Isolation and molecular characterization of entomopathogenic nematode,Heterorhabditissp.from an arable land in Nigeria
2021-10-22FisayoDARAMOLAOsarenkhoeOSEMWEGIEStephenOWASamuelORISAJOEvbuomwanIKPONMWOSAElizabethALORI
Fisayo Y.DARAMOLA,Osarenkhoe O.OSEMWEGIE,Stephen O.OWA,Samuel B.ORISAJO,Evbuomwan IKPONMWOSA,Elizabeth T.ALORI
1 Department of Microbiology,Landmark University,Omu Aran 370102,Kwara State,Nigeria
2 Crop Protection Division,Cocoa Research Institute of Nigeria,Ibadan 200257,Oyo State,Nigeria
3 Department of Crop and Soil Sciences,Landmark University,Omu Aran 370102,Kwara State,Nigeria
Abstract The occurrence of entomopathogenic nematodes (EPNs) in arable soil samples from Nigeria was investigated using Baermann extraction tray and insect-bait (White’s trap) techniques. Isolates were tested for infectivity using the larvae of Galleria mellonella (greater moth) and Tenebrio molitor (mealworm). The study revealed a new species of Heterorhabditis (MT371593) in soil samples that were randomly collected from an arable farmland cultivated with cassava TMS-30572 at the Teaching and Research Farm of Landmark University,Nigeria. Amplification of the internal transcribed spacer region (ITS) of the ribosomal DNA produced a nucleotide sequence of 933 base pairs (bp). A BLASTN search of GenBank showed that the sequence of the Nigerian isolate is identical at 99% similarity to that of Heterorhabditissp.from Thailand. Infectivity test of the isolate showed 100% mortality against T.molitorlarvae within 48 h of exposure while only 80% mortality was recorded for G.mellonellaafter 1 week of exposure. This is the first account of Heterorhabditis sp.in Nigeria. The varying degrees of infectivity against mealworm and greater moth observed in this study proved that the Nigerian isolate of Heterorhabditis sp.could potentially be an attractive option in the management of insect pests of cash crops.
Keywords:biological control,EPNs,Heterorhabditissp.,ribosomal DNA, Tenebrio molitor
1.Introduction
Entomopathogenic nematodes (EPNs) are obligate soilinhabiting parasites of many insects. They are ubiquitous in both cultivated and uncultivated soils all over the world. EPNs are widespread in their distribution and have been reported in all the continents of the world except Antarctica (Campos-Herreraet al.2012;Bhatet al.2020). The potential of EPNs to acts as natural enemies and their ability to suppress the population of insect pests of agricultural importance has made them attractive options as biological control agents in pest management. Their application has formed part of a more integrative approach towards reducing crop losses and frequent damage to crops,including direct injury from pests and damage that comes indirectly from the impact of chemical insecticides as well as other agronomic practices aiming at mitigating against insect pests.
More recently,research into the biocontrol potential of EPNs is gaining more attention and has opened up opportunities to explore the rich diversity of nematode species that are beneficial to human and could have commercial value. In Europe and many other developed countries,EPNs have been commercialized and successfully used for pest management (Lacey and Georgis 2012;Laceyet al.2015;Malan and Ferreira 2017). In countries such as Australia,Japan,China,and those in North America and Europe,commercial applications of EPNs have been successful. Also,many high-value plantations have been protected from the devastating effects of insect pests by the strategic application of EPNs (Ehlers 1996;van Zyl and Malan 2014;Malan and Moore 2016;Gulcuet al.2019;Kapranaset al.2020).
EPNs have potential value to be a non-toxic alternative to chemical pesticides,in cases where resistance to insecticides has developed (Ehlers 2001),enabling producers to use an additional biological resource to control pests in an environmentally friendly manner (Plattet al.2020). Biological control using EPNs could be incorporated into existing or emerging IPM strategies (Abd-Elgawad 2019) by developing compatible methods (Shapiro-Ilanet al.2014,2017;Abd-Elgawad 2017a,b) that are complimentary to chemical nematode management methods (Stevens and Lewis 2017) and synergistic with other agricultural inputs in IPM programmes (Laznik and Trdan 2014,2017;Malan and Moore 2016;Bajcet al.2017;Gulcuet al.2019;Kapranaset al.2020). When properly applied,EPNs can control a variety of soil-dwelling insect pests (Hiltpold 2015) as well as aboveground herbivorous insects (Shapiro-Ilanet al.2017). However,different factors comprising soil pH,texture,aeration,temperature and atmospheric CO2can affect the efficacy of EPNs against many aboveground insect pests (Shapiro-Ilanet al.2012;Hiltpoldet al.2020).Lacey and Georgis (2012) gave a detailed account of the successful commercialization and application of EPNs for the control of various insect pests above and below ground. Reports from South Africa also show that extensive research has been done on the biocontrol potential of EPNs against codling mothCydia pomonellaL.(Lepidoptera:Tortricidae),false codling mothThaumatotibia leucotretaL.(Lepidoptera:Tortricidae),wax mothGalleria mellonellaL.(Lepidoptera:Pyralidae) and mealwormTenebrio molitorL.(Coleoptera:Tenebrionidae) (De Waalet al.2011;van Zyl and Malan 2014;Odendaalet al.2016;Malan and Ferreira 2017). Although Galleria larvae is the most commonly used insect bait for EPNs (Woodring and Kaya 1988),there are indications that mass production and commercial application of EPNs have been achieved with mealworm larvae as the insect host (Shapiro-Ilanet al.2012;van Zyl and Malan 2014;Rahooet al.2019).
Increased awareness on the use of EPNs as effective non-chemical alternatives to insect pest control has resulted in more surveys being conducted across the globe in order to identify and isolate new species that are more virulent and locally adapted to the environmental conditions of the region (Tarascoet al.2015;Yanet al.2016;Kouret al.2020). In Nigeria,studies on the potential use of indigenous EPNs for biological control of insect pests are attracting attention and some species belonging to the families Heterorhabditidae and Steinernematidae have been described from some states within the country. They include:Heterorhabditis bacteriophoraPoinar (Nematoda:Heterorhabditidae),H.indicaPoinar,Karunakar and David (Rhabditida:Heterorhabditidae),an unidentifiedHeterorhabditissp.andSteinernema feltiaeFilipjev (Rhabditida:Steinernematidae) (Akyaziet al.2012;Aliyuet al.2015,2016;Claudius-Cole 2018).
The isolation of indigenous and native species of EPNs is important for searching for potential biocontrol options that are commercially valuable,antagonistic to indigenous insect pests,and adaptable to a range of environmental conditions. Accurate distribution and identification of these indigenous species coupled with a good understanding of their nematode-host interaction capacity are key considerations for their long-term commercial production. This exploit is likely to contribute to the global initiatives in promoting agriculture and thereby sustainably resolving the challenges of poverty and hunger.
This current investigation is therefore aimed at identifying local EPNs that could be incorporated as biocontrol agents into existing Integrated Pest Management (IPM) programs. It also aims to provide baseline information that demonstrates the biocontrol potential of the Nigerian isolate ofHeterorhabditissp.againstT.molitor.
2.Materials and methods
2.1.Site description
Samples were randomly collected from different locations of arable farmlands at the Teaching and Research Farm of Landmark University,Omu-Aran,Nigeria. The farm is located at 8.1248630°N,5.0764680°E and 143 m a.s.l. This region is typically covered by tropical savannah vegetation with an annual rainfall of 1 364 mm and mean temperature of about 25.3°C. The farm was cultivated with cassava (Manihot esculenta)variety TMS-30572 for two years,after which the land was abandoned to fallow. Soil samples for this study were collected,two years into the fallow period. Conventional tillage was practiced and crops were harvested two years after planting.
2.2.Soil sampling and nematode isolation
A total of 30 core soil samples were randomly collected from 1 ha each of a cassava plantation,fallowing cassava and uncultivated farmlands. The samples were collected to a depth of about 20 cm into the soil. The soil samples were then bulked and mixed thoroughly with a hand spade,and composite samples were taken from the mixture for nematode assay. Modified method of Coyneet al.(2014) was used for nematode extraction and the set-up was left undisturbed in the laboratory. Several infective stage juveniles (IJs) of the EPNs were observed from the extraction tray after 48 h. Morphological observation for the identification of the nematodes and their morphological features was done under a Leica DM2000 compound microscope.
2.3.Trapping for entomopathogenic nematodes
Entomopathogenic nematodes were isolated from soil samples in the laboratory by trapping with mealworm as the insect bait (Bedding and Akhurst 1975;De Waalet al.2011). Ten mealworm larvae were added to soil samples in separate 500 mL plastic containers. The containers were closed with lids and incubated in a dark room at 25°C. Dead larvae were removed from the soil after a period of 6-7 days and transferred to a modified White’s trap (White 1927). This was kept at room temperature until the emergence of IJs. The infective juveniles were then harvested after a period of 7 days,maintained by recycling throughT.monitor(Dutkyet al.1964) and stored in vented 500 mL culture flasks at 14°C.
2.4.Test for infectivity
Six Petri dishes were lined with filter paper,andT.molitorlarvae were placed in 3 Petri dishes,while fiveGalleria mellonellalarvae were placed in the remaining 3 Petri dishes. A total of 1 mL of water,which contained about 800 IJs of the EPNs,was added into each Petri dish. Each dish was sealed with parafilm and placed in an incubator at 25°C. The insects were examined after 48 h for mortality by means of dissection (Plattet al.2018).
2.5.Nematode identification
Identification of the nematodes was done using both morphological observations and molecular characterization.Nematodes were dissected from infectedT.molitorcadavers and all life stages of the nematodes (IJs,males,females,and hermaphrodites) were observed under a high power Leica DM2000 Compound Microscope (Leica Microsystems,USA). However,molecular analysis was considered as the primary approach for identifying the species of the nematode. Molecular characterization of the isolates was performed by analysis of the ITS rDNA sequences.
2.6.DNA extraction and molecular identification of nematodes
DNA was extracted from single young female nematodes. Nematodes were picked with sterile needles and placed on the side of 0.2 mL Eppendorf PCR tubes that contained 30 μL lysis buffer (500 mmol L-1MgCl2,10 mmol L-1DTT,4.5% Tween 20,0.1% gelatine and 3 μL proteinase K at 600 μg mL-1). Thereafter,the nematodes were aseptically cut into many parts with the aid of a sterile insulin needle. The PCR tubes were kept at -80°C for 15 min,and then incubated at 65°C for 1 h and at 95°C for 10 min in a thermocycler.
PCR for the amplification of the ITS regions of the ribosomal DNA was done in a thermocycler using KAPA2G™ Robust Hotstart Ready Mix (KAPA Biosystems,USA) with the primer sets described by Vrainet al.(1992) which comprised of a forward primer 18S (5´-TTGATTACGTCCCTGCCCTTT-3´) and reverse primer 26S (5´-TTTCACTCGCCGTTACTAAGG-3´). The cycling condition included:1 cycle of 94°C for 7 min,followed by 35 cycles of 94°C for 60 s,50°C for 60 s and 72°C for 60 s and a final extension of 72°C for 10 min. The PCR products were separated on 1.5% agarose gel stained with ethidium bromide and visualised under UV light with a transilluminator imaging system.
2.7.Sequencing and phylogenetic analysis
The PCR products were purified using the Nucleo-Fast Purification System (Macherey Nagel,Waltham,Massachusetts,USA). The purified DNA products was sequenced in both directions with the Big Dye Terminator V1.3 Sequencing Kit,followed by the use of electrophoresis on the 3730xl DNA Analyser (Applied Biosystems,USA) at the DNA Sequencing Unit (Central Analytical Facilities,Stellenbosch University,South Africa). Sequence editing and assembly was done using the software for biological sequence alignment editor,Bioedit 7.2 (Hall 1999),and the newly obtained sequence was compared for similarity against other sequences ofHeterhorhabditiswhich were obtained from GenBank using BLASTN (Altschulet al.1997). The newly obtained sequence was deposited on the GenBank with accession no.MT371593.
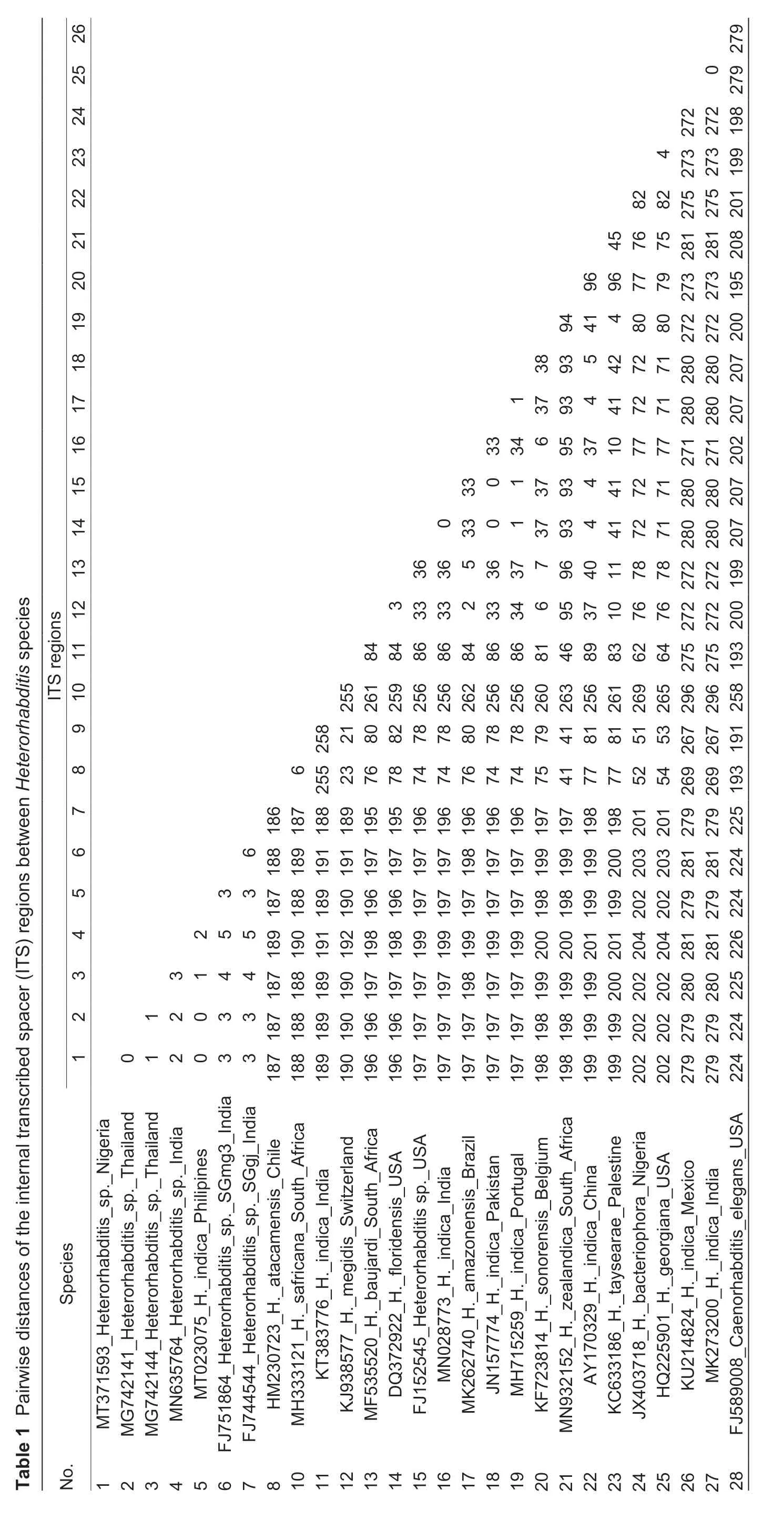
The phylogenetic analysis involved 16 nucleotide sequences ofHeterorhabditiswithCaenorhabditis elegansMaupas (Nematoda:Rhabditidae) as outgroup.Sequences were aligned using Multiple alignment program for amino acid or nucleotide sequences,MAFFT version 7 (Katoh and Standley 2013). Evolutionary analyses were conducted in MEGA X version 10.0.5 (Kumaret al.2018) and the confidence intervals for the various branching patterns in the trees were measured using bootstraps (Felsenstein 1985) with 1 000 replicates. Estimates of the evolutionary divergence between sequences were done using pairwise distance and the number of base differences per sequence are shown in Table 1. Evolutionary analyses were conducted in MEGA X (Kumaret al.2018).
3.Results
A preliminary investigation showed that theG.mellonemalarvae were not killed after 48 h of exposure to the EPNs.However,80% mortality was observed after 8-10 days of incubation. IJs were recovered from the cadavers after two weeks of incubation. The mealworm larvae (T.molitor) however proved to be a better bait forHeterorhabditissp.with a 100% mortality rate after 48 h of incubation (Figs.1 and 2). All life stages ofHeterorhabditissp.were observed from the samples obtained fromG.mellonellaandT.molitorcadavers. Males were also observed from the second generation of the EPNs.
3.1.Description
Hermaphrodite (the first generation hermaphrodite) has a body that is long and robust,takes a C shape when relaxed with heat. The head is slightly rounded and continuous with the body and has 6 distinct protruding pointed lips surrounding the oral aperture. Cheilostome is short with refractile rhabdions. Amphidial pore is obscure. The esophagus is rhabditoid,corpus is cylindrical,metacorpus is undifferentiated and basal bub is pyriform with a distinct valve. Nerve ring surrounds the isthmus posterior to the basal bulb. The vulva is located near the middle of the body and has protruding vulva lips. The tail is tapering to a pointed terminus (Fig.3). The second generation female is amphimictic. The genital tract is didelphic and reflexed while the vulva is located close to the middle of the body and has protruding vulva lips. The tail is conoid and tapers to a pointed end. Eggs are oval and arranged in visible rows in the hermaphroditic females (Figs.4 and 5). Males from the second generation have single testis,reflexed twice. Spicules are paired,separate and slightly curved. Gubernaculum is curved ventrally between the spicules.Bursa is peloderan and adorned by the complement of nine pairs of genital papillae.

Fig.1 Dead Tenebrio molitor larvae after exposure to Heterorhabditits sp.in Petri dish.
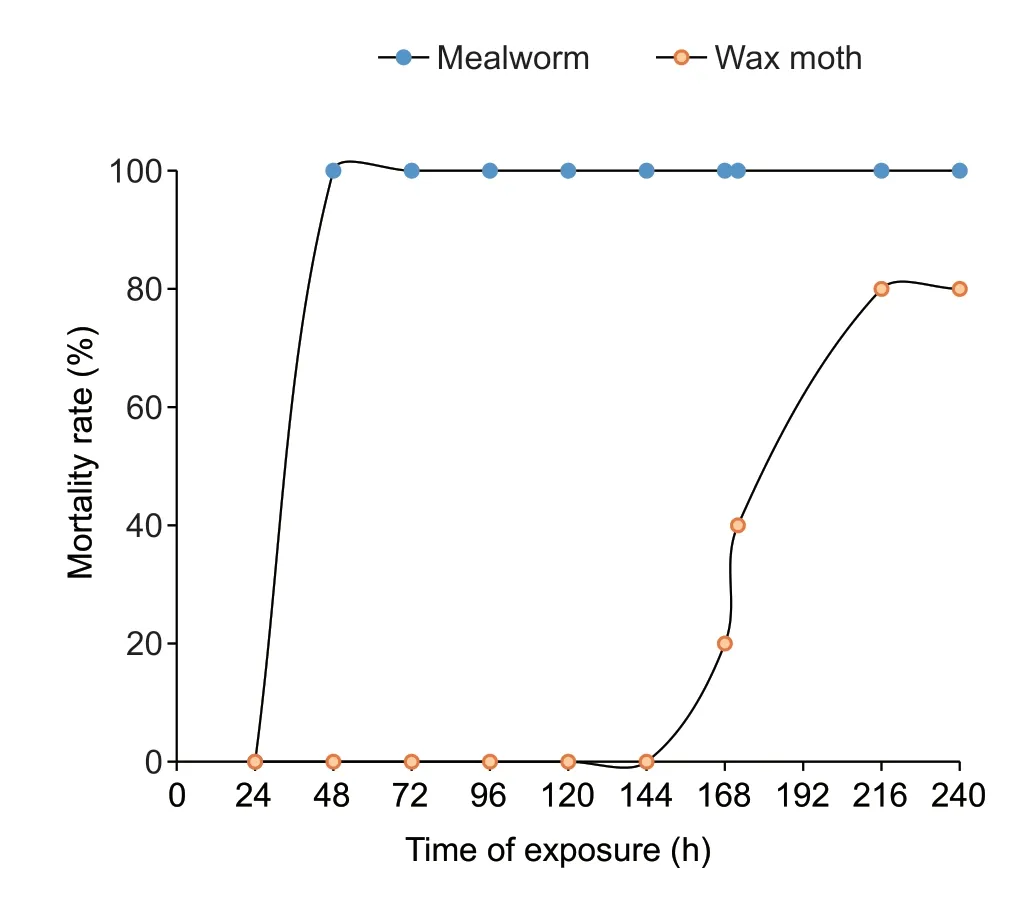
Fig.2 Mortality rate of Galleria mellonellaand Tenebrio molitor after exposure to Heterorhabditis sp.
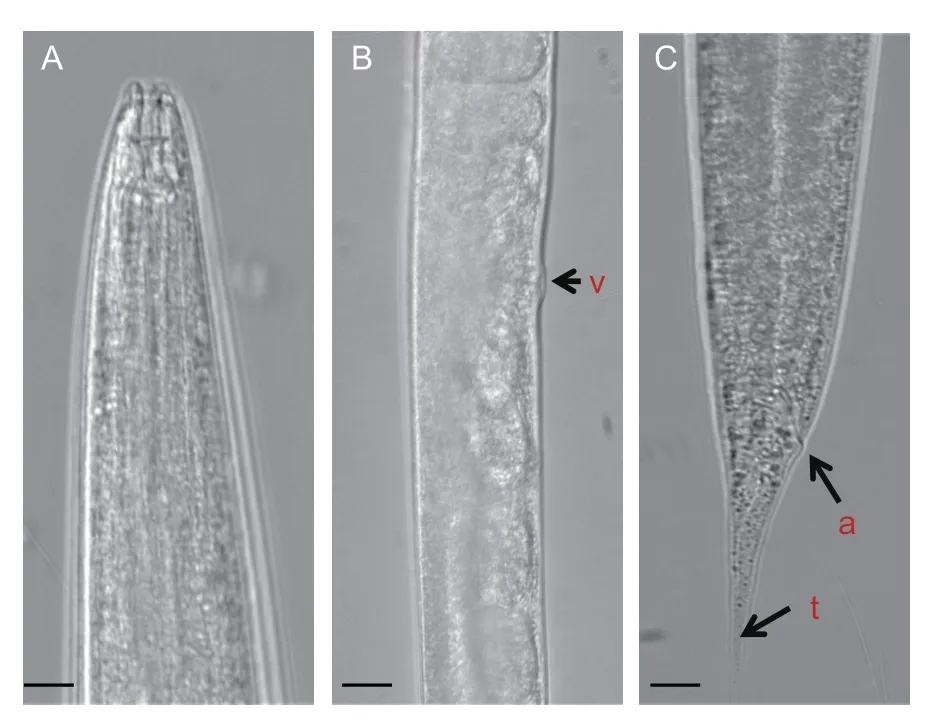
Fig.3 Light micrographs of Heterorhabditis sp. A,head region.B,mid-body region. C,tail region. v=vulva;a=anus;t=tail. Scale bar=50 μm.
The infective juveniles were observed with slightly curved slender bodies that gradually tapered at the posterior and were ensheathed in the cuticle of the second-stage juvenile.The lip region is continuous and longitudinal ridges run through most of the body length. Mouth and anus are closed. The tail is pointed.
3.2.Phylogenetic analysis
The result of the amplification of the internal transcribed spacer regions (ITS) produced a nucleotide sequence of 933 base pairs (bp) which comprise of the partial 18S,ITS1,5.8S,ITS2,and partial 28S. A BLASTN search of GenBank revealed that the sequence obtained from the Nigerian isolate was identical at 99% similarity to that ofHeterorhabditissp.from Thailand. The pairwise distances of the ITS rDNA regions betweenHeterorhabditisspecies are shown in Table 1.
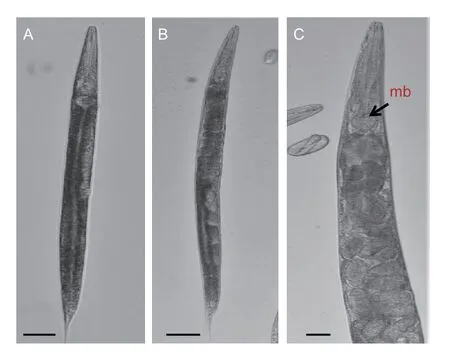
Fig.4 Lightmicrographs of female Heterorhabditis sp. A,young female. B-C,hermaphroditic female. mb=median bulb.
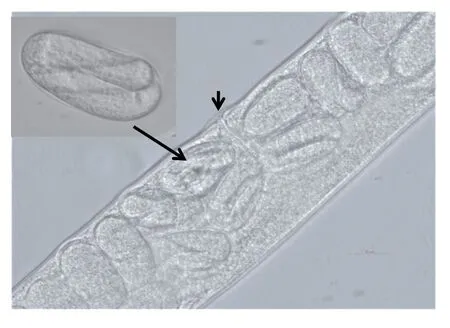
Fig.5 Lightmicrographs of eggs within and outside an adult female Heterorhabditis sp. Vertical arrow indicates the position of the female vulva while the slanted arrow shows the eggs within an adult female.
Phylogenetic analysis was based on the maximum parsimony (MP) method. The sequence of the ITS rDNA region confirmed that the Nigerian isolate was grouped with other isolates ofHeterorhabditis,when compared with other species of the genus. The phylogenetic relationships of the Nigerian isolate in comparison with the sequences of other closely relatedHeterorhabditisare given in Fig.6.
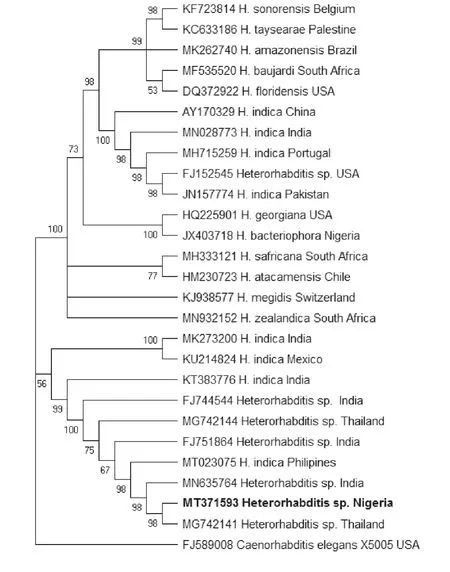
Fig.6 Phylogenetic relationships among Heterorhabditisspecies,based on analysis of the internal transcribed spacer (ITS) region with maximum parsimony (MP),using Caenorhabditis elegans as the outgroup. Newly obtained sequences are indicated by bold letters.
4.Discussion
Entomopathogenic nematodes are economically important groups of nematodes reported to have great potential for controlling insect pests of crops (Malanet al.2011;Lacey and Georgis 2012). Reports also attested to their successful use as biological control agents and suppression of insect populations (Shapiro-Ilanet al.2002;Shapiro-Ilan and Gaugleret al.2002;Nguyenet al.2006). An extensive survey for EPNs has been conducted worldwide and research into their biocontrol potential has been reported in all the continents of the world except Antarctica (Popiel and Hominick 1992). In Africa,EPNs have been reported from ten countries including Egypt,Kenya,Ethiopia,Tanzania,Benin,Morocco,South Africa,Algeria,Cameroon,and Nigeria (Akyaziet al.2012;Bhatet al.2020). EPNs in the family Heterorhabditidae have been described as effective biological control agents of insect pests (Gaugler and Kaya 1990;Stocket al.1996). The family contains one genus,Heterorhabditis,with currently more than 21 species described worldwide (Nguyen 2007;Bhatet al.2020).
In Nigeria,research in EPNs is still in its infancy,with only a few species being reported in the country (Akyaziet al.2012;Aliyuet al.2015;Claudius-Cole 2018). In the current investigation,Heterorhabditissp.was identified from soil samples that were obtained from arable land cultivated with cassava. Molecular identification and phylogenetic analysis of this nematode showed that it has not been previously described from Nigeria and the isolate clustered with other species ofHeterorhabditisthat were described from Thailand. Nguyen and Hunt (2007) had linked the occurrence ofHeterorhabiditisto tropical regions. The climatic conditions of Thailand and Nigeria are similar,with a rainy season which generally runs from mid-May to October and dry season from November to February. Similar climatic conditions could have contributed to the occurrence of EPNs in these two geographical locations.
The phylogenetic analysis also revealed variability betweenHeterorhabditissp.observed in this present study andH.bacteriophora(Akyaziet al.2012) previously reported from Nigerian soil. The isolates fall within separate clades and the pairwise distance revealed a base pair difference of about 202 nucleotides,thus indicating an appreciable difference and divergence between the two species. In addition,the estimate of the p-distance analysis indicated that the Nigerian population is not highly divergent from the undescribed species reported from Thailand and India. The Nigerian isolate revealed for the first time in Nigeria by the present study,is identical toH.indicathat is described from the Philippines and clusters with manyH.indicasequences along with some undescribed species in the phylogenetic tree. This actually puts the Nigerian isolate in theH.indica“sub-group”withH.indicaas a sister taxon. The outcome is expected,asH.indicais frequently encountered in warm tropical climates (Banuet al.2005;Chaeraniet al.2018) which the Nigerian climate typifies. This is characterized by relatively high temperature,high humidity and abundant rainfall. This study assumes that due to the divergent geographical distance among the sites of occurrence of the“indica-subgroup”across the globe,there is a possibility for the isolates ofH.indicato be“latitudinal clades”,or that they might be different species altogether. This phenomenon has been suggested by some authors (Dolginet al.2008;Dolinskiet al.2008). Similarly,it may be attributed to intragenomic rRNA polymorphisms which can be prevalent across wide geographic ranges with slight differences in polymorphism diversity as proposed by Qinget al.(2019).However,more extensive studies need to be done on the Nigerian isolate in order to substantiate this claim.
This confirms the fact that there are many uncharacterized and understudied populations ofHeterorhabditiswith possible potentials for biological control still existing in nature. It also reaffirms the need to conduct more detailed and diversified studies of nematodes in general and EPNs in particular for an updated national inventory of biological control. This can serve as a baseline reference in research prospecting biotechnological means to harness indigenous species of EPNs and other nematodes for the benefits of humanity (Lacey and Georgis 2012). While very little is currently known about their distribution in the continent,molecular results showed that this is the first account of the isolate in Nigeria and possibly in the West African subregion.
The biocontrol potential of EPNs had been established for the control of many insect pests including termitesOdontermes obesus(Isoptera:Termitidae),mealy bugsPlanococcus ficus(Hemiptera:Pseudococcidae),stem borersScirpophaga incertulasWalker (Pyralidae:Lepidoptera),moths (Lepidoptera:Pyralidae),Corcyra cephalonicaStainton (Lepidoptera:Pyralidae) and weevilsPhlyctinus callosus(Coleoptera:Curculionidae) (Divya and Sankar 2009;Malan and Ferreria 2017). Its applications have been reported to form part of the integrated management system deployed to tackle the menace of insect pests’ attack of pre-and post-harvest crops. Aliyuet al.(2015) used greater wax moth as the bait trap in an investigation that demonstrated the potential bio-insecticidal property ofSteinernema-bacteria complex. Claudius-Cole (2018) also demonstrated the potential of some EPNs for the control of stem borer,Sesamia calamistisHampson (Lepidoptera:Noctuidae),in Nigeria. In the current study,the infectivity potential of the new species ofHeterorhabditissp.was investigated and an interesting result was obtained that validates the isolate as a potential biocontrol of mealworm larva by showing 100% mortality within 48 h of exposure.This is in agreement with some research in South Africa where different aspects relating to the efficacy of mealworm as a suitable insect host for commercial production of EPNs have been investigated (van Zyl and Malan 2014,2015).
According to Popiel and Hominick (1992),EPNs have a preferred host range and are not equally efficient in infecting all insects. In the current investigation,theHeterorhabditissp.showed greater virulence to mealworm larva than wax moth that is commonly used as bait insect in EPN surveys. van Zyl and Malan (2014) posited that the use ofG.mellonellaas bait to obtain EPNs from soil has led to the limited information available on the natural host range of these nematodes. According to Lacey and Georgis (2012),the selection of an EPN for the control of a particular insect pest is determined by factors such as the nematode’s host range,host finding or foraging strategy,soil conditions,application methods,tolerance of environmental factors and their effects on survival and efficacy (Shapiro-Ilanet al.2006). This could only suggest that mealworm fall within the host infectivity of the isolated EPN from Nigeria. Native and indigenous EPNs could therefore provide a rich and valuable resource for biocontrol options because of their adaptability to local environmental conditions. This could also account for their potential effectiveness.
5.Conclusion
This study confirms the presence of an entomopathogenic nematode,Heterorhabditissp.in Nigerian soil. The molecular identification and phylogenetic analysis of this nematode show that it is closely related to those previously described from Thailand. The Nigerian isolate from this study shows a 100% mortality against mealworm,T.molitorlarvae. Therefore suggesting that the EPN is a potential biocontrol agent,which can be useful in the management of insect pests of agricultural importance.
Acknowledgements
The authors are grateful to the Management of Landmark University for financial assistance and to Prof.A.P.Malan of the Department of Conservation Ecology &Entomology,Stellenbosch University,South Africa for providing guidance with molecular identification.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- lmpacts of climate change on drought risk of winter wheat in the North China Plain
- Triple bottom-line consideration of sustainable plant disease management:From economic,sociological and ecological perspectives
- A rice geranylgeranyl reductase is essential for chloroplast development
- Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters
- Does nitrogen application rate affect the moisture content of corn grains?
- Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean
