Nitrogen Removal Performance of Continuous Anoxic/Oxic System Using Activated Sludge and Sludge Biofilms
2021-10-20GHORIFaheemAhmedCHENHongYUXinSHEShuaiqi佘帅奇XUEGangCHENShanping陈善平SABIHAYousuf萨比哈
GHORI Faheem Ahmed (欧 凡), CHEN Hong(陈 红)*, YU Xin(于 鑫), SHE Shuaiqi(佘帅奇), XUE Gang(薛 罡),2, CHEN Shanping(陈善平), SABIHA Yousuf(萨比哈)
1 College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China 2 Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China 3 Shanghai Institute for Design & Research on Environmental Engineering Co., Ltd., Shanghai 200232, China 4 College of Basic Medical Sciences, Dalian Medical University, Dalian 116044, China
Abstract: Nitrogen is the most important component for living beings while the excessive discharge of organic and inorganic nitrogen may create severe environmental problems. In this study, a continuous anoxic/oxic (A/O) reactor adopting activated sludge and sludge biofilms in the anoxic and oxic zones was applied for total nitrogen (TN) and chemical oxygen demand (COD) removal, and the efficiencies of nitrification and denitrification were compared as well. Results showed that when using activated sludge, the effluent concentrations of NH4+-N, NO3--N, NO2--N, TN and COD were inconsistent and fluctuated greatly, and the removal efficiencies of corresponding nitrification, denitrification and TN were also unstable; the obtained average COD removal efficiency was 85%. While using sludge biofilms, the acquired effluent concentrations of TN and COD became stable and constant. The nitrification, denitrification, TN and COD removal efficiencies were 96%, 84% and 65% and 94%, respectively. Bacterial community analysis of sludge biofilms indicated that the genus Arcobacter was the major denitrifiers in the anoxic zone with relative abundance of 76.1%, and in the oxic zone the abundances of Acinetobacter, Hydrogenophaga and Nitrospira responsible for complete nitrification were 20.05%, 7.6% and 3.7% respectively. The high abundance of nitrifying bacteria and denitrifiers were related with the high and stable nitrogen and COD removal.
Key words: nitrogen removal; suspended sludge; biofilm; anoxic/oxic system
Introduction
Nitrogen is a key component for living creatures while the unnecessary discharge of organic as well as inorganic nitrogen species may cause serious environmental problems[1]. Nitrogenous compounds are main source of water body deterioration because excessive load of nitrogen into watercourses contributes to increasing eutrophication and relates impacts on water quality[2-4]. To lessen the amount of nitrogen containing compounds entering watercourses, physical, chemical and biological removal methods were adopted in wastewater treatment plants.
The physical-chemical approaches for nitrogen removal are less desirable since they need high operating and maintenance cost and will cause secondary waste generation[5-6]. Thus, due to low operating cost, less adverse effect on the subsequent environment and high efficiency in nitrogen removal from wastewater, the biological methods are extensively used[7-8]. To date, the traditional biological nitrogen removal method using activated sludge is the most widely used due to its easy handling. It is the suspended growth biological method for nitrogen removal in term of nitrification and denitrification[9], but this traditional suspended sludge system has disadvantages of low nitrogen removal efficiency because of the difficult accumulation of nitrifying bacteria with a long generation time[10].
To solve this problem, attached growth processes biofilm methods have been widely introduced for treating wastewater and can allow nitrification and denitrification simultaneously[11]. In general, the biofilm method is a well-defined technology that includes compact media to the suspended growth reactor and offers biofilm attachment surfaces; this increases the microbial concentration along with contaminant degradation rates including nitrogen[12-13]. Moreover, biofilms are compact biostructures which appear on all surface that are regularly in contact with wastewater, and biofilm methods supplement the mechanism of population and the control of reaction rate to a certain degree[11, 14]. Biofilms can protect bacterial activity against adverse conditions[15]. Overall, the biofilm method has numerous benefits including flexibility in operation and change in the environment. It requires high biomass concentration which results in less space demanding and longer biomass residence time. This can reduce the recalcitrant of the wastewater, and decrease the hydraulic retention time. The production of sludge is less than activated sludge mthod[16-17]. Hence, the application of biofilm-based wastewater treatment has developed as a favorable option for nitrogen removal[16].
Biological nitrogen removal always includes nitrification and denitrification processes which demand aerobic and anoxic environment, respectively. Therefore, anoxic-oxic (AO) technology is the most commonly applied and mainly in the form of suspended activated sludge system. To enhance the nitrogen removal efficiency of AO system, some researchers introduce biofilm in oxic stage, which will make anoxic zone exist as well, and the nitrification and denitrification will happen simultaneously in oxic tank[18-19]. Therefore, the total nitrogen (TN) was removed in oxic tank beside the anoxic tank. Additionally, the maintenance of microbial on biofilm can accumulate the bacteria of long-generation time, which is beneficial for nitrifying bacteria growth. Moreover, high microbial concentration accumulated in biofilm both in oxic and anoxic tanks is in favour of nitrogen removal. Since biofilm shows several advantages different from suspended sludge, it is expected that better nitrogen removal performance can be achieved when anoxic stage also applies biofilm.
In this study, a continuous anoxic/oxic (A/O) reactor was developed with the aim of comparing the nitrification, denitrification, TN and chemical oxygen demand (COD) removal performance using activated sludge and sludge biofilms. These comparisons can give suggestions for the process of optimizing nitrogen removal efficiency in practice.
1 Materials and Methods
1.1 Synthetic wastewater

1.2 Sludge and biofilm
The seeded sewage sludge of the AO system in this study was collected from the sedimentation tank in a municipal sewage treatment plant (Shanghai, China). The sludge was stored and settled down in a plastic box within 24 h at 4℃, and then the concentrated sludge was seeded. The combined polypropylene packing material was used as the carrier of biofilm in anoxic and oxic tanks. The diameter of plastic discs is 80 mm.
1.3 Set-up and operation of the reactor
A continuous flow A/O reactor was constructed as shown in Fig. 1, which contained a settling and effluent tank as well. The total working volume was 8 L, and the volume ratio of anoxic and oxic zone was 1∶3. The air in the oxic zone was supplied by air blowers fixed at the bottom of the reactor which permitted oxygen to be diffused from air to wastewater. No air was supplied in anoxic tank, and the sludge and wastewater were mixed by mechanical stirring. When using biofilm in anoxic tank, the mechanical stirring was stopped to avoid the disruption of biofilm growth. The whole reactor was operated by an on/off control system.
At the start, the activated sludge system was set up and operated. The A/O reactor was seeded with activated sludge which was obtained from the Songjiang wastewater treatment plant, Shanghai, China. The working volume of anoxic and oxic tanks was 2 L and 6 L, respectively, and the concentration of mixed liquor suspended solids (MLSS) in each tank was approximately 3000 mg/L. The A/O reactor was operated continuously at hydraulic retention time hydraulic retention time (HRT) for 8 h, 2 h for anoxic tank and 6 h for the oxic tank. The influent synthetic wastewater was firstly pumped into the anoxic zone and then the oxic zone. The mixture of suspended sludge and the wastewater flowed from the anoxic zone to the oxic zone, and then settled into the clarifier unit. Finally, the effluent was collected in an effluent tank. The settled sludge from clarifier was returned to the anoxic zone with a returning ratio of 100% of the influent. In general, the nitrification liquid in the oxic tank should be returned to anoxic tank. In this study, the nitrification liquid returning was modified as returning from the effluent to the anoxic tank in order to avoid the disruption of sludge contained in nitrification liquid, and the nitrification liquid returning ratio was 200% of the influent. The peristaltic pumps were used to control influent, sludge return, and nitrification liquid returning. The pH of influent was adjusted to 7.5. The reactor was maintained at room temperature, and the dissolved oxygen (DO) concentration in the oxic tank was maintained around 3 mg/L. After operating the A/O system in the form of suspended activated sludge, combined packing material was introduced to anoxic and oxic tanks, therefore the biofilm and suspended sludge could co-exist in this system. Other operation conditions kept the same. The performances of COD and nitrogen removal between suspended sludge system and biofilm system were compared.
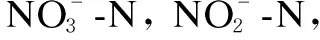
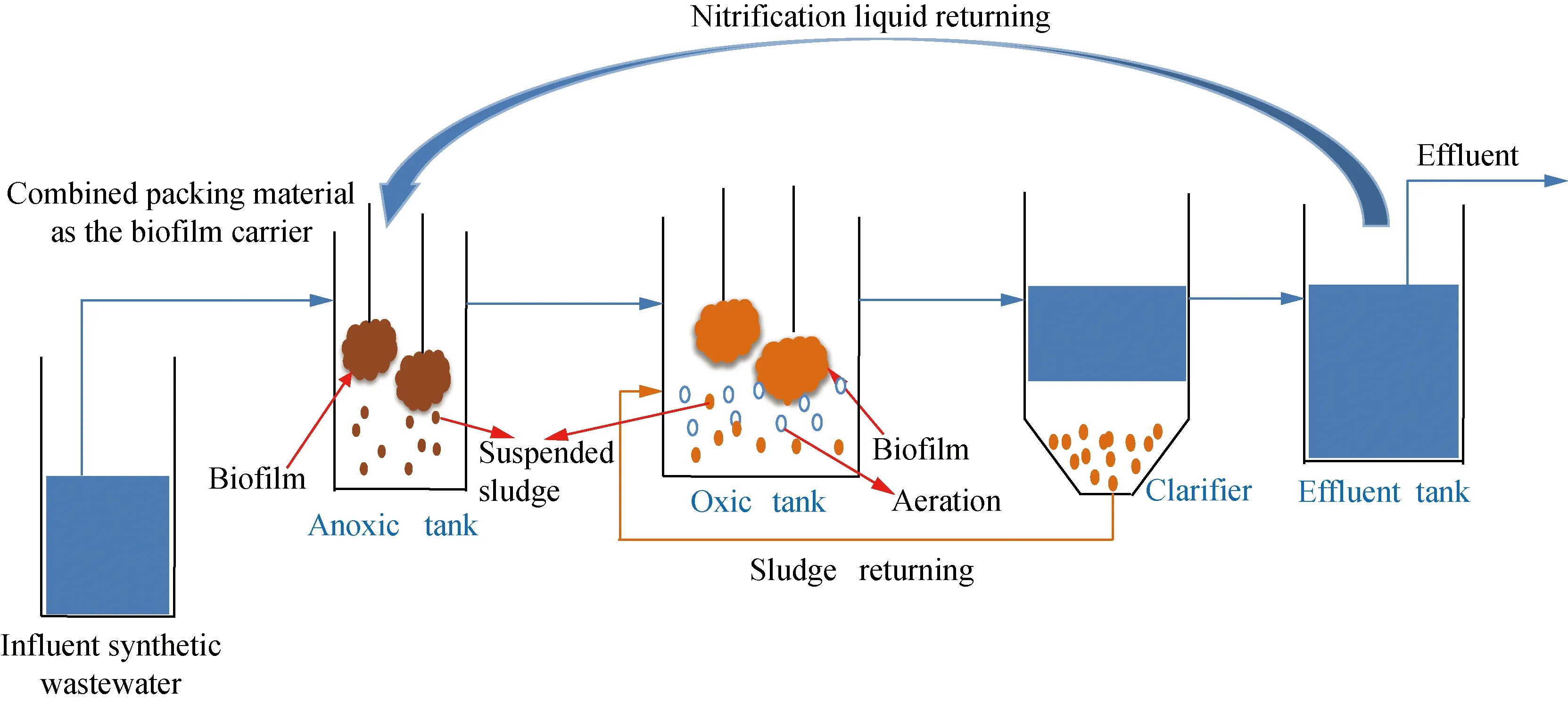
Fig. 1 Schematic diagram of continuous A/O reactor
1.4 MiSeq sequencing
Illumina MiSeq sequencing technique was done for the analysis of the microbial community. The DNA was extracted from sludge samples A and B which were taken from the biofilms presented in the anoxic and oxic zones using the FastDNA SPIN kit (Qbiogene, Carlsbad, CA, USA) following the manufacturer’s protocol. The purity and concentration of the DNA extracts were measured using a NanoDrop microvolume spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware, USA), and then the MiSeq sequencing procedure was performed. The fusion primers 515F: 5’-GTGCCAGCMGCCGCGGTAA-3’ and 907R: 5’-CCGTCAATTCMTTTRAGTTT-3’ were used to amplify the bacterial 16S V4-V5 region. The polymerase chain reaction (PCR) system contained 25.0 μL of reaction mixture, which included 1.0 μL of 20 ng/μL templates, 5 μL of buffer (5×), 2.0 μL of 2.5 mmol/L dNTP, 5.0 μL of GC high enhancer (5×), 1.0 μL of each primer (10 μmol/L), 0.25 μL of pyrobest DNA polymerase, and 9.75 μL of ultra-pure water. The PCR conditions were as follows: initial denaturation at 98 ℃ for 2 min; followed by 25 cycles of denaturation at 98 ℃ for 30 s, annealing at 50 ℃ for 30 s, and extension at 72 ℃ for 30 s; and a final extension at 72 ℃ for 5 min. The sludge bacterial 16S amplicon sequencing was performed using the Illumina MiSeq platform by the Personal Biotechnology Co. (Shanghai, China).
1.5 Processing of sequencing data
Raw sequences from this research were sorted based on the specific barcodes of the sludge samples using the Pipeline Initial Process tool at the Ribosomal Database Project (RDP). The raw sequencing data for the two samples were deposited with the NCBI Sequence Read Archive under accession number SRP302675 at the website of https://www.ncbi.nlm.nih.gov/sra. To obtain high-quality sequences, segments that were ambiguous or homologous or with lengths shorter than 250 bp were trimmed. The remaining sequences were checked for potential chimeras using Qiime (Quantitative Insights Into Microbial Ecology, v1.8.0), and the chimeras were deleted using Usearch. The resulting sequences were then grouped into operational taxonomic units (OTUs) using the 97% identity threshold (3% dissimilarity level). The reads flagged as chimeras were extracted out, and the non-chimera reads were included in a database of effective reads for each sample. The Shannon, Chao and abundance-based coverage estimator (ACE) indices were calculated to compare the microbial diversity and richness among the five samples according to Ref.[21].
2 Results and Discussion
2.1 Nitrogen removal performance
2.1.1Totalnitrogenremovalperformance
In this continuous A/O reactor, the anoxic zone was stirred by a mechanical stirrer at a constant rate at dissolved oxygen (DO) concentration of 0.2 mg/L for creating anoxic condition and the air was continuously supplied in the oxic zone at 3 mg/L of DO concentration to maintain the oxic condition. The NH+4-N, NO-3-N, NO-2-N and COD contents were determined each day. As synthetic wastewater does not contain organic nitrogen, total nitrogen was considered as the total sum of NH+4-N, NO-3-N, NO-2-N.
Data was collected after a culturing period of 12 d using activated sludge or sludge biofilms. Figure 2(a) shows the effluent concentrations of NH+4-N, NO-3-N, NO-2-N, and TN using activated sludge, and Fig. 2(b) exhibited the performance of sludge biofilm system. When biofilm was introduced to oxic and anoxic tanks, the content of suspend sludge decreased day by day, and some unstable suspend sludge was washed out and discharged via sludge discharge. After several days using biofilm, the sludge was attached on biofilm stably. The influent concentrations of NH+4-N and TN in synthetic wastewater were 47 mg/L and 50 mg/L, and NO-2-N was very low. Both in suspended sludge system and biofilm system, the concentration of NH+4-N in the effluent was declined while NO-3-N concentration increased, showing that the influent NH+4-N was mainly converted into NO-3-N. When the returning ratio of nitrification liquid is 200%, the theoretical highest TN removal efficiency is 66.7%. For suspended sludge A/O system, the effluent TN concentrations fluctuated greatly, ranging from 21.2 to 42.0 mg/L, and the corresponding TN removal efficiency was from 16.0% to 57.5% with an average value of 42.2%, far below the theoretical TN removal efficiency; additionally, the deterioration of nitrification with higher NH+4-N accumulation in effluent occurred from 14 d to 17 d, resulting in the TN removal efficiency decrease dramatically[22]. As shown in Fig. 2 (b), when applying sludge biofilms in the anoxic and oxic zones, it was observed that most of NH+4-N was removed and transformed into NO-3-N as the effluent concentration of the NO-2-N was very low (0.08 mg/L). The concentration of NO-3-N remained relatively constant with small change between 18.8 to 24.0 mg/L. Also, the TN effluent kept stable with an average removal efficiency of 65%, which was close to the theoretical TN removal efficiency. When using activated sludge, after operating for a long time, the sludge particles became loose and the settleability of the suspended sludge easily became poor. The unstable sludge characteristics might relate to the unstable nitrification and poor TN removal. When using biofilm, the system became more stable, and the high concentration of bacteria in oxic and anoxic tanks need more NH+4-N to grow, which would also contribute to the TN removal besides the denitrification. Therefore, when adding the combined packing material, the biofilm system showed higher and more stable TN removal capacity.
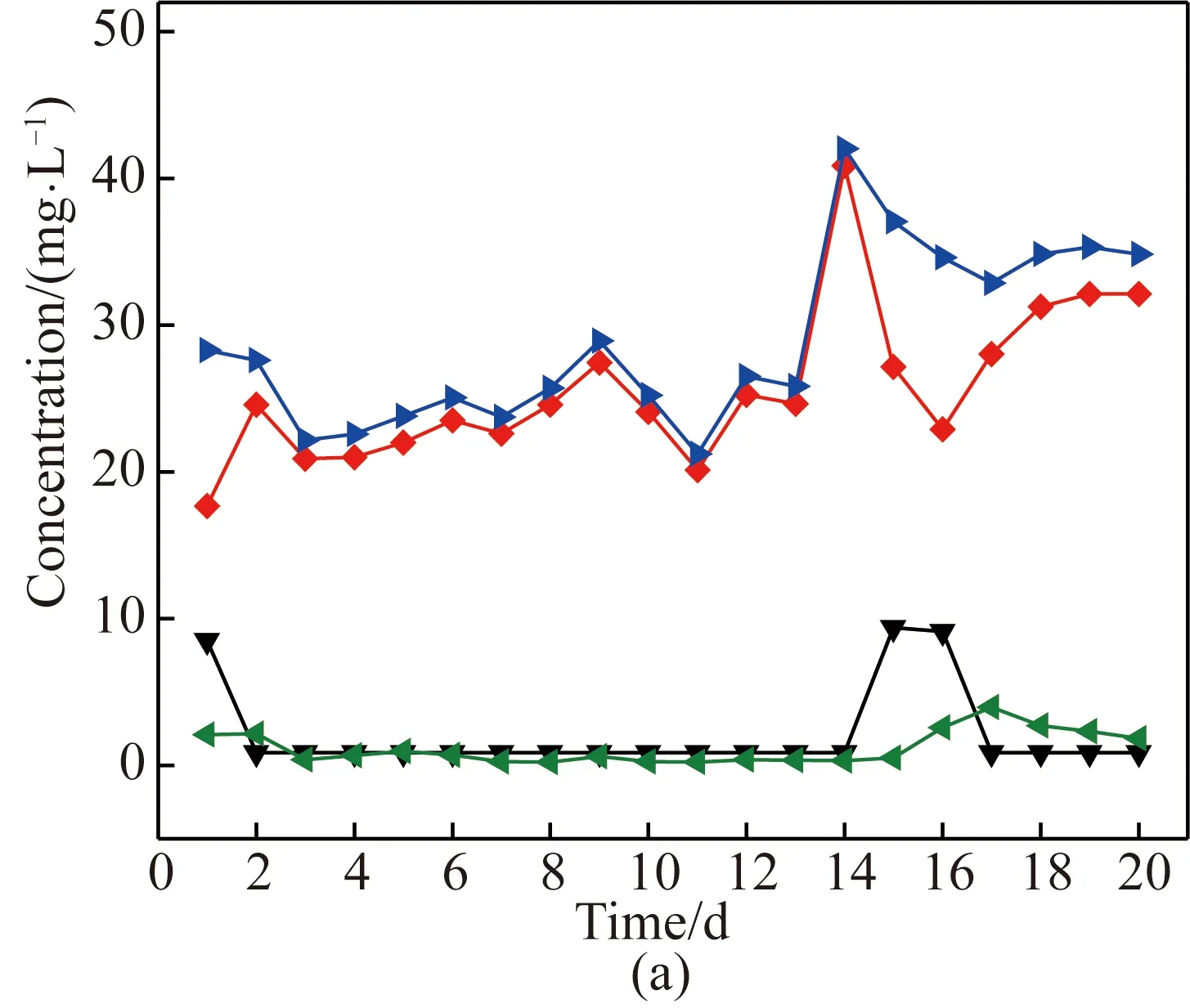

Fig. 2 Effluent concentrations of NH4+-N, NO3--N, NO2--N and TN using (a) activated sludge; (b) sludge biofilms
2.1.2Nitrificationperformance


Fig. 3 Nitrification performance using (a) activatedsludge; (b) sludge biofilms
It can be seen from Fig. 3(a) that the influent NH+4-N to the oxic zone was varied and the effluent concentration of NO-3-N OxicEffNO-3-N converted from influent NH+4-N was also fluctuated greatly during the whole period of using suspended activated sludge. The concentration of NO-2-N in the effluent (OxicEffNO-2-N) was low and constant at 0.3 mg/L; however, after 14 d of operation the NO-2-N concentration increased and reached 2.3 mg/L. The nitrification efficiency increased from 54% to 93% and then falled to 49%. Our findings revealed that incomplete and unstable nitrification occurred in suspended sludge nitrification system. When nitrification in the oxic tank became poor, the returning nitrification liquid will contain low concentration of NO-3-N. The organics in the anoxic tank cannot be used as the carbon source efficiently, and is oxidized to CO2. In this study, it was found that activated suspended sludge was easy washed out sometimes, the sludge concentration would decrease, and then sludge returning ratio should be increase. This is the also the reason for the unstable nitrification performance. After adding the combined packing material as shown in Fig. 3(b), the influent NH+4-N concentration to the oxic zone was constant and stabilized at 19.5 mg/L, and the effluent concentration of NO-3-N reached 20.0 mg/L. The effluent concentration of NO-2-N was very low (0.08 mg/L) as well. The biofilm system showed good nitrification with an average efficiency of 96 %, which was good for easy operation. It could be seen that the stable and better nitrification performance was obtained when sludge biofilms were used.
2.1.3Denitrificationperformance
Denitrification is an anoxic process in which NO-3-N is used by denitrifying bacteria as an electron acceptor. As shown in Fig. 4(a), denitrification efficiency using activated sludge was gradually altered and did not reach the stable phase during the whole period. The maximum denitrification efficiency of about 68% was obtained on the 9th day, while the minimum efficiency was only less than 10%. The results indicated that the purpose of stable denitrification was hardly achieved by using activated sludge. In experiment scale, the total volume of the AO tank is only 8 L. The small volume might be the reason of the fluctuated removal efficiency. This suggested that when the scale of wastewater treatment plant was small, stable operation might be difficult to achieve. In sludge biofilm system as shown in Fig. 4(b), the denitrification efficiency increased from 71% and became constant at 84% with an anoxic effluent concentration of NO-3-N 2.3 mg/L, which meant that most of the NO-3-N from nitrification liquid return/effluent return was consumed by heterotrophic denitrifying bacteria presented in the anoxic zone. The results indicated that the use of sludge biofilms exhibited better and more stable denitrification performance.
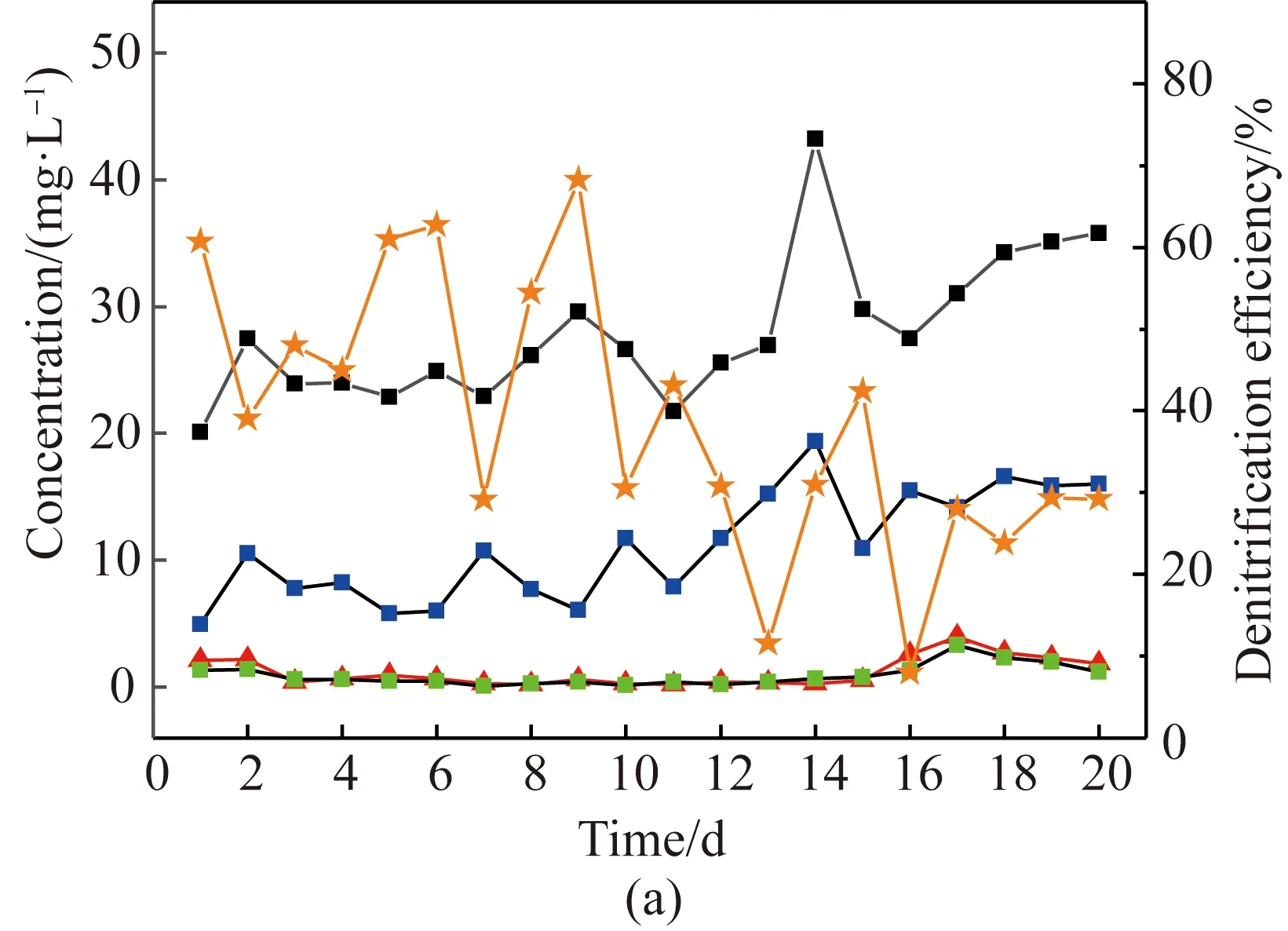

Fig. 4 Denitrification efficiency using (a) activatedsludge; (b) sludge biofilms
2.2 Organic removal performance
In AO system, organic matter can be removed as well, and its removal performances were compared as shown in Fig. 5. With influent COD concentration of 400 mg/L using both activated sludge and sludge biofilms, the final effluent COD using activated sludge was less than 50 mg/L, but after 12 d the effluent COD concentration was fluctuated and increased to 90 mg/L. The average COD removal efficiency of 85% was obtained during the whole period of using activated sludge. In anoxic stage, the effluent COD content was unstable, ranging from 101 mg/L to 254 mg/L, showing a similar fluctuation rule as that of denitrification efficiency. As to biofilm sludge system, the COD concentration after anoxic treatment was between 75.81 mg/L to 132 mg/L, and kept very stable after 6 d. In this stage, part of COD was used as denitrification carbon source to guarantee the stable and effective denitrification process, and part of COD was degraded. Since the influent COD was acetate, a kind of organics easy to be biodegraded, its majority was removed in anoxic stage, and further degradation was occurred in oxic stage. The final effluent COD was only less than 25 mg/L with an average COD removal efficiency of 94 %, which showed better COD removal performance than that of suspended sludge system.
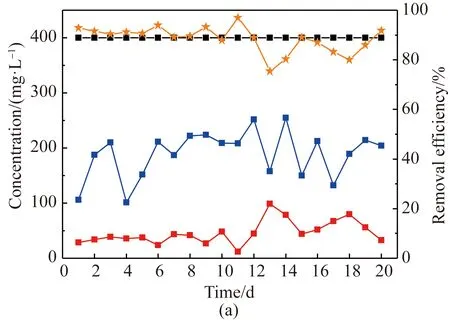
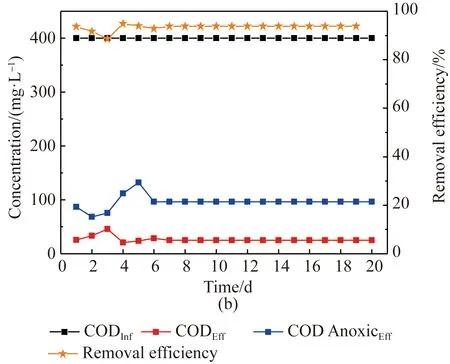
Fig. 5 COD concentration of influent, effluent and its removal efficiency using (a) activated sludge; (b) sludge biofilms
2.3 Microbial community structure and relative abundance in sludge biofilms
Microbial community plays a significant part in nitrogen and organic elimination during biological wastewater treatment processes. In order to investigate the influence of bacterial community structure on nitrification and denitrification, the sludge samples from biofilms presented in the anoxic and oxic zones were taken and analyzed via high throughput Illumina MiSeq sequencing. Figure 6 shows the relationship of sequence 16S rDNA in phylum, family, and genus levels.
The results in Fig. 6(a) indicated that phylumproteobacteriawere predominant in both samples with an abundance of 18.6% in the anoxic zone and 79.1% in the oxic zone. The relative abundance of phylaEpsilonbacteraeotain the anoxic and oxic zones was 71.6% and 5.1% respectively. The third highest abundance in the anoxic and oxic zones wasBacteroidetesaccounting for 5.1% and 9.1%, respectively. The bacteria which are involved in the denitrification reactions belong primarily toProteobacteria[9]. Therefore, the anoxic zone contained a large percentage of phylumproteobacteriathan the oxic zone. Furthermore, the relative abundance of phylumNitrospiraewhich is essential for nitrification reaction[23]accounted for 3.8% in the oxic zone while 0 in the anoxic zone.
As shown in Fig. 6(c), the genus level was further investigated. It could be seen that theArcobacterhad the highest proportion (76.1%) in the anoxic zone. TheAcinetobacteraccounted the highest at 20.05% in the oxic zone. The genusArcobacterandAcinetobacterare demonstrated as the contributor to the denitrification process[28]. The genusHydrogenophagabelonging to the phylaProteobacteria, a kind of facultative autotroph correlated to the denitrification process, was higher in the oxic zone (7.6%) than anoxic zone (3.03 %), which may show possibly concurrent nitrification-denitrification. TheDechloromonasgenus accounted for 2.5% in the anoxic zone, which could decrease the production of sludge[29]. The other genera present in the oxic zone wereSphaerotilus(2.2%),Sulfurimonas(0.029%),UKL13-1 (2.01%),Runella(3.2%), andNitrospira(3.7%). The presence of nitrite-oxidizing bacteriaNitrospirain the oxic zone is responsible for full nitrification.
As discussed above, the bacteriological community plays a vital role in nitrification, denitrification and nitrogen removal performance. The bacteriaSphaerotilus,Sulfurimonas,UKL13-1,Runella,Zoogloea, andNitrospira(nitrite oxidizing bacteria) were existed in biofilms present in the oxic zone, which were responsible for nitrification process. TheArcobacter,Acinetobacter,HydrogenophagaandDechlorobacterare demonstrated as denitrifying bacteria occurred in the biofilms of anoxic zone, which contributed to denitrification process. So the biofilm AO system showed stable and high nitrogen removal efficiency.

Fig. 6 Taxonomic configurations of categorized bacteriological populations in sludge biofilms discovered by IlluminaMiSeq sequencing at (a) phylum level; (b) family level; (c) genus level
3 Conclusions

