Application Research on K/S Value in Determination of Reactive Dyes Fixation Rate
2021-10-22FENGLongHAOQingqing郝晴晴HUXuemin胡雪敏YANGWenxiu杨文秀
FENG Long (冯 龙), HAO Qingqing (郝晴晴), HU Xuemin (胡雪敏), YANG Wenxiu (杨文秀)
College of Textile and Garments, Hebei University of Science and Technology, Shijiazhuang 050018, China
Abstract: Reactive dyes are the main dyes in printing and dyeing of cellulosic fibers. Reactive dyes fixation rate is a vital indicator to measure the degree of the covalent bond between cellulose and reactive dyes. However, the determination of the fixation rate is tedious and time-consumptive. Based on the theory of reactive dyes dyeing and application of modern computer color matching technology, the relationship between K/S value and the fixation rate with the reactive dyes on cotton fabric was studied. The feasibility of K/S value instead of the traditional washing method for the determination of reactive dyes fixation rate was proved. In this study, the K/S value of the fabric has an excellent linear relationship to the reactive dyes fixation rate obtained by the washing method. The reactive dyes fixation rate can be obtained through the K/S correction value.
Key words: reactive dye; pure cotton fabric; fixation rate; Kubelka-Munk function; K/S value
Introduction
Reactive dyes with the advantages of bright color, complete chromatogram, and simple processes are the paramount dyes in printing and dyeing of cellulosic fibers[1-2]. Reactive dyes fixation rate is an important indicator to evaluate the degree of the covalent bond between cellulose and reactive dyes, which reflects the utilization of reactive dyes. There are two methods for determining the fixation rate of reactive dyes. One is the washing method, the other is the dissolution with the acid method. Both processes are cumbersome in operation and inaccuracy[3]. TheK/Svalue indicating the shade of the surface of the dyed fabric is the most important parameter in modern computer color matching technology.Kis the absorption coefficient of opaque object;Sis the scattering coefficient of opaque object. TheK/Svalue of the fabric has a favorable linear relationship to dye concentration on dyed textile[4]. Before that, theK/Svalue has been applied to the characterization of the fixation rate, but due to the limitation of dye concentration, there is a big error in dyeing on high concentration dyes[5]. In this study, take reactive red B-2BF, reactive light-yellow B-6GLN and reactive dark blue B-2GLN as an example, to research the relationship betweenK/Svalue of reactive dyes in the fabric and the fixation rate by washing method. The reactive dyes fixation rate can be obtained through theK/Scorrection value, and it has high reliability in the range of each dye concentration gradient.
1 Theoretical Basis
1.1 Reactive dyes fixation rate
In the alkaline environment, Cello anions cellulose anions (cello-) react with the electron deficient carbon atoms on the active groups by nucleophilic displacement or nucleophilic addition reactions, and form covalent bonds with them to achieve the purpose of dyeing[6]. Reactive dyes fixation rate is the percentage of the dyes that react with fibers to the total amount of the input dyes, which can be calculated by[7]
(1)
whereXis the fixation rate,D0is the amount of dyes, andD1is the amount of dyes bonded to the fiber.
1.2 Kubelka-Munk function and K/S value
Kubelka-Munk function is the foundation of computer color measurement and the matching Kubelka-Munk function can be calculated by[8-9]
(2)
whereRλis the reflectivity of the material. The Kubelka-Munk function delimits theK/Svalue. TheK/Svalue is proportional to dye concentration absorbing in the dyed fabric[10-11].
1.3 Experimental theory
TheK/Svalue represents the number of reactive dyes connecting with fibers by the covalent bond. The more amount of reactive dyes and cellulose fiber, the greater theK/Svalue is, so there is an absolute correlation between the reactive dyes fixation rate and theK/Svalue[12]. By theK/Svalue and fixation rate of reactive dyes on cotton fabric, theK/Svalue of dyed fabric can be used instead of the traditional method for determining reactive dyes fixation rate. In this study, the fixation rate of reactive dyes is determined by the washing method.
2 Experiments
2.1 Experimental materials
2.1.1Mainexperimentalmaterials
Pure cotton fabric (132 g/m2mass density) was purchased from Yongsheng Cotton Textile Mill, Handan, China. Reactive red B-2BF, reactive light-yellow B-6GLN and reactive dark blue B-2GLN were provided by Zhejiang Longsheng Group Co., Ltd., Jiaxing, China. Sodium chloride (NaCl) and sodium carbonate (Na2CO3) were supplied by Tianjin Yongda Reagent Co., Ltd., Tianjin, China.
2.1.2Experimentalinstruments
The dye depth was tested by Color i5D meter (X-rite, USA). The absorbance was measured by visible spectrophotometer V-5600 (Shanghai Metash Instrument Co., Ltd., Shanghai, China). Digital display constant temperature water bath pot HH-4 (Changzhou Jintan Shuangjie Experimental Instrument Factory Co., Ltd., Changzhou, China) was used for keeping constant temperature during dyeing.
2.2 Experimental methods
Three primary colors, reactive red B-2BF, reactive light-yellow B-6GLN and reactive dark blue B-2GLN were selected for dyeing under different dyeing concentrations. TheK/Svalue and the fixation rate of dyed fabric were measured after dyeing and fixation.
2.2.1Determinationoffixationrateofreactivedyesbywashingmethod
In this method, the amount of dyes in dyeing residue (including washing solution and soap liquid) after dyeing and fixing is used to calculate indirectly[13]. The fixation rateXof reactive dyes by the washing method is obtained by
(3)
whereA1andV1are absorbance and volume of diluted standard solution, respectively;A2andV2are absorbance and volume of the diluted dye residue, respectively.
2.2.2DeterminationofK/Svalueofdyedcottonfabric
TheK/Svalue of cotton fabric dyed with reactive dyes is determined at the maximum absorption wavelength according to the color standard by Color i5D Color tester. Five tests were carried out for each dyeing sample, and the average value was calculated. The larger theK/Svalue is, the darker the surface color is. This means higher dye uptake.
2.2.3RelationbetweenK/Svalueoffabricandfixationrateofreactivedyes
Setting theK/Svalue and the fixation rate asXandYrespectively, the relationship between theK/Svalue and the fixation rate is studied. By fitting the function, the relationship between theK/Svalue and the fixation rate can be accurately reflected. The accuracy of the fitting equation was verified under different initial dye concentrations.
2.3 Experimental prescription and process conditions
The temperature of dyeing bath (bath ratio 1∶50) was raised to 60 ℃ at the rate of 1-2 ℃/min, NaCl (20 g/L) and Na2CO3(10 g/L) were added after dyeing for 60 min, and then the dyed fabric was washed with warm water and cold water successively[14].
The fabric was put into 95 ℃ soaping solution (Na2CO32 g/L, soaping agent 2 g/L, bath ratio 1∶100) for 10 min. And the fabric was washed and dried[15].
3 Results and Discussion
3.1 Correlation between K/S value and fixation rate of reactive red B-2BF
The experimental scheme and principle are shown in Fig. 1.

Fig. 1 Experimental scheme and principle
When the concentration of reactive red B-2BF is 1%, 2%, and 4% on weight of fabric (owf), the relationship between theK/Svalue and the fixation rate is shown in Fig. 2. According to the different relationships between theK/Svalue and the fixation rate under different concentrations of reactive red B-2BF, theK/Svalue and fixation rate were linearly fitted to obtain the fitting formula and fitting degree[16].
As shown in Fig. 2, when the concentration of reactive red B-2BF is 1%, 2%, and 4% owf, the fixation rateXincreases with the increase of the fabric’sK/Svalue. Table 1 shows that when the concentration of reactive red B-2BF is 1%, 2%, and 4% owf, the fitting degree betweenK/SandXis 0.995 5, 0.987 0 and 0.985 5, respectively. The closer the fitting degree is to 1, the better the linear relation is. Therefore, there is a great linear relationship between theK/Svalue and the fixation rate of dyed cotton fabric under different reactive red B-2BF dye concentrations[17].


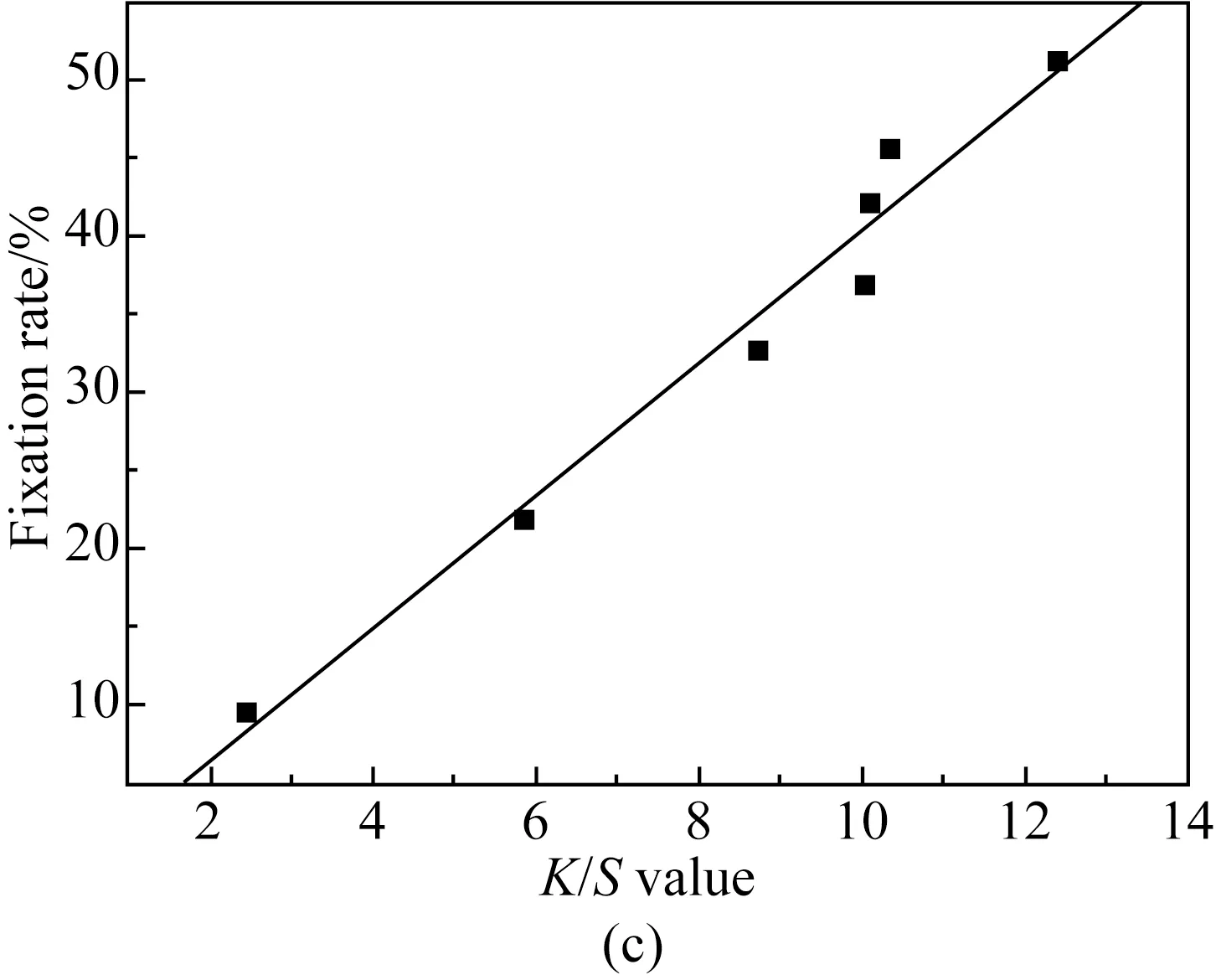
Fig. 2 Relationships between K/S value and fixation ratewith reactive red B-2BF of different concentrations:(a) 1%; (b) 2%; (c) 4%

Table 1 Fitting formula and fitting degree of reactive redB-2BF dyes at different concentrations
3.2 Correlation between K/S value and fixation rate of cotton fabric dyed by reactive light-yellow B-6GLN
Figure 3 displays the relationship between theK/Svalue and the fixation rate of reactive light-yellow B-6GLN at 1%, 2%, and 4% owf. According to the different relations between the dyeingK/Svalue of reactive light-yellow B-6GLN under different concentrations and the fixation rateXmeasured by the washing method, the relational formula between theK/Svalue and fixation rate was obtained for linear fitting. The fitting formula and the fitting degree are shown in Table 2.
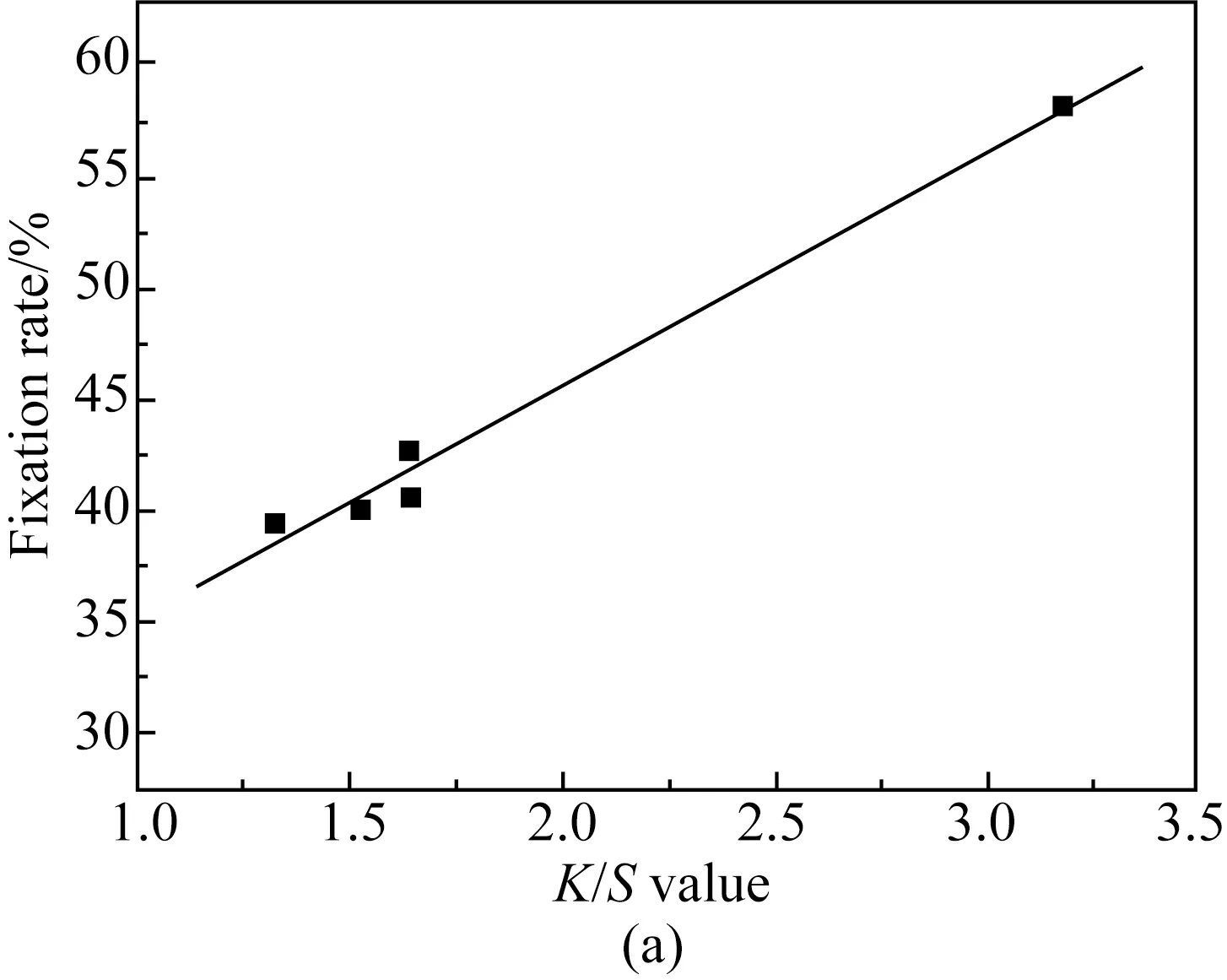


Fig. 3 Relationships between K/S value and fixation ratewith reactive light-yellow B-6GLN under differentconcentrations: (a) 1%; (b) 2%; (c) 4%

Table 2 Fitting formula and fitting degree of reactivelight-yellow B-6GLN at different concentrations
As shown in Fig. 3, the fixation rateXincreases with the increase of theK/Svalue of the fabric when the concentration of reactive light-yellow B-6GLN is 1%, 2%, and 4% owf. As shown in Table 2, when the concentration of reactive light-yellow B-6GLN is 1%, 2%, and 4% owf, the fitting degree betweenK/SandXis 0.993 1, 0.967 5 and 0.997 5. TheK/Svalue of dyed cotton fabric with different concentrations of reactive light-yellow B-6GLN dye also shows a great linear relationship with the fixation rate.
3.3 Relationship between K/S value and fixation rate of dyed cotton fabric by reactive dark blue B-2GLN
When the concentration of active blue B-2GLN is 1%, 2%, and 4% owf, the relationship between theK/Svalue and the fixation rate is shown in Fig. 4.
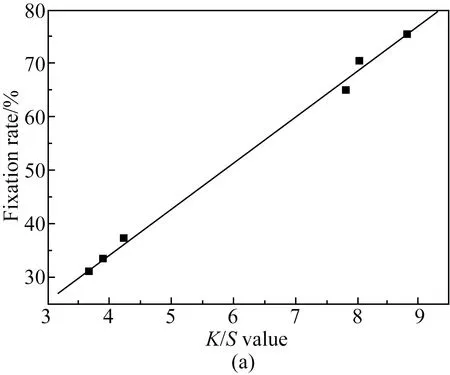
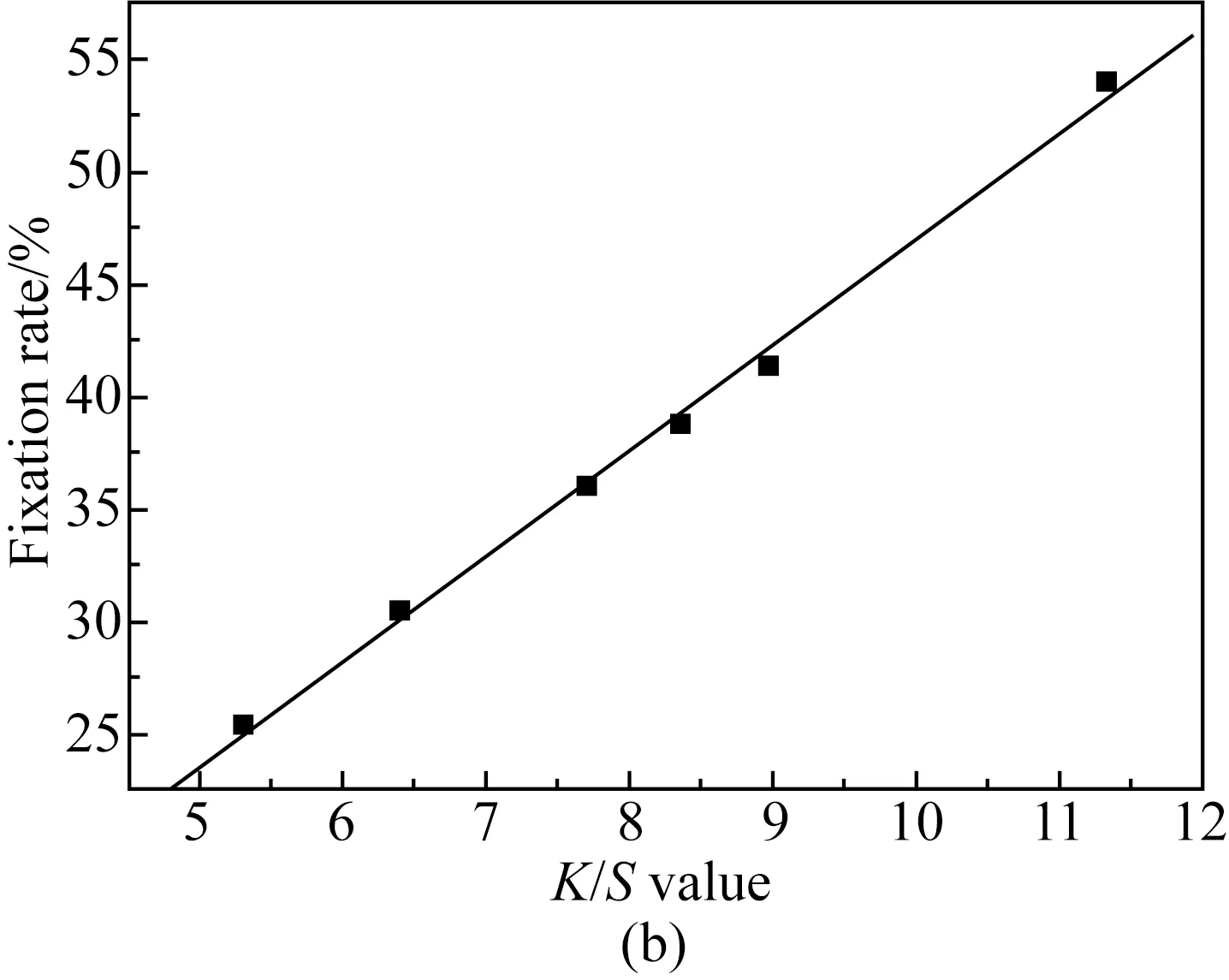

Fig. 4 Relationship of K/S value and fixation rate of cotton fabrics dyed with different concentrations of reactive dark blue B-2GLN: (a) 1%; (b) 2%; (c) 4%
According to the different relationship between the dyeingK/Svalue of active blue B-2GLN under different concentrations and the fixation rateXmeasured by the washing method, the relationship was analyzed by linear fitting. The fitting formula and fitting degree are shown in Table 3.

Table 3 Fitting formula and fitting degree of reactive dark
As shown in Fig. 4, when the concentration of reactive dark blue B-2GLN dyes is 1%, 2%, and 4% owf, the fixation rateXmeasured by the washing method increases with the increase ofK/Svalue of the fabric. As can be seen from Table 3, when the concentration of reactive dark blue B-2GLN is 1%, 2%, and 4% owf, the fitting degree betweenK/SandXis 0.998 4, 0.998 4, and 0.987 1, respectively. TheK/Svalue of dyed cotton fabric with different reactive dark blue B-2GLN concentrations also shows a good linear relationship with the fixation rate.
According to the experiments, the relationship between theK/Svalue and the fixation rate of reactive dyes can be determined. By measuring theK/Svalue, the fixation rateXof reactive dyes can be calculated according to the relationship.
Reactive red B-2BF, reactive light-yellow B-6GLN, and reactive dark blue B-2GLN belong to reactive B series dyes mainly containing two active groups, which have high reactive activity in the dyeing process, high binding force with the fiber and good stability. It is difficult to change the high correlation between the measuredK/Svalue and the fixation rate. The fitting degreeRrepresents the reliability of the solid color ratio calculated byK/Sin the range of values. The closer it is to 1, the more accurate the final result is.
In conclusion, because of the existence of fitting equation, the fixation rate can be easily obtained by theK/Svalue. As shown in Fig. 5, the relationship between dye uptake andK/Svalue has nothing to do with dye concentration, so this experimental method can be applied to various scenarios. The fitting degree between dye uptake andK/Svalue is higher than 0.95 regardless of dye concentration. This provides a wider prospect for the application ofK/Svalue in characterizing the dye uptake of reactive dyes. TheK/Svalue of dyed fabric has a linear relationship with the fixation rate of reactive dyes, which is entirely consistent with the simplified Kubelka-Munk function. The value ofK/Sis directly proportional to the dye concentration on the dyed fabric. The value ofK/Sindicates the number of dyes on the fabric. The larger the value ofK/Sis, the deeper the color will be. The smaller the value ofK/Sis, the lighter the color is, which means the dye absorption of colored substances is low[18].

Fig. 5 Fitting degree histogram of three dyes in different concentrations
4 Conclusions
(1)K/Svalue has a linear relationship with the fixation rate of reactive dyes determined by the washing method.K/Svalue can be used to represent the fixation rate of reactive dyes. The determination method of theK/Svalue of fabric is simple, fast, and accurate.
(2) The fixation rate of reactive dyes can be obtained by calculating the linear relationship between theK/Svalue of the fabric and the fixation rate of reactive dyes determined by the washing method. The linear relationship between theK/Svalue and fixation rate of reactive dyes with different shades of red, yellow, and blue was different.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Influence Mechanism of Clothing Anchor Features on Consumers’ Purchase Intention
- Performance Evaluation of a Molten Carbonate Fuel Cell-Graphene Thermionic Converter-Thermally Regenerative Electrochemical Cycles Hybrid System
- Long Text Classification Algorithm Using a Hybrid Model of Bidirectional Encoder Representation from Transformers-Hierarchical Attention Networks-Dilated Convolutions Network
- Estimating Mechanical Vibration Period Using Smartphones
- Meta-Path-Based Deep Representation Learning for Personalized Point of Interest Recommendation
- Design and Characterization of Electrical Connections for Conductive Yarns
