A clinical protocol:A double-blinded,randomized controlled trial on the effect of traditional Chinese medicine formula Shoutai Pill in the treatment of threatened abortion
2021-10-20LiLiJingYanSongWenXiuYangChengChengJiYiMingCheMengJieWangYingXinWangNingZhang
Li Li,Jing-Yan Song,Wen-Xiu Yang,Cheng-Cheng Ji,Yi-Ming Che,Meng-Jie Wang,Ying-Xin Wang,Ning Zhang*
1Shandong University of Traditional Chinese Medicine,Jinan 250014,Shandong,China;2Affiliated Hospital of Shandong University of Traditional Chinese Medicine,Jinan 250014,Shandong,China.
Abstract Background:Threatened abortion is a common complication in early pregnancy,accounting for about 20% of all clinically confirmed pregnancy.It is the main cause of early abortion.Vaginal bleeding is the main clinical manifestation,which seriously affects the mental health and quality of life of pregnant women.Currently,there is effective treatment for this condition.A recent meta-analysis showed that Shoutai Pill (ST Pill),a traditional Chinese medicine (TCM)formula,can effectively decrease the rate of threatened abortion.However,high heterogeneity was found among the studies included in the meta-analysis,this conclusion on the efficacy of TCM is not definitive.Although several have been conducted,some of them do not describe randomization and blinding methods.To address these problems,this article proposes an improved clinical treatment scheme based on ST Pill,which is to be tested through a well-designed randomized controlled trial,for the treatment of threatened abortion.Methods:This is a double-blinded,randomized,placebo-controlled trial to be conducted in a public Three-A hospital in mainland China.A total of 200 people will be enrolled.Using computer-generated random numbers,the participants will be randomly divided into two groups at a ratio of 1:1 (treatment group (treated with ST Pill group) and placebo group).Both groups will receive medication to the end of the 20th gestational week or 1 week after vaginal bleeding stops,depending on which is longer.Participants in the treatment group will be treated with ST Pill (20 pills/ time,once a day),and those in the placebo group will receive a placebo drug which is similar in appearance and smell with ST Pill.The main observation index is the live birth rate.Discussion:Although the efficacy of ST Pill in threatened abortion is well-known,no study has tested its efficacy through a double-blinded,randomized trials.Therefore,there is an urgent need for a standardized randomized double-blinded controlled trial to evaluate the clinical efficacy of ST Pill.ST Pill is likely to be a convenient and effective TCM pill for the prevention of threatened abortion.
Keywords:Shoutai Pill;threatened abortion;traditional Chinese medicine;randomized controlled trial
Background and rationale
Threatened abortion is a prevalent complication occurring in early pregnancy (majorly in the first 20 weeks of pregnancy),accounting for 15% -20% of all clinically recognized pregnancies [1].The clinical manifestations are vaginal bleeding,with or without abdominal spasm pain,while the cervix is closed and the fetus is still alive in the uterine cavity.
Based on modern medicine,the causes of threatened abortion are complex and primary includes embryonic chromosome abnormalities,endocrine disorders,reproductive organ abnormalities,reproductive tract infections and immune dysfunction,etc.Miscarriages induced by abnormal embryonic chromosome structure and quantity in the first trimester of pregnancy are the most common,also,balanced translocation of chromosomes can easily trigger recurrent spontaneous abortion [2].Moreover,miscarriages caused by genetic factors usually fail to preserve the fetus,eventually causing inevitable miscarriage.
Luteal insufficiency is the most important endocrine factor causing threatened abortion.Other endocrine disorders including polycystic ovary syndrome,thyroid dysfunction,placental dysfunction,poor local decidual secretion[3],or severe uncontrollable diabetic factors can trigger miscarriage.Abnormal reproductive organs including uterine deformities,uterine and pelvic tumors,and uterine adhesions influence the implantation and development of embryos and lead to abortion.Maternal factor is the primary trigger of late threatened abortion,where infection plays a key role.Reports indicate[4]that more than 90% of late abortion is related to infection during the 24th and 28th week of pregnancy.For recurrent threatened abortion,immune factors account for 50%-70%,primarily including autoantibodies (specifically antiphospholipid antibodies,blocking antibodies and germ cell-related antibodies,etc.) and abnormal immune function.Besides the prevalent factors mentioned above,environmental factors (including formaldehyde,chemicals,high temperature and radiation,etc.),bad living habits (such as picky eating,smoking,drinking and staying up late,etc.),systemic diseases(including severe anemia,chronic nephritis,etc.),severe deficiencies in nutrition or trace element etc.,can cause threatened abortion.Also,mental and psychological aspects of excessive tension,anxiety,fear,and other negative emotions affect the state of female reproductive organs aggravating the symptoms of threatened abortion.
Several studies indicate that vaginal bleeding in early gestation is linked to poor pregnancy outcomes.For threatened abortion,clinicians routinely recommend bed rest.However,a Cochrane review[5] provides insufficient evidence supporting the theory that bed rest prevents miscarriage.Lack of moderate physical activity can also trigger other complications including thromboembolic events,back pain,and muscle atrophy.Further,vaginal bleeding frequently makes pregnant women feel stressed and anxious,which is even more unfavorable to pregnancy.Therefore,it is essential to actively prevent and cure threatened abortion.Regarding its treatment,progesterone,dydrogesterone[6],human chorionic gonadotropin[7],uterine muscle relaxant[8],anti-D immunoglobulin[9][10] etc.are often used in western medicine.Nonetheless,scholars suggest that the treatment of threatened abortion with western medicine is prone to a series of side effects including dizziness and headache,nausea and vomiting,insomnia etc.which might affect the normal life of patients.Based on the current situation,a traditional Chinese medicine (TCM) pill which is not overly strong and easy to administer might be an effective approach for the treatment of threatened abortion.
Shoutai Pill is a traditional Chinese medicine formula with a long history pioneered by Mr.Zhang Xichun,a well-known physician in the Qing Dynasty.The pill was first recorded in "The record of Chinese Medicine and Western Medicine",and presently the most used and effective prescription among several TCM formulas for the treatment of threatened abortion.The original prescription was used for the treatment of habitual abortions,and practitioners of later generations applied in the treatment of conditions including bleeding during pregnancy,fetal irritability,retarded growth of fetus etc.At present,the pill is primarily used for diseases including threatened abortion,unexplained recurrent spontaneous abortion etc.[11][12].ST Pill comprises Parasitic Loranthus,Dipsacus Asperoids,Cuscuta Seed,and Ass-hide Gelatin,which tonifies the kidney and protects the fetus.Modern studies reveal that ST Pill inhibits the contraction of uterine smooth muscle,enhances the luteinizing function of pituitary-ovary,increases the levels of estrogen and progesterone in patients[13],the expression of estrogen and progesterone receptors[14].Therefore,the pill enhances and regulates the endocrine system of pregnant women as well as promotes the development of the uterus and fetus.Besides,whether taking ST Pill during gestation causes adverse effects on the intellectual and physical development of the fetus remains unreported[15].Nevertheless,no large-scale and high-quality double-blinded randomized placebo-controlled trials have been reported on the efficacy of ST Pill in the treatment of threatened abortion.As such,it is important to conduct scientific and rigorous clinical trials to establish whether ST Pill is effective for patients diagnosed with threatened abortion.
A previous meta-analysis[16] revealed that ST Pill had a high clinical effective rate in the treatment of threatened abortion.However,the studies included in the meta-analysis were heterogeneous.Many of studies had small sample size,unclear random methods,without indicating the dose of Chinese herbal medicine ingredients.Therefore,their findings could not draw a clear conclusion on the therapeutic effect of the TCM formula.So far,no large well-designed randomized double-blinded controlled trial has been conducted to test the efficacy of the ST Pill.Given the lack of effective treatment for threatened abortion,ST Pill (a TCM prescription) is expected to offer a satisfactory solution for clinical gynecologists.Therefore,this randomized controlled trial (RCT) aims to explore the efficacy and safety of ST Pill.
Methods
Aim
This study aims to explore the therapeutic effect of the traditional Chinese medicine formula Shoutai Pill in the treatment of threatened abortion.
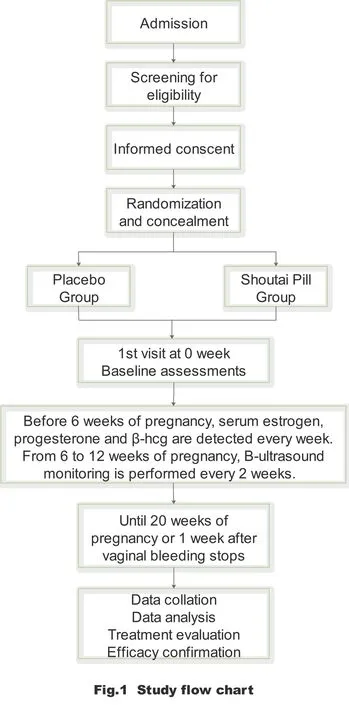
Trial design and setting
This is a prospective,randomized,placebo-controlled,double-blinded,superiority trial with a 1:1 allocation ratio between the experimental and the control groups.This work will be conducted at the Reproduction and Genetics Center of Shandong Hospital of traditional Chinese Medicine.Based on the sample size estimation,100 people will be enrolled in the treatment group and another 100 in the control group.The Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) checklist is provided as Additional file 4.
Threatened miscarriage is defined as vaginal bleeding,with or without abdominal pain,provided the cervix is closed and the fetus remains viable and inside the uterine cavity[17].
Inclusion criteria
The inclusion criteria for the study include:
(1)individuals meeting the diagnostic criteria for threatened abortion;
(2) within 20 weeks of pregnancy;
(3) aged between 20-40 years;
(4) agree to participate in this clinical trial study and comply with the treatment.
Exclusion criteria
The exclusion criteria for the study include:
(1) complicated with uterine leiomyoma,adenomyosis,uterine benign and malignant tumor,reproductive tract malformation;
(2) complicated with serious medical diseases including heart disease,abnormal liver and kidney function,hematological disease,etc.;
(3) complicated with abnormal endocrine function including thyroid,adrenal gland and pituitary gland;
(4) unable to cooperate with researchers,including mental and intellectual abnormalities;
(5) have recently taken other fetus protection drugs;
(6) individuals with habits such as smoking and drinking,long-term exposure to radioactive environment or certain chemicals (including pesticides,lead,arsenic,mercury,etc.).
Criteria for culling and shedding cases
The criteria for culling and shedding cases include:
①the subjects have poor medical compliance and fail to complete the full treatment as required;
②automatic withdrawal from the study and lost visitors;
③complicated with other system diseases during treatment;
④ultrasound monitoring suggested that hydatidiform mole,ectopic pregnancy,multiple pregnancies or missed abortion were harmful to the health of mothers.
Interventions
TCM ST Pill formula preparations
Traditional Chinese herbal medicine is prepared by boiling in water.Herbal medicine harbors a strong smell and acts violently,thus it potentially causes nausea,difficulty in swallowing among pregnant women,and might disturb the fetus.The pill prepared with honey counters the strong smell of some herbs,easy for patients’ acceptance,with slow and long-lasting effect,thus more suitable for pregnant women.Here,based on the Good Manufacturing Practice for Pharmaceutical Products (GMP) standard,the pill will be prepared by the combination of traditional and modern technology.The modified ST Pill will comprise Pilose Asiabell Root 120g,Radix Astragali 120g,Rhizoma Rioscoreae 120g,Cuscuta Seed 120g,Vinegar Rhizoma Cyperi 60g,Parasitic Loranthus 60g,Dipsacus Asperoids 60g and Ass-hide Gelatin 60g.Using ultra-fine pulverization technology to turn traditional Chinese medicine into fine powder,the pill will be prepared by mixing gelatin Ass-hide Gelatin and the powders.The weight of each pill should be about 0.3g (dry weight).Patients will take 20 pills per time,once a day.The daily dose of the modified ST Pill will be packaged separately for convenient administration to the patients.
Placebo preparation
The placebo will be produced by the Pharmacy Department of Shandong Hospital of traditional Chinese Medicine.They have a placebo production technology that mimics the color of TCM formula,outlook,and smell,but without any effective ingredients,hence,no therapeutic effect.The appearance and flavor of the placebos are indistinguishable from the ST Pill.The daily dose of the placebo will be packaged in individual sachets under the GMP license for easy consumption.The placebo restricts participants from recognizing their allocation into the placebo group,potentially causing decreased patient compliance.On the other hand,it prevents the potential benefits of vitamin placebo for patients.Patients in the control group will receive similar doses of placebo as the treatment group.
Study process
The reproductive center will evaluate the condition of each patient meeting the entry requirements.Based on the random numbers generated by the computer,the participants will be randomly divided into two groups,the treatment and the control groups.Both groups will receive the drugs from the time of diagnosis of threatened abortion.The treatment group will be treated with a modified ST Pill and the control group will receive the placebo until 20 completed gestational weeks or 1 week after vaginal bleeding has stopped.Nurses will conduct a telephone follow-up at 20 weeks of pregnancy for each patient to ascertain the abortion rate.Also,another telephone follow-up will be conducted within the following year.In the first 10 weeks of pregnancy,blood samples will be drawn once a week to detect E2,P and β-HCG.During 6-12 weeks of pregnancy,B-ultrasound monitoring will be performed every 2 weeks.
Treatment success criteria:defined as embryo survival,pregnancy continues.Treatment failure:defined as abortion (including complete abortion and incomplete abortion) or missed abortion.
Adherence
Further,telephone follow-up will be conducted every two weeks to enhance the compliance of participants.A free opportunity for quadruple Down's screening will be conducted during 15-18 weeks of gestation for patients with good compliance.Whether patients with poor compliance will be excluded depending on the situation.
Concomitant care
For the first 90 days,all participants will be not allowed to take other TCM prescription and nutritional supplements related to the protective effect of the fetus.This is because these drugs/supplements might confuse the efficacy of the ST Pill formula.
Blood tests
In the first 10 weeks of pregnancy,E2,P and β-HCG will be detected once a week,because their levels are closely related to the development of the embryo.Additionally,during the period of taking medicine,blood routine tests,liver and kidney function tests will be performed every 4 weeks to assess the safety of the TCM prescription and placebo.
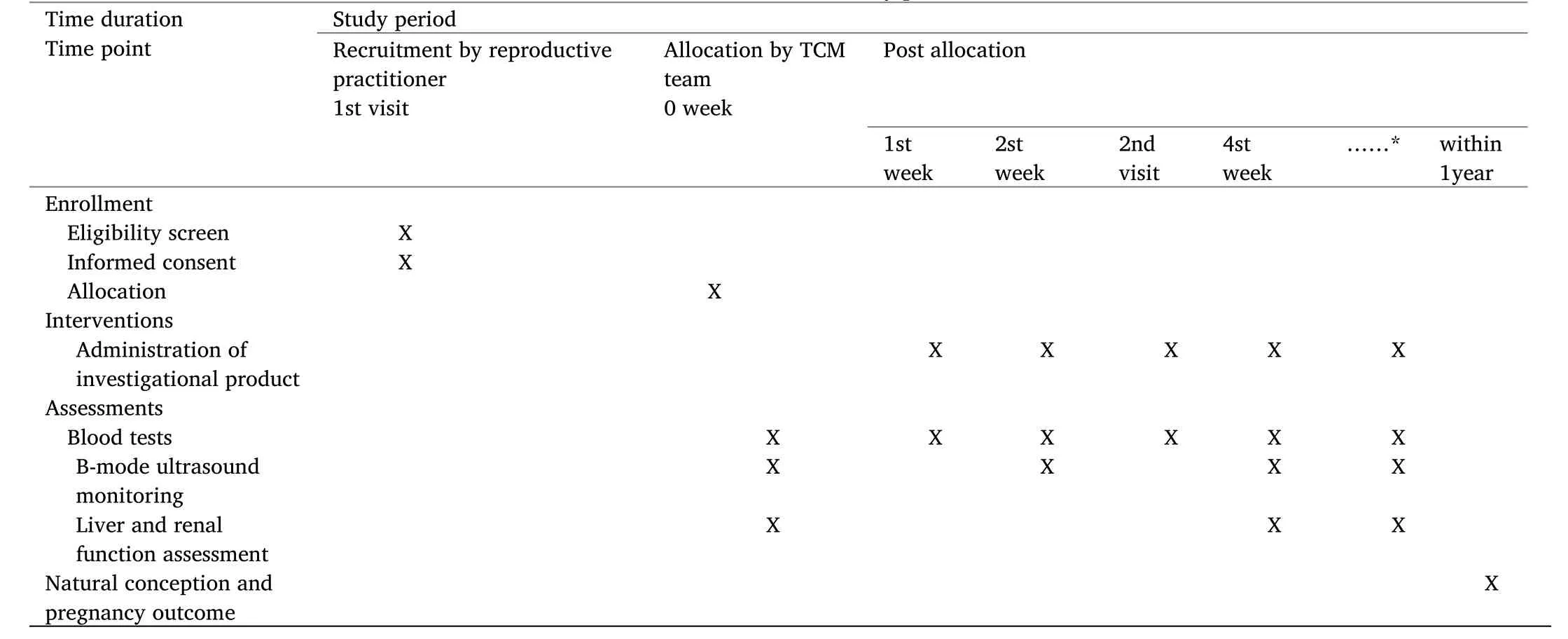
Table 1 Schedule of the study process
Serum E2,P,β-HCG determination
Exactly 5mL venous blood will be drawn from all participants in the morning.After centrifugation and serum extraction,E2,P and β-HCG will be detected by chemiluminescence.Instrument:DXI800 automatic chemiluminescence immunoassay analyzer (manufacturer:Beckman Coulter Co.,Ltd.).The Beckman Coulter's original reagent will be used,and detection will be performed strictly following the instructions of the kit.
Ultrasonic monitoring
B-ultrasound monitoring will be performed every 2 weeks in 6 to 12 weeks of pregnancy as the most intuitive method to monitor the development of embryos.
Outcome measurements
The primary outcome will be the live birth rate.Secondary objectives will be the abortion rate before 20 weeks of pregnancy,preterm birth rate,cesarean section rate,neonatal outcome,and the safety assessment of the TCM formula.
Live birth rate=Number of live births in the group/Total number of the group;Abortion rate before 20 weeks of pregnancy=Number of abortion before 20 weeks of pregnancy/Total number of the group;Preterm birth rate=Number of preterm birth/Total number of the group;Cesarean section rate=Number of cesarean section/Total number of the group;Neonatal outcomes included low birth weight infants,neonatal infections,neonatal hypoglycemia,etc.
Natural conceptions and pregnancy outcome
The occurrence of miscarriage,continued pregnancy,and live birth rate will be recorded.Participants will be followed up by telephone at the end of 20 weeks of pregnancy or 1 week after vaginal bleeding has stopped and within a year after study.
*In the first 10 weeks of pregnancy,E2,P and β-HCG will be detected once a week.During the period of taking medicine,blood routine test,liver and kidney function tests will be performed every 4 weeks.In 6 to 12 weeks of pregnancy,B-ultrasound monitoring will be performed every 2 weeks.
Retention
Once participants were enrolled,the investigators and staff will report data serum E2,P,β-HCG levels,and hepatorenal function detection.The follow-up nurses will maintain the interest of the participants in the study through fortnightly follow-up calls,reminding participants to arrive in time before the next visit,and recording feedback from participants (including,but not limited to,recent physical discomfort).
Sample size calculation
This is a randomized controlled trial,where the treatment group is the ST Pill group,while the control group is the placebo group.The observed outcome index will be early pregnancy abortion rate of the study subjects.Based on the literature,the prevalence rate of the treatment group is expected to be 10%,and that of the control group will be 31%,with the bilateral α=0.05 and the control degree being 90%.The sample size of the treatment group (n=73) and the control group (n=73) are calculated using the PASS 15 software.Considering that approximately 20% of the subjects will be lost or fail to undergo a complete follow-up,92 subjects will be required for the treatment and control groups.A total of 184 subjects will be enrolled in this study but 100 people will be recruited in each group.Therefore,the entire recruitment process will take about 12-18 months to complete.
Recruitment
The population will be women diagnosed with threatened abortion.Once diagnosed based on the above diagnostic criteria,the research assistant will contact the patient,inform her of the general content of the study and invite her to the study.If the patient is willing to participate,the receiving doctor will further inform her of the project,ask her to sign the informed consent form.
Randomization and allocation concealment
The enrolled patients will be assigned to two groups with random numbers generated by a computer when diagnosed with threatened abortion.ST Pill and the placebo will be prepared in a similar package.The trial will follow the updated guidelines from the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement[18] and the CONSORT guidelines for TCM to report the parallel-group randomized trials[19].Blindness can only be relieved under special circumstances when the problem cannot be solved through ongoing randomization.Relieving blindness shall be performed by an authorized investigator who will report all non-blinding incidents and their causes on the corresponding case report form (CRF).
Quality assurance
The Data Monitoring Committee (DMC) has been established with members including an independent chairman from the Clinical Research Management Office,an independent member from Obstetrics and Gynecology and an independent member from the Institute of Chinese Medicine.The DMC will be primarily responsible for monitoring data,interim analysis,evaluating post-intervention adverse events and cases of hepatorenal dysfunction,reviewing core trial processes and documents,as well as discussing any revisions to the major research protocols.
Data management
All information regarding the participants will be carefully recorded on CRF.The errors will be crossed out,corrected,and signed by the appropriate investigator.The research data in the CRF will be entered into a double-entry form and coded into the corresponding electronic form.
The hard copy of the CRF will be stored in a separate room and locked in a filing cabinet,and only authorized investigators will have access to these information.The electronic CRF will be stored in an encrypted server using advanced encryption standards,only accessed by authorized investigators.Researchers will minimize the number of personnel dealing with subject data to guarantee confidentiality of sensitive data.
Data analysis
Continuous data will be presented as means ± standard deviation(SD).Patients with compliance less than 70% (taking ST Pill for less than 10 days in a cycle of two weeks) will be excluded.Such participants will be automatically excluded from the final analysis but will be included in the intention-to-treat analysis.Researchers will check compliance every two weeks based on the diaries of participants and telephone follow-up.The full analysis set (FAS) will include all randomized patients and will be conducted based on the principle of intention-to-treat.The per-protocol set will include all patients of the FAS who will complete the follow-up without violating the study protocol.
For baseline comparison,an independent Student t-test or Mann-Whitney U test will be performed to evaluate the mean or median difference between the treatment and placebo groups with continuous data,depending on the normal distribution of data.A Chi-square test will be conducted to compare frequencies.For longitudinal data with baselines,repeated measures analysis of covariance (ANCOVA) shall be conducted to evaluate the average differences within and between groups.All tests will be double-tailed,and the significance level will be defined as a value of <0.05.
Ethical considerations
This study protocol and informed consent form have been approved by the Reproductive Medicine Clinical Research Ethics Committee(CREC) of the affiliated Hospital of Shandong University of Traditional Chinese Medicine,with the reference number of SDTCM/E-2021.02.26 (Additional file 1).All subjects will be comprehensively explained on trial and their consent will be obtained before joining the study.The patient will sign a written informed consent form (Additional files 2 and 3).Blood test is a direct,invasive,less harmful,and most accurate method to detect sex hormones.Collecting blood samples will cause a slight irritation to the skin and the risk of infection is relatively low.The ST Pill is a mild prescription that has been used for many years with no apparent side effects or known toxicity.The liver and kidney function of each patient will be tested before the start of the study and at the end of ST Pill treatment to ascertain the safety of the TCM formula.
This study complies with the Declaration of Helsinki and the Guideline for Good Clinical Practice of the International Conference on Harmonization.
Discussion
About 20% of pregnant women are diagnosed with threatened abortion,vaginal bleeding,abdominal pain and other symptoms.Modern medical study shows that the level of serum sex hormones is closely related to early threatened abortion.Estradiol is an important hormone to maintain pregnancy,which can reflect the function of the corpus luteum and the quality of follicles.Progestin is an important hormone for maintaining early pregnancy and an important indicator for predicting threatened abortion.β-HCG can effectively inhibit uterine contractions.So,they can indicate us if the fetus develops well.Meanwhile,B-ultrasound monitoring will also be performed.Currently western medical interventions such as bed rest and progesterone supplement are routinely used to protect the fetus,but with unsatisfactory outcomes.It is therefore necessary to develop treatments with fewer side effects and high acceptance rate among pregnant women.ST Pill has been used for decades to protect and stabilize the fetus,nourish the kidney and Qi,and effectively prevent treat threatened abortion.Modern pharmacological research shows that the active ingredient flavonoids in Cuscuta play a role in protecting fetus by regulating the endocrine-immune balance of the mother and fetus[20].The saponins in Dipsacus can also play an effective analgesic effect by enhancing the expression and function of adrenergic receptors[21].The Modified Shoutai Pill can improve uterine hemodynamics,promote sex hormone level recovery,correct pathological offset of Th1/Th2 cytokines and improve the success rate[22].Therefore,it can effectively improve the clinical symptoms of pregnant women and promote the prognosis of pregnancy.However,no randomized controlled trial has been conducted to confirm its efficacy.The proposed RCT will be conducted by a group of experienced experts from different fields such as,reproductive medicine,traditional Chinese medicine,and endocrinology.This pioneer project aims to clarify the clinical efficacy of ST Pill in the prevention of threatened abortion.
Trial status
This trial has been registered as Version 1.0,27/02/2021 (ChiTR);registration number:ChiCTR2100043700.The actual study start date is 01/05/2021;the anticipated study end date is 31/01/2024.The recruitment start date is 01/06/2021;while the anticipated recruitment end date is 31/01/2023.
Ethics approval and consent to participate
The study protocol and informed consent form have been approved by the Reproductive Medicine Clinical Research Ethics Committee (CREC) of the affiliated Hospital of Shandong University of Traditional Chinese Medicine,with a reference number of SDTCM/E-2021.02.26(Additional file 1).The researcher comprehensively will explain the experiment to all the subjects and obtain their consent before joining the study.Each patient will sign a written consent form(Additional files 2 and 3).
杂志排行
Clinical Research Communications的其它文章
- Effect of feedback health education on postoperative rehabilitation of patients with lumbar disc herniation:a cluster randomised trials
- Clinical observation of Guipi Decoction Combined with non steroidal drugs on sleep in elderly patients with acute traumatic pain and Qi deficiency constitution
- Effects of crude thymus gland extract on some physiological and biochemical parameters in local rabbits
- A case of resection of giant decidual polyp in first trimester
