kg N ha-1.Azospirillum brasilensegreatly improved the N2-fixation activities in the rhizosphere when the N application rate was lower than 90 kg N ha-1. The soil respiration rates and microbial biomass N contents were affected by the interactions between the rhizobacteria inoculations and the N application rate. Co-inoculation with a mixture ofA.brasilenseandP.fluorescensgreatly improved the N supply capacities in the rice rhizosphere and obviously increased the rice grain yield. To improve the environmental quality and soil fertility deteriorated by excessive N application,co-inoculation of rice seedlings with a mixture ofA.brasilenseandP.fluorescenscan be an alternative measure to improve the N supply potential and rice productivity.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (2016YFD0200801,2016YFD0200805),the National Natural Science Foundation of China (31872857),the Foundation of State Key Laboratory of Rice Biology,China National Rice Research Institute (2017ZZKT10404),and the Zhejiang Provincial Natural Science Foundation of China (LY16C130007).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- lmpacts of climate change on drought risk of winter wheat in the North China Plain
- Triple bottom-line consideration of sustainable plant disease management:From economic,sociological and ecological perspectives
- A rice geranylgeranyl reductase is essential for chloroplast development
- Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters
- Does nitrogen application rate affect the moisture content of corn grains?
- Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean
