Follicle-stimulating hormone is expressed in ovarian follicles of chickens and promotes ovarian granulosa cell proliferation
2021-10-22BlYulinYANGShuyanWANGHaiyanCHANGGuobinCHENGuohong
Bl Yu-lin,YANG Shu-yan,WANG Hai-yan,CHANG Guo-binCHEN Guo-hong
1 College of Animal Science and Technology,Yangzhou University,Yangzhou 225009,P.R.China
2 Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education,Yangzhou University,Yangzhou 225009,P.R.China
3 Department of Biochemistry,Capital Institute of Pediatrics,Beijing 100020,P.R.China
4 Aquacultural College,Rizhao Polytechnic,Rizhao 276826,P.R.China
Abstract Follicle-stimulating hormone (FSH),an important hypothalamic-pituitary-gonadal axis (HPG) hormone,is secreted by the pituitary gland. This study confirms that FSH is expressed in chicken follicles at different stages,and positive FSHβ mRNA signals were stronger (P<0.05) in granulosa cells than in oocytes. The 369 bp coding sequence of FSHβ in ovaries is 100% identical to that in the pituitary gland. The experiment in vitro revealed that the ovary possessed FSH secretory capacity. Further,FSHβ mRNA was significantly upregulated (P<0.05) in follicles and significantly higher (P<0.05) than that in the pituitary gland by approximately 2-23 times with the development. The number of granulosa cells decreased significantly (P<0.05) in the cells with siRNA treatment,confirming that the ovarian FSH could promote granulosa cell proliferation. This view was supported by cell cycle analysis and CCND2 and CCNE2expression. Further research indicated that no difference (P>0.05) was observed between the number of granulosa cells treated with FSHβ siRNA and in exogenous FSH.However,the number of granulosa cells without FSHβ siRNA transfection was significantly higher (P<0.05) for exogenous FSH. This finding suggests that the proliferative effect of exogenous FSH on ovarian granulosa cells depend on endogenous FSH. This study demonstrated that the FSH gene was expressed in chicken follicles and promoted ovarian granulosa cell proliferation,which enriched the theory on HPG axis.
Keywords:FSH,expression,follicle,granulosa cell proliferation,chicken
1.lntroduction
Classical theory states that the hypothalamic-pituitarygonadal (HPG) axis in animals forms a closed self-feedback system to maintain the relative stability of the endocrine system and regulate reproductive actions under the action of the nervous system (Suet al.2015). Follicle-stimulating hormone (FSH),a highly heterogeneous glyco-protein,is secreted by the pituitary gland (Liet al.1949). FSH has two subunits,FSHα and FSHβ,with the latter performing most of the FSH functions (Volonteriet al.2013;Huanget al.2015;Zhenget al.2017). FSH,a biomacromolecule,cannot pass through the cell membrane;thus,the effect of FSH must be mediated by the specific receptor FSHR (Ulloa-Aguirreet al.2018). After binding to FSHR,FSH induces several reproductive reactions (including granulosa cell proliferation and gametogenesis)viathe secondary messengers,such as Ca2+and cAMP (Xiaet al.1994;Downs and Hunzicker-Dunn 1995;Denget al.1998;Suet al.1999;Orisakaet al.2006;Yang and Roy 2006;Casarini and Crépieux 2019).
FSH can initiate primary follicular development and plays a crucial role in growth follicle development by promoting proliferation and differentiation of follicular cells (Campbellet al.1995). The ovaries include numerous follicles,mainly consisting of germ cells,granulosa cells,and interstitial cells. Granulosa cells affect oocyte growth,differentiation,and maturationviagap junctions to promote follicular development (Eppiget al.2001). A large number of factors,including gonadotropins,regulate granulosa cell proliferation. In granulosa cells,FSH activates the extracellular regulatory protein kinaseviathe cAMP-PKA/PKC pathway (Nemeret al.2018) and upregulates the expression levels ofcyclinD2,P450arom,3β-HSD,andP450sccgenes to promote granulosa cell proliferation and estrogen synthesis (Escamilla-Hernandezet al.2008;Kayampilly and Menon 2009;Yivgi-Ohanaet al.2009).
FSHR is located in multiple tissues (Konget al.2018;Chruscielet al.2019;Liznevaet al.2019;Olejaret al.2020) and mediates the regulation of FSH on various biological processes,such as fat metabolism,bone metabolism,and tumorigenesis (Cuiet al.2012;Liuet al.2015,2017;Jiet al.2018;Shiet al.2018;Bergandiet al.2019),revealing the diversity of FSH physiological functions. The expression of FSH in ovarian follicles of chickens at different stages has not been reported,and the effect of FSH on the proliferation of ovarian granulosa cells is not clear. It was hypothesized that FSH would be expressed in ovarian follicle s of chickens and promote ovarian granulosa cell proliferation. Therefore,the objective of the present study was to test the hypothesis by investigating the expression of FSH in ovarian follicles of chickens at different stages and the effect of FSH on the proliferation of ovarian granulosa cells.
2.Materials and methods
2.1.Animals and sample collection
Twelve female Beijing-You chickens in their laying cycle were purchased from the Poultry Institute,Chinese Academy of Agricultural Sciences. The ovary and pituitary gland of each bird were collected and used as the control. The follicles with the size of 0.3 mm (primary stage),4 mm (growth stage,white),7 mm (F6 stage,yellow) were separated from the ovary (Sturkie 2012). The samples were stored at 4°C after fixation with 4% paraformaldehyde or at -80°C for subsequent experiments.
2.2.Reverse transcription -PCR (RT-PCR)
Total RNA was respectively isolated from the ovarian tissue and the follicles at three developmental stages (the primordial follicles,growth follicles,and mature follicles) using TRIzol Reagent in accordance with the manufacturer’s protocol (Invitrogen,USA). Total RNA from the pituitary tissue and the genomic DNA of chicken were used as positive controls. The obtained RNA was dissolved at 1 μg μL-1after removing any genomic DNA. Up to 2 μg total RNA was used to synthesize the cDNA with a 20 μL system in accordance with the instructions provided by the manufacturer (Promega,USA). Specific primers (Table 1) were designed to amplify the fragment ofFSHβmRNA. RT-PCR was performed in a final volume of 50 μL of PCR mixture containing 25 μL of 2× PCR mix (TaKaRa Biomedical Technology (Beijing) Co.,Ltd.,Beijing,China),1.0 μL (10 mmol) of each primer,1 μL of cDNA,and ddH2O.The products were visualized after electrophoresis in 2.0% agarose gels,and then were sequenced (Tianyi Huiyuan,China) to verify authenticity.

Table 1 The specific primers for RT-PCR and Q-PCR in this study
2.3.In situ hybridization (lSH)
Growth follicles from three birds were selected and then mounted on cork disks by using Tissue-Tek optimal cutting temperature compound. Serial cryostat sections measuring 10 μm (-20°C) were cut and prepared from three birds.Sequential sections were divided into three sets and used forFSHβmRNAin situhybridization (ISH) following the established methodology (Gjerdrumet al.2001). The antisense probe was hybridized to 738-1 190 bp of theFSHβmRNA region (GenBank accession no.NM_204257).
2.4.lmmunohistochemistry (lHC)
Paraffin-embedded sections (10 μm) of the growth follicles and pituitary glands from three birds were dewaxed in xylene (twice for 5 min) and 100% ethanol (twice for 2 min). The sections were then rehydrated using a graded ethanol series (95,70,50,and 30%) for 2 min each and finally rinsed with distilled water. Slides were incubated in citrate buffer (10 mmol L-1citric acid,0.05% Tween 20,pH 6.0) at 95-100°C for 10 min. After they were washed twice with phosphate-buffered saline (PBS),the slides were blocked with 3% bovine serum albumin (BSA) in PBS for 30 min. The slides were incubated in a humidified chamber overnight with 1:100 dilutions of rabbit anti-chicken FSH (purchased from Huijia Biological Technology Co.,Ltd.,Xiamen,China) at 4°C. The slides were then washed thoroughly and treated with 1:100 dilutions of horseradish peroxidase-conjugated goat antimouse immunoglobulin at room temperature for 1 h,washed 3 times in PBS,and developed with 3.3´-diaminobenzidine for 10-15 min in the dark. The sections were subsequently counterstained with hematoxylin. The slides of pituitary glands were treated with the rabbit anti-chicken FSH as the negative control.
2.5.Western blotting (WB)
The FSH level in the growth follicles and pituitary glands from the same individual also was detected by WB. The total protein levels of all samples were assayed with a BCA Protein Assay Kit (Beyotime,Shanghai,China). The following monoclonal antibodies were purchased from Huijia Biological Technology Co.,Ltd.(Xiamen,China),and their working concentrations were as follows:the anti-β-Tubulin (1:500),anti-FSH (1:1 000).
2.6.lmmunogold electron microscopy
The growth follicles were fixed with 3% glutaraldehyde,dehydrated in graded ethanol and embedded in the LR White resin. After polymerization at 55°C,10 nm sections were cut and treated with 0.36 mmol L-1sodium periodate for 5 min and then marked with colloidal gold. The copper networks of the sections were incubated twice with 50 mmol L-1glycine (prepared with PBS) for 5 min,washed twice for 5 min with porphobilinogen buffer (PBS,0.5% BSA,and 0.0005% gelatin buffer),incubated overnight with rabbit anti-chicken FSH (as above),and washed six times with PBG buffer. After incubation with the secondary antibody for 1 h,silver-enhancing treatment was conducted by incubating twice with 50 mmol L-1glycine for 5 min,washed twice with ultrapure water for 5 min,incubated with silver-intensifying liquid for 15 min in the dark,and stained with acetic acid and uranium after washing 6 times with ultrapure water at 40°C. The specimens were observed using an H7500 electron microscope (Hitachi,Japan) (Semeshinet al.2002).
2.7.Granulosa cell isolation and treatment
Granulosa cells were collected from the growth follicle in accordance with published methods (Leoniet al.2018). The cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Gibco,Grand Island,NY,USA) and incubated in a 5% CO2incubator at 37°C. The medium was changed every 2 d. Some primary granulosa cells were plated in six-well culture dishes and collected for 48 and 96 h to detect the FSH mRNA and protein levels in the cells and cultured medium. Other primary granulosa cells were passaged after reaching 80% confluence,and passage 2 was used for the subsequent siRNA transfection experiments.
FSHβsiRNAs were constructed after sequencing by RiboBio Co.,Ltd.(Guangzhou,China;GenBank accession no.NM_204257). After some cells in 96-or 6-well culture dishes reached 20% confluence,theFSHβsiRNAs were transfected,with si-NC as the control. Other granulosa cells transfected or not withFSHβsiRNA,were also selected,and 20 mIU mL-1FSH (Huijia Biological Technology Co.,Ltd.,Xiamen,China) was added. All cells were collected and detected in the subsequent experiments after culturing for 72 h.
2.8.Enzyme-linked immunosorbent assay (ELlSA)
FSH concentrations in the granulosa cells or the cultured medium were measured using a chicken-specific FSH ELISA Kit (Huijia Biological Technology Co.,Ltd.,Xiamen,China). Cell samples were homogenized at room temperature and centrifuged (1 000×g,20 min) at 4°C to separate the debris and the pellet. The supernatant was frozen immediately at -80°C until assayed. The assay was conducted in accordance with the manufacturer’s protocols and suggested dilutions to optimize accuracy.
2.9.3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The cells were cultured in 96-well plates with different treatments for 72 h. The cell counts were subsequently determinedvia3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. About 20 μL of MTT (5 mg mL-1,Solarbio,Beijing,China) was added into each well,and the plates were incubated at 37°C for 4 h. After the supernatant was removed,formazan crystals were dissolved in 200 μL of dimethylsulfoxide (Solarbio,Beijing,China),and absorbance was measured at 490 nm. To ensure experimental accuracy,8 wells were replicated per group.
2.10.Flow cytometry
Cells transfected or not withFSHβsiRNA were collected for 48 h to analyze the cell cycle by using a FACSCanto II flow cytometer (Becton,Dickinson and Company,Franklin Lake,NJ,USA). The cells were then mixed with 0.25% Triton X-100 and 5 μL propidium iodide (Solarbio,Beijing,China) and incubated at room temperature for 30 min in the dark. The cells were resuspended in 0.5 mL of PBS and immediately analyzed.
2.11.Real-time quantitative PCR (qPCR)
Three follicles in 3 stages and 3 pituitary glands from all 12 birds were used. The isolated granulosa cells were subjected to qPCR to detect the target gene expression. The specific primers used are listed in Table 1. Each 25 μL PCR mixture contained 12.5 μL of 2× iQ™ SYBR Green Supermix,0.5 μL (10 mmol) of each primer,and 1 μL of cDNA. The mixtures were incubated in the iCycler iQ Real-time Detection System (ABI7500,USA) programmed to conduct 40 PCR cycles (95°C for 15 s and 60°C for 35 s). A melting curve was constructed to verify that only a single PCR product was amplified. The samples were assayed in triplicate with standard deviations of the CT values not exceeding 0.5 on a within-run basis. Each analysis was repeated at least twice. Negative controls (without template) and positive controls (pituitary tissue) were included with each assay.
2.12.Statistical analyses
The data of FSH contents between granulosa cells and cultured medium as well as the data of cell number and mRNA levels ofFSHβ,CCDN2andCCNE2between FSH siRNA and si-NC and cell number between FSH and no FSH were analyzed using Student’s unpairedt-test in SAS Software (version 8.2,SAS Institute,Cary,NC,USA,2001).Other data were analyzed by GLM in SAS. The differences between treatments were analyzed by the least significant difference (LSD). Each replicate served as an experimental unit. Differences were considered statistically significant atP<0.05.
3.Results
3.1.ldentification of FSH gene expression in chicken ovary
After the specific primers were optimized and the RT-PCR products were confirmed by sequencing,FSHβmRNA was detected at the transcript levelviaRT-PCR to determine whether theFSHgene was expressed in the chicken ovarian tissue. As shown in Fig.1-A,the expected 497 bp products ofFSHβfrom the ovary,follicles at different developmental stages,and pituitary gland were verified by electrophoresis,and a 1 433-bp product was found from the chicken genomic DNA.
TheFSHgene was detectedviaISH.FSHβtranscripts were found in both granulosa cells and oocytes of the growth follicles;however,more positive signals ofFSHβmRNA were found in the granulosa cells than in the oocytes (Fig.1-C). IHC and WB results also showed that FSH-positive signals were observed in the growth follicles (Fig.1-C). Similarly,FSHβ protein was detected in the cytoplasm by immunogold electron microscopy (Fig.1-D).
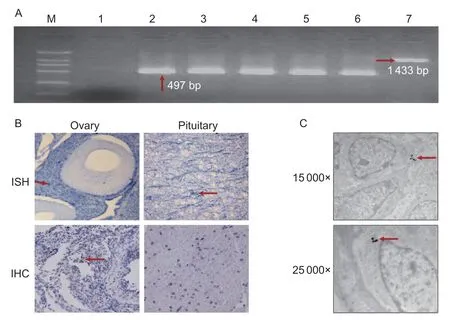
Fig.1 Expression of FSHβ mRNA in chicken ovarian follicles at different stages and in pituitary. A,FSHβ mRNA expression was detected by RT-PCR,and the expected products were verified by electrophoresis in different tissues. M,DL2000 marker;lane 1,negative control (no template);lane 2,positive control (pituitary);lanes 3-6,ovary tissue,primary follicles,growth follicles,and mature follicles,respectively;lane 7,genomic DNA. B,in situ hybridization (ISH) and Immunohistochemistry (IHC) detection:positive hybridized signals of FSHβ mRNA were found in the growth follicles,the pituitary gland was the positive control;and positive signals of FSH protein appear as brown staining in the growth follicles,the pituitary gland was the negative control,without anti-FSH (inverted microscope,200×). C,immunogold electron microscopy detection of FSH protein in the growth follicle:positive signal appears as black staining in the cytoplasm of granulosa cells (electron microscopy,15 000× and 25 000×).
3.2.Characterization of FSHβ expression in ovary
By using two pairs of specific primers,the full-length coding sequence (CDS) of the 369 bpFSHβwas cloned from chicken ovaries by RT-PCR. Alignment analysis indicated that both the FSH nucleotides and amino acid sequences in the ovary were 100% identical with those in the pituitary gland (Fig.2-A),and the CDS ofFSHβin the ovary encoded a polypeptide of 123 amino acids.
The isolated granulosa cells were cultured for 48 and 96 h. TheFSHβmRNA and protein were detected by qPCR or ELISA.FSHβmRNA was expressed in the granulosa cellsin vitro,but no significant difference in expression between the 48 and 96 h culturing duration (Fig.2-B). Similarly,FSH protein was detected in the culture medium of the granulosa cells after culturing for 48 and 96 h;no significant difference in expression level was found between the 48 and 96 h culturing duration (Fig.2-C).
The abundances ofFSHβmRNA in the follicles at different developmental stages were also determined by qPCR. The expression levels ofFSHβmRNA in the follicles at different stages were upregulated with the development,and such upregulation was statistically significant (P<0.05 orP<0.01),with the highest being in the mature follicles and the lowest in the primary follicular development (Fig.2-D). AllFSHβmRNA levels were significantly higher (by approximately 2-23 times) in the follicular tissue than in the pituitary tissue (P<0.05 orP<0.01).
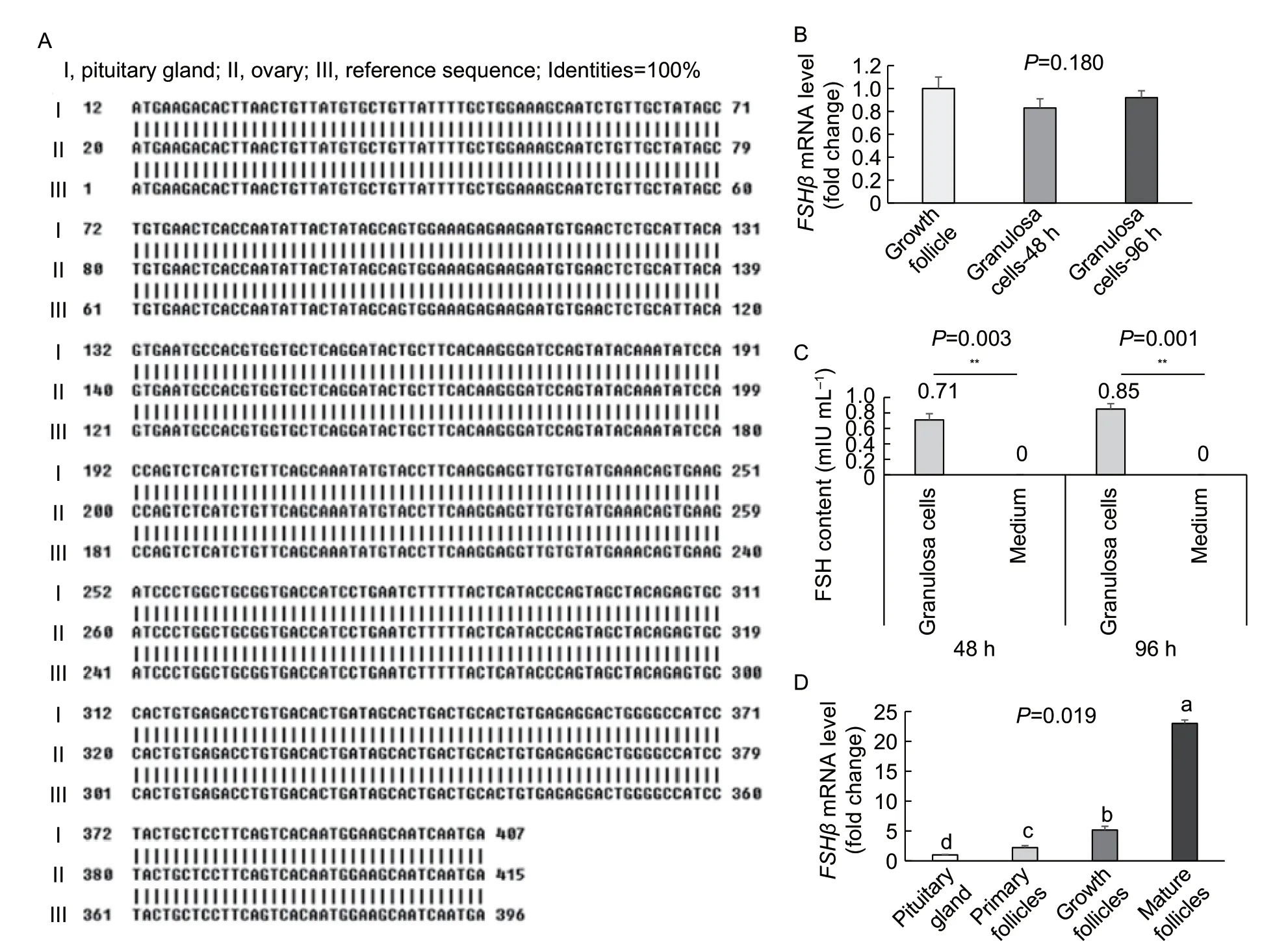
Fig.2 Expression characteristics of FSH gene in chicken ovarian granulosa cells in vivo and in vitro. A,a 369-bp CDS of FSHβ mRNA was cloned from the growth follicles of chicken ovaries via RT-PCR,which encoded a 123 amino-acid polypeptide and had 100% identity with the pituitary tissue. B and C,mRNA and protein levels of FSH gene in granulosa cells or cultured medium in vitro for 48 and 96 h. D,FSHβ mRNA levels in ovarian follicles at different stages and in the pituitary gland (in vivo). Data are presented as the mean±SE,n=3. **,P<0.01. Values with different letters (a to d) indicate significant differences (P<0.05).
3.3.Effects of FSH from chicken ovary
To determine whether FSH from the ovary affected the granulosa cells,an interference experiment was conducted.FSHβsiRNA was used to interfere withFSHmRNAin vitro. After treatment for 72 h,the cell count was lower in the siRNA-treated granulosa cells than in the untreated control cells,and the difference was statistically significant (P<0.05),accompanied by decreases inFSHβandFSHRmRNA (P<0.01) at 48 h (Fig.3-A). As shown in Fig.3-B,the percentage of siRNA-transfected cells was lower in the S phase and higher in the G1 phase. Genetically,the mRNA levels ofCCND2andCCNE2were significantly downregulated (P<0.01) in the siRNA-transfected cells than in the untransfected control cells (Fig.3-C).
On the basis ofFSHβsiRNA interference for 12 h,the granulosa cells were treated with the exogenous FSH for another period of 60 hin vitro;no significant difference in cell number was indicated between the siRNA-transfected cells and control cells without siRNA transfection (Fig.3-D). The same exogenous FSH treatment was applied to the granulosa cells withoutFSHβsiRNA interference,and the cell counts were significantly (P<0.05) higher in the granulosa cells than control cells without siRNA transfection (Fig.3-D). The results suggest that the proliferative effect of exogenous FSH on granulosa cells depends on the endogenous FSH.
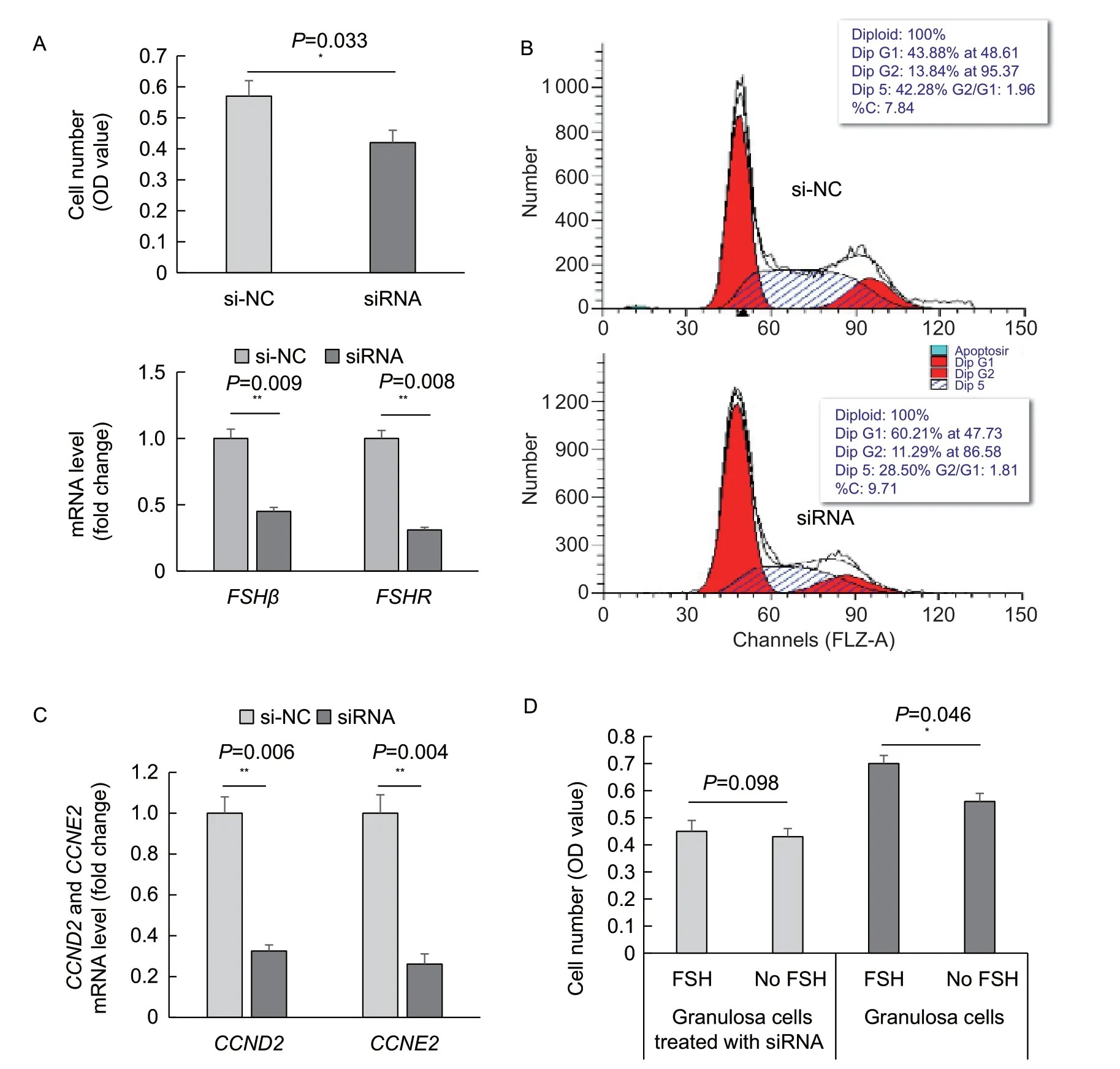
Fig.3 Effect of FSH silencing or exogenous FSH on ovarian granulosa cell proliferation and mRNA levels of FSHβ, CCDN2 and CCNE2. A,FSHβ mRNA and number of granulosa cells treated with FSH siRNA or si-NC (as a control). B,detection of cell cycle of granulosa cells treated with FSH siRNA or si-NC by FCM. C,mRNA levels of CCND2 and CCNE2 in granulosa cells treated with FSH siRNA or si-NC for 48 h. D,cell numbers of granulosa cells with treatment by exogenous FSH from pituitary (20 mIU mL-1) plus the siRNA of FSHβ,or only with treatment by exogenous FSH (20 mIU mL-1). Data are the mean±SE;n=3 (FCM and Q-PCR) or 8 (MTT). *,P<0.05;**,P<0.01.
4.Discussion
FSH is secreted by the pituitary gland (Liet al.1949) and plays a crucial role in follicular development by promoting cell proliferation and differentiation (Campbellet al.1995).Results of the present study support the hypothesis that FSH is expressed in ovarian follicles of chickens and promote ovarian granulosa cell proliferation. In the present study,FSHwas found to be expressed in chicken ovary,which differs from the current theory regarding the HPG axis.The expression and biological effects of FSH from chicken ovarian tissue were further exploredin vivoandin vitro,and it was shown that FSH could promote ovarian granulosa cell proliferation. The new findings from this study could enrich the theory on the HPG axis and help to elucidate a new mechanism of FSH in regulating reproduction in chicken ovary tissues.
FSHβ was the main subunit performing FSH functions (Volonteriet al.2013;Huanget al.2015;Zhenget al.2017).Thus,FSHβgene expression in chicken ovary was detected in-depth as a new discovery.FSHβmRNA was first found in the follicles at the primary,growth,and maturation stages by RT-PCR. Further research using the growth follicles was conducted by ISH. The result revealed the presence ofFSHβmRNA in the granulosa cells and oocytes;positive signals were stronger in the granulosa cells than in the oocytes. Similarly,FSH was found in the granulosa cells and oocytes of ovary by IHC. It was known that the FSH secreted by the pituitary gland would directly target the ovary. Thus,this study selected the growth follicles to explore the FSH expression in the ovary by immunogold electron microscopy.FSH was also found inside the granulosa cells. Combined results onFSHβmRNA and protein confirmed that theFSHgene was expressed in the granulosa cells,which differed from the current HPG theory.
On the basis of the aforementioned results,characterization of FSH expression was also explored. The CDS ofFSHβin the pituitary gland encodes a polypeptide of 123 amino acids. By using the different primers,a 369-bp full-length CDS ofFSHβmRNA was cloned from ovarian tissue. Both the FSH nucleotides and amino acid sequences in the ovary were 100% identical with those in the pituitary gland. In addition,FSH is the secreted glycoprotein by the pituitary gland (Liet al.1949);thus,cell experimentsin vitrowere also performed. The results revealed that bothFSHβmRNA and protein cultured for 48 or 96 h were expressed inside the granulosa cells,and FSH protein was detected in the culture medium. These results support the hypothesis that ovarian FSH exhibits secretory capacity and suggest that ovarian FSH may have the same function as the pituitary FSH.
As an important indicator of FSH expression,the abundance ofFSHβmRNA in the follicles was also analyzed by qPCR,using the pituitary gland as the control.FSHβmRNA levels in follicles at different stages significantly increased with follicular development,which was consistent with the increase in granulosa cells (Eppiget al.2001).Notably,allFSHβmRNA levels in the follicles at different stages increased by 2-23 times relative to that in the pituitary gland,which prompted further research on the functionality of the ovarian FSH.
Regarding the effect of the pituitary FSH on cell proliferation (Hernández-Coronadoet al.2016;Zhanget al.2017;Sirotkinet al.2019),FSHβmRNA interference in granulosa cells was conducted to identify its functionin vitro. AfterFSHβinterference in granulosa cells,theFSHβmRNA level was downregulated,the cell count was reduced,and the percentages of granulosa cells in the S and G1 phases exhibited the corresponding changes. These results confirmed that ovarian FSH could accelerate the G1/S phase transition in granulosa cells. CCND2 and CCNE2 could promote the cell cycle (Zhouet al.2016;Meinsohnet al.2017;Gaoet al.2018),and the downregulation ofCCND2andCCNE2mRNA levels with the interference ofFSHβconfirmed that the ovarian FSH positively regulated the proliferation of granulosa cells in chicken.
The pituitary FSH plays a crucial role in follicle development by promoting the proliferation and differentiation of follicular cells (Campbellet al.1995). An additional experiment showed that after granulosa cells with or withoutFSHsiRNA transfection were treated with exogenous FSH for 72 h,the number of granulosa cells withFSHβsiRNA transfection did not differ,but the number of granulosa cells withoutFSHβsiRNA transfection increased significantly for exogenous FSH treatment. These results indicate the importance of ovarian FSH which the effect of exogenous FSH on granulosa cells depends on.
5.Conclusion
Results of the present study show that FSH was expressed in chicken follicles and enhanced ovarian granulosa cell proliferation,which enriches the HPG theory and helps to elucidate FSH regulation of reproduction in chicken ovary tissues.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31802057),the Second Batch of Special Grant from China Postdoctoral Science Foundation (2020TQ0252),the Postdoctoral Research Funding Project of Jiangsu Province,China in 2020 (2020Z213),and the Open Project Program of Joint International Research Laboratory of Agriculture and Agri-Product Safety,the Ministry of Education of China (JILAR-KF202017).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures were performed in accordance with the Administration Act of Experimental Animals Use and Care in Jiangsu Province,China (#115th Jiangsu Province Government Notice in 2008).
杂志排行
Journal of Integrative Agriculture的其它文章
- lmpacts of climate change on drought risk of winter wheat in the North China Plain
- Triple bottom-line consideration of sustainable plant disease management:From economic,sociological and ecological perspectives
- A rice geranylgeranyl reductase is essential for chloroplast development
- Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters
- Does nitrogen application rate affect the moisture content of corn grains?
- Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean
