Synthesis of Bimodal Mesoporous TiO2 -PTA/BMMS and Its Enhanced Performance in the Photocatalytic Oxidative Desulfurization
2021-10-09YangLinaZhangXiCuiLichengXuMeizhenLiJian
Yang Lina; Zhang Xi; Cui Licheng; Xu Meizhen; Li Jian
(School of Petrochemical Engineering, Liaoning Petrochemical University, Liaoning 113001)
Abstract: With the bimodal mesoporous silica (BMMS) acting as the support and the composite of TiO2 with phosphotungstic acid (PTA) functioning as the active constituent, TiO2‐PTA/BMMS was synthesized by the two‐step impregnation route.This catalyst was applied in the photocatalytic oxidative desulfurization (PODS) process, with dibenzothiophene serving as the model sulfur compound.PODS proceeds in one pot, in which H2O2 acts as the oxidant and methanol plays the role of the solvent.TiO2‐PTA/BMMS was characterized by XRD, N2 adsorption and desorption, XRF,FTIR, UV‐vis, SEM, EDS and TEM techniques.It showed that the introduction of PTA contributes higher order, higher surface area and pore volume to the bimodal mesoporous support.With TiO2‐PTA/BMMS used as the catalyst under the UV irradiation, the desulfurization rate can reach 99.6%.This result is obviously higher than that achieved by TiO2/BMMS.The catalyst also has no significant drop in catalytic activity after eight runs of reusing.In such catalytic system, the synergistic effects of this photocatalytic oxidation and the extraction with the methanol serving as the solvent played an indispensable role.
Key words: phosphotungstic acid; bimodal mesoporous silica; titanium dioxide; photocatalytic desulfurization; UV irradiation;dibenzothiophene
1 Introduction
Deep desulfurization of diesel is receiving worldwide attention in the research community due to the increasingly stringent regulations and fuel specifications in many countries out of environmental protection concerns[1‐3].The photocatalytic oxidative desulfurization(PODS) is considered to be one of the promising super‐deep desulfurization methods for fuel oil[4‐6].Compared with hydrodesulfurization (HDS), one advantage of PODS is its high efficiency for the removal of benzothiophene (BT), dibenzothiophene (DBT) and their derivates, and the other advantage is its low cost due to the mild operating conditions[7‐8].Many efforts on the development of PODS catalysts have been reported in the past few years[9‐10], such as TiO2[11], Bi2WO6[12], MgO/TiO2[13], CoP/CdS[14], g‐C3N4@Ag/BiVO4[15], α‐Fe2O3[16],Ag/TiO2/Fe2O3[17], etc.Among them, TiO2has been considered as the most appropriate photocatalysis agent due to its high photosensitivity, non‐toxic nature, low cost, and easy availability.It is well known that TiO2may exist as the amorphous or crystalline form.Compared to the crystalline material, the amorphous TiO2has larger specific surface area and richer surface hydroxyl groups/chemisorbed water, which is preferentially used as a catalyst.However, the amorphous TiO2band gap is wider than the crystalline one, so that it can perform well only under the UV irradiation.In order to make full use of the visible light, the wide band gap between TiO2and the fast recombination of photo-excited electron-hole pair is still a problem to be resolved[18‐19].
Loading is one of the feasible strategies to improve the photo‐efficiency of TiO2.Among many porous materials,the mesoporous silica is a good selection as an ideal support for TiO2because of its high specific surface area and large pore size for the diffusion of the large sulfur molecule such as DBT and its derivatives[20‐22].
Compared with the mono‐modal mesoporous materials,the bimodal mesoporous silica (BMMS) with 2D or 3D hierarchical pore structures can provide more opportunities for sulfur compounds (i.e.BT and DBT)to approach the catalytic sites and increase the reaction rate[23].The large pores of BMMS can permit better diffusion of the molecules, and the small pores can offer more active sites for the adsorption and catalytic reaction[24‐25].Hence, BMMS is a good selection as a PODS catalyst support.
Making a composite of TiO2with other metals or nonmetal compounds is also a practical way to improve the photocatalytic performance based on the tailoring of the band gap of TiO2[26].As an efficient electron trap,phosphotungstic acid (PTA) has been widely adopted to form composite with TiO2for the purpose of improving overall efficiency of photocatalytic process[27‐28].Such composite has the excellent performance in PODS, while the almost insolubility of PTA/TiO2in the polar solvent also endows it with a good recovering ability[29‐30].
Hence, an idea to load the TiO2/PTA onto BMMS jumped into our mind after studying the above‐mentioned reports.In this paper TiO2‐PTA/BMMS catalysts were synthesized with the two‐step impregnation method.In the PODS system containing H2O2serving as the oxidants and methanol acting as the solvent, TiO2‐PTA/BMMS was applied as the catalyst for the removal of DBT in the model fuel under UV irradiation.TiO2‐PTA/BMMS has an enhanced photocatalytic activity over both TiO2and TiO2/BMMS due to the merits of the bimodal mesoporous support and the electron trapping of PTA.It also has remarkable stability because of the almost insolube nature of TiO2‐PTA in the polar solvent and diffusion advantage of the support.The mechanism of the photocatalysis was proposed and proved and the synergistic function of any component of the PODS system was also confirmed.
2 Experimental
2.1 Materials and Methods
All chemicals were used directly in our experiments without further purification.Tetraethylorthosilicate(TEOS, AR), phosphotungstic acid (H3PW12O40, PTA,AR), tetrabutyl titanate(TBT, AR), ethanol (AR),methanol (AR), hydrogen peroxide (H2O2, 30%),dibenzothiophiophene (DBT, 98%), hydrochloric acid (2.0 mol/L), dodecane (AR), and Na2SO4·10H2O (AR) were all purchased from the China National Pharmaceutical Group.In addition, P123 (AR) and F127 (AR) were purchased from the Sigma‐Aldrich Corporation.
2.2 Synthesis of bimodal mesoporous silica
As referred to in the published literature[31], BMMS was prepared with P123 and F127 serving as the templates and TEOS serving as the silica source.
2.3 Synthesis of TiO2
8.0 mL of distilled water were added into a beaker containing a mixture containing 30.0 mL of ethanol and 8.0 mL of TBT under vigorous stirring, and the white flocs were obtained immediately.Aging was carried out for 24.0 h at room temperature after continuous stirring for 1.0 h, and then the beaker was placed in a water bath at 40 ℃ under constant stirring for 6.0 h to evaporate ethanol, then the white powder was dried at 100 ℃ for 10.0 h to obtain the amorphous TiO2particles.
2.4 Synthesis of TiO2-PTA
A certain mass of PTA was dispersed in 45.0 mL of ethanol by ultrasonic dispersion for 0.5 h to form the solution A.Then 4.0 mL of TBT were dispersed in 15.0 mL of ethanol under stirring until being fully dissolved, and 4.0 mL of deionized water (DI) were added into the solution under vigorous stirring, the obtained mixture was magnetically stirred at room temperature for 1.0h to form the solution B.
To prepare TiO2‐PTA, the solution B was poured into the solution A.After being subject to magnetic stirring for 0.5 h at ambient temperature the mixed solution was aged for 24.0 h, and then the solution C was formed.After evaporating the ethanol at 40 ℃ for 6.0 h under constant stirring, the powder was dried at 100 ℃ for 10.0 h to yield the TiO2‐PTA.
2.5 Synthesis of TiO2-PTA/BMMS
TiO2‐PTA/BMMS was prepared with the equal volume impregnation method.In general, a certain amount of BMMS was dispersed into a specified amount of ethanol solution of PTA under stirring based on the ethanol adsorption ratio, and the concentration of the PTAsolution was determined by the loading percentage of PTA in the catalyst.Then the impregnated sample was dried at 100 ℃ for 12.0 h to obtain the sample of BMMS loaded with PTA (PTA/BMMS).
TiO2‐PTA/BMMS was prepared by adding 1.0 g of PTA/BMMS in 50.0 mL of ethanol solution of TBT.The same synthesis steps as mentioned in Section 2.3 were followed.Finally, the obtained sample was dried at 100℃ for 12.0 h.
2.6 Characterization of catalysts
X‐ray diffraction (XRD) patterns were obtained on a D/max‐IIIA X‐ray diffractometer (Rigaku, Japan) with Cu‐Kα radiation operating at 35 kV and 30 mA.The composition of the catalyst was identified by a ZSX‐100(Rigaku, Japan) X‐ray fluorescence spectrometer (XRF).The specific surface area, the pore volume and the pore diameter of all the samples were calculated based on N2adsorption‐desorption isotherms acquired at 77 K by an ASAP 2420 (Micromeritics, USA) system.The Fourier transform infrared spectra (FTIR) were recorded on a Nicolet 380 spectrometer (Thermo Fisher Scientific)in the range of 4000‒400 cm‐1at a resolution of 4 cm‐1.The UV‐vis diffuse reflection spectra (UV‐vis) were recorded on a UV‐vis spectrometer (UV‐2450, Shimadzu)in the range of 200‒800 nm.The surface morphology was studied by a scanning electron microscope (SEM)(Hitachi SU 8010) operated at an acceleration voltage of 15 kV, and then the elements of the samples were analyzed with an energy dispersive spectrometer (EDS).The transmission electron microscope (TEM) images of catalyst samples were obtained on a JEM‐2010CX (JEOL,Japan) electron microscope operating at 200 kV.
2.7 Photocatalytic oxidative desulfurization
Model fuel with a sulfur concentration of 200 μg/g was prepared by dissolving DBT in dodecane.The PODS reaction was operated in a beaker under magnetic stirring at ambient temperature using a 25‐W UV lamp as the source of irradiation and the distance from the source to the beaker was 5 cm, while the catalyst (0.4%, calculated according to the mass ratio of PTA‐TiO2to model fuel),hydrogen peroxide (with O/S mole ratio=10: 1), 10 mL of model fuel, and 10 mL of methanol were put into in a beaker under stirring.The beaker was placed in the dark under constant magnetic stirring for 30 min firstly to establish the adsorption‐desorption equilibrium of DBT on catalysts without H2O2.After adding H2O2and turning on the UV light, the mixture was stirred for 3.0 h and was separated by centrifugation.
After the PODS reaction, DBT was converted into corresponding oxides, which were extracted into the solvent, and then the sulfur content of the model fuel was measured on a WK‐2D microcoulomb comprehensive analyzer.The operating parameters are as follows: The integral resistance is 400 Ω, the bia voltage ranges from 146 mV to 149 mV, the burning furnace temperature is 850 ℃, the evaporation section temperature is 750 ℃, the stable section temperature is 650 ℃, the oxygen flow rate is 150 mL/min, and the nitrogen flow rate is 260 mL/min.Based on the sulfur contents of the model fuel before and after PODS, the desulfurization rate (η) of the model fuel can be calculated by Eq.(1):

whereC0is the sulfur content of the feed, andCis the sulfur content of the product after photocatalytic reaction.
2.8 Stability test
The catalyst at the bottom of the beaker was separated from the reaction mixture by filtration and was washed with the deionized water and ethanol, dried at 100 ℃ for 24 h and then was directly used for the next run.
2.9 Active species trapping experiments
In the typical PODS process the model fuel, CH3OH,TiO2‐PTA/BMMS (with a PTA/TiO2mass ratio of 1: 7), photocatalyst, and H2O2were added into the beaker in sequence, then 1.0 mmol/L isopropanol(IPA),p‐benzoquinone (p‐BQ), and disodium ethylenediaminetetraacetate (EDTA‐2Na) were also added into the beaker, respectively, for detecting hydroxyl radicals (·OH), superoxide radicals(·O2-) and holes (h+)[32].
3 Results and Discussion
3.1 Characterizations
3.1.1 XRD analysis
The small angle XRD patterns of TiO2/BMMS and TiO2-PTA/BMMS are shown in Figure 1.The distinct double‐peaks were obviously found for both TiO2/BMMS and TiO2‐PTA/BMMS in the range of 2θ=0.89˚‒1.00˚,indicating that there were two kinds of long range ordered mesoporous structures.Double‐peaks were related to(100) and (110) planes, respectively, corresponding to a typical hexagonal SBA‐15 and a cubic‐structured SBA‐16.For TiO2‐PTA/BMMS such double‐peaks characteristic is clearer and a shift of the first peak to the small angle is also found, which can be attributed to PTA’s post modification of the mesoporous silica.As a kind of strong acid, PTA has an impact on the sol‐gel process of BMMS synthesis, and may make the mesoporous pores of the silica rearrange, which would consequently change the order of the mesopores and the crystal cell size of silica[33].

Figure 1 Small angle XRD of TiO2/BMMS and TiO2-PTA/BMMS
The wide angle XRD patterns of TiO2‐PTA/BMMS, TiO2/BMMS and TiO2are presented in Figure 2.It can be seen that there was no obvious diffraction peak for the amorphous TiO2.For TiO2‐PTA/BMMS and TiO2/BMMS,wide peaks around 2θ= 22.5˚ appeared, corresponding to the amorphous silica.No distinct peaks for PTA were observed for a too small amount of PTA to be detected in TiO2‐PTA/BMMS or for the high dispersion of it in BMMS.For TiO2/BMMS no peaks appeared except that peak of the amorphous silica, indicating that the active component has been dispersed quite well.

Figure 2 Wide angle XRD of TiO2, TiO2/BMMS and TiO2-PTA/BMMS
3.1.2 N2 adsorption-desorption analysis
N2adsorption‐desorption isotherms and BJH pore size distributions of BMMS, TiO2/BMMS and TiO2‐PTA/BMMS are shown in Figure 3(A) and Figure 3(B),respectively.According to the isotherm shape and pore diameter distribution, all the three catalysts still belonged to typical type IV isotherms with a characteristic H1hysteresis loop in thep/p0region of 0.40‒0.55 and a characteristic H2hysteresis loop in thep/poregion of 0.55‒0.70, which further proved the bimodal mesoporosity of the samples, and these results were consistent with the XRD characterization results.
However, it is also found in Table 1 that the addition of TiO2and PTA into BMMS caused a smaller surface area and pore volume.The distribution peaks of TiO2‐PTA/BMMS became wider than those of BMMS and TiO2/BMMS in Figure 3(B), suggesting the reduced uniformity of the pore size after the introduction of TiO2‐PTA.And the most probable pore diameter for TiO2‐PTA/BMMS is a little larger than BMMS, which is consistent with the XRD results.
Based on the detailed pore parameters of three samples depicted in Table 1, it can be seen that the specific surface area of the catalyst after loading is significantly greater than that of TiO2, and TiO2/BMMS.However, such decrease for TiO2‐PTA/BMMS is not as intense as that of TiO2/BMMS.
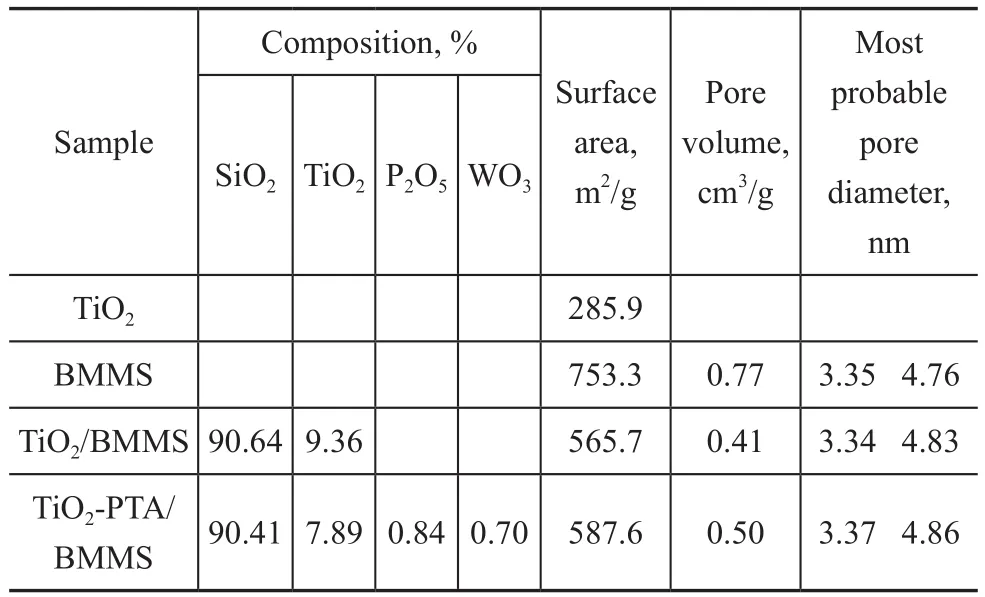
Table 1 Physical pore structure parameters of samples
3.1.3 FITR spectrometric analysis
Figure 4 shows the FTIR spectra of the samples.The characteristic peaks of TiO2at around 400‒800 cm‐1were assigned to the O‐Ti‐O lattice[34], and the characteristic peaks at around 1390‒1660 cm‐1were assigned to the symmetry deformation of C‐H in ‐CH2‐ and CH3‐ groups or the vibrational peak of H‐O in H2O molecules[35‐36].The strong and broad peaks at around 1077 cm‐1and810 cm‐1for BMMS, TiO2/BMMS and TiO2‐PTA/BMMS were assigned to the symmetric vibration of Si‐O‐Si.The band at around 968 cm‐1was attributed to the stretching vibration of Si‐O bond in Si‐O‐Ti.There was no obvious difference between the characteristic peaks of TiO2/BMMS and TiO2‐PTA/BMMS.Upon comparing with BMMS, the peak at around 1077 cm‐1became wider for TiO2/BMMS and TiO2‐PTA/BMMS.The characteristic peaks for the Keggin structure of PTA usually appeared at 1077 cm‐1, 983 cm‐1, 889 cm‐1, and 810 cm‐1[37], however,such peaks were not found from two samples.This might be related to its small amount in the sample or to the overlapping of its characteristic peaks with the strong peaks of the support.
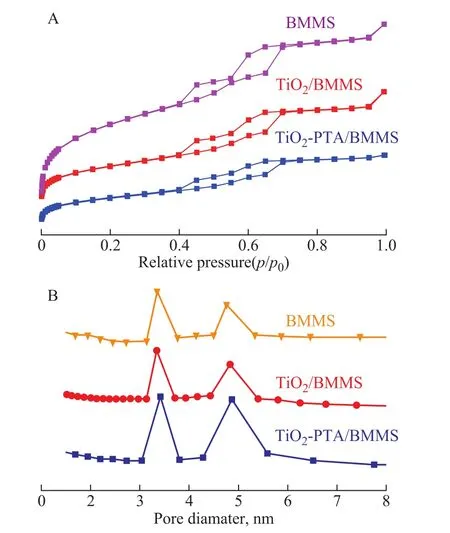
Figure 3 N2 adsorption-desorption isotherms (A) and BJH pore size distribution (B) of BMMS, TiO2/BMMS and TiO2-PTA/BMMS
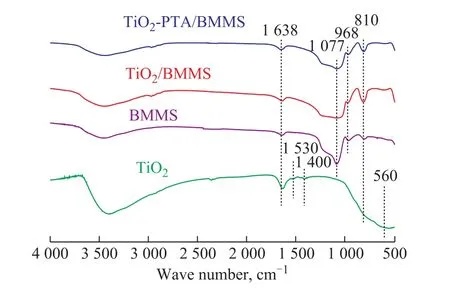
Figure 4 FTIR of TiO2, TiO2/BMMS and TiO2-PTA/BMMS
3.1.4 UV-vis spectrometric analysis
Figure 5 (A) displays the UV‐vis diffuse reflectance spectra of PTA, TiO2‐PTA/BMMS, TiO2/BMMS, TiO2and BMMS.It can be seen that five catalysts all have intense absorption at 200‒400 nm and the absorption of TiO2‐PTA/BMMS in UV‐vis region is stronger than TiO2.The enhancement of absorbance in the UV‐vis region increases the number of photo‐generated electrons and holes to participate in the photocatalytic reaction,which can enhance the photocatalytic activity of the catalysts[38‐39].
Compared with TiO2, PTA and BMMS, the onset absorption spectrum of TiO2‐PTA/BMMS shows a red shift.Then the forbidden band width of the sample can be calculated by the following Eq.(2):

whereα,h,ν,AandEgare the absorption coefficient,the Planck constant, the light frequency, a constant and the band gap energy, respectively.The value ofnis determined by the transition type (n=2 for indirect semiconductor, andn=1/2 for direct semiconductor).Figure 5 (B) shows the energy intercept of a plot of(αhν)2vs.hνfor a direct transition.After calculation,the band gap energy (hν) of TiO2, PTA, TiO2/BMMS and TiO2‐PTA/BMMS can be obtained, which is 3.23 eV, 3.01 eV, 3.03 eV and 2.97 eV, respectively.Based on the band gaps it can be concluded that the introduction of TiO2and PTA together into BMMS can decreasehνmore significantly than just introducing TiO2into BMMS.
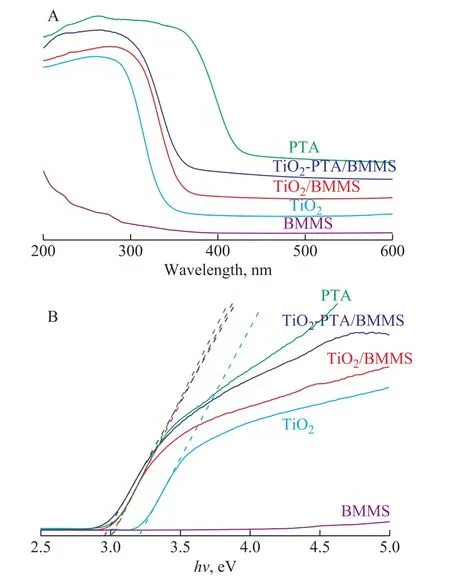
Figure 5 UV-vis diffuse reflectance spectra (A) and estimated band gap (B) of TiO2, PTA, BMMS, TiO2/BMMS and TiO2-PTA/BMMS
3.1.5 SEM and EDS analyses
Figure 6 displays the SEM images of TiO2‐PTA, TiO2-PTA/BMMS and BMMS.It is obvious that TiO2-PTA/BMMS still retains the spike‐like morphology of BMMS[40].And a seldom spherical TiO2‐PTA is distributed on the outer surface of BMMS.Five elements including Ti, Si, O, P and W have been detected in TiO2-PTA/BMMS, with the distribution of these elements presented in Figure 7.
3.1.6 TEM analysis
In order to further observe the pore structure of TiO2-PTA/BMMS, the TEM images are shown in Figure 8.It is not difficult to find the coexistence of the hexagonally arranged mesopores and the cubically arranged mesopores in BMMS and the pore size of the former and the latter is about 5.0 nm and 3.0 nm, respectively.The observed typical bimodal mesoporous structure is consistent with the N2adsorption and desorption results.The TEM image of TiO2‐PTA/BMMS complies with that of pure BMMS.

Figure 6 SEM images of TiO2-PTA(A), TiO2-PTA/BMMS(B) and BMMS(C)
3.2 Photocatalytic oxidative desulfurization performance
3.2.1 Photocatalytic activity comparison
Comparison of the photocatalytic activity among the bulk TiO2and the supported catalysts including TiO2/BMMS and TiO2‐PTA/BMMS was carried out, with the results shown in Figure 9.Among them, TiO2‐PTA/BMMS possesses higher photocatalytic activity than TiO2/BMMS and the bulk TiO2, and the desulfurization rate can reach 97.6% as depicted in Figure 9(A).Such excellent performance of TiO2‐PTA/BMMS can be ascribed to the bimodal mesoporous structure of the support, which makes the large sulfur molecules like DBT diffuse freely.At the same time, the higher desulfurization rate may also be related to the narrower forbidden band width of the catalyst and the increased absorbance in the ultraviolet region.With the special structure of PTA, TiO2‐PTA/BMMS has a strong ability to accept electrons, which means that the photon‐absorbing electrons can be caught by heteropoly acid anions to reduce the possibility of recombination of electron-hole pairs and improve the photocatalytic efficiency of nanocrystal TiO2[41].Consequently TiO2‐PTA/BMMS demonstrates a highest photocatalytic activity.
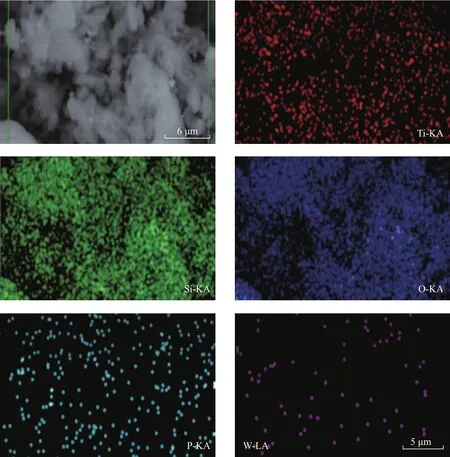
Figure 7 EDS photographs of TiO2-PTA/BMMS
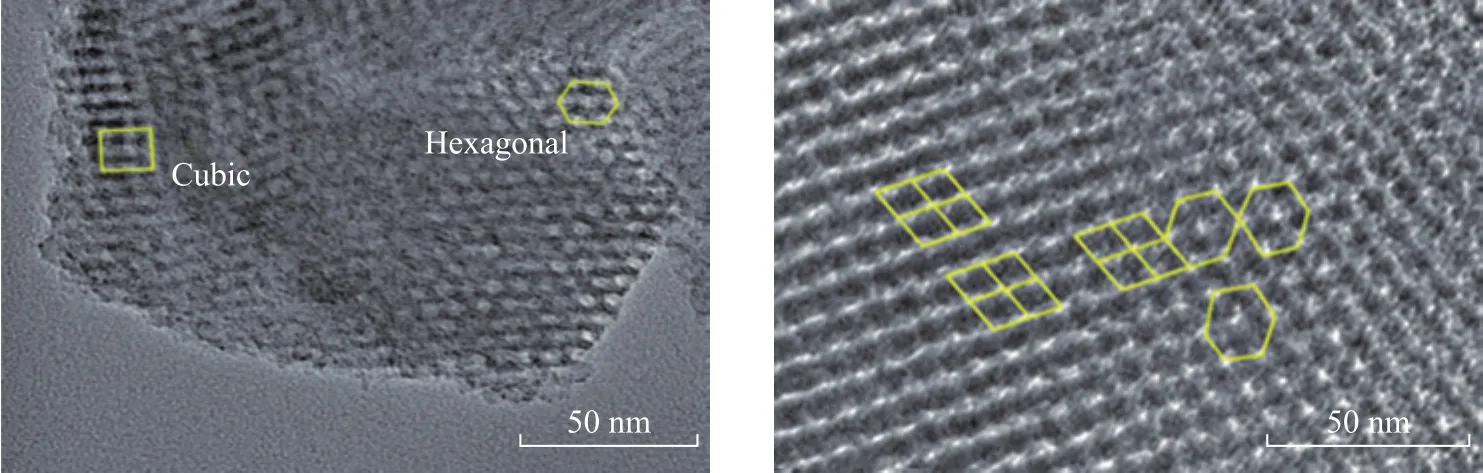
Figure 8 TEM images of TiO2-PTA/BMMS(A) and BMMS(B)
The photocatalytic oxidation of DBT is assumed to be the pseudo‐first‐order reaction and the sulfur content of model fuel can be expressed by Eq.(3):

in whichCandC0have been explained in Eq.(1), andkis the pseudo‐first‐order reaction rate constant (min‐1).The correlations of time‐course variation of -ln (C/C0) to the reaction time are represented in Figure 9(B).Values of-ln (C/C0) for all catalysts show a clear linear increase with the reaction time, suggesting a pseudo‐first‐order reaction.Meanwhile, the reaction rate constants and the slopes of the straight lines would decrease in the following order:TiO2‐PTA/BMMS > TiO2/BMMS > TiO2.
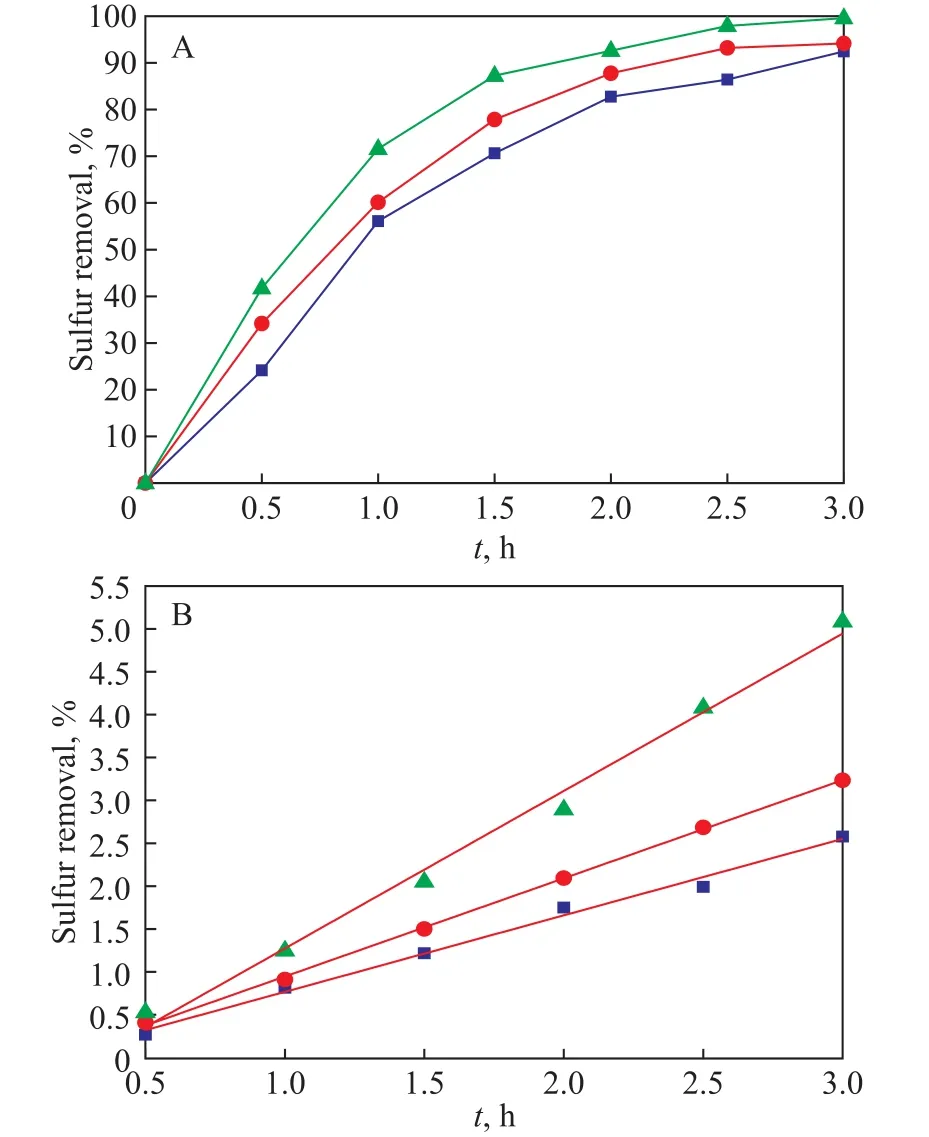
Figure 9 Sulfur removal (A) and pseudo-first-order kinetic fitting curves (B) of TiO2, TiO2/BMMS and TiO2-PTA/BMMS
3.2.2 Stability of TiO2-PTA/BMMS
Stability of TiO2‐PTA/BMMS was studied, with the results shown in Figure 10.It can be found that the catalyst does not exhibit significant loss of activity and the desulfurization rate still retains more than 90% after eight runs of repeated PODS experiments, indicating the good catalytic stability of TiO2‐PTA/BMMS.
3.2.3 Photocatalytic oxidative desulfurization mechanism
The radicals trapping experiment was conducted to well explain the photocatalytic mechanism.Active mediates including hydroxyl radicals (·OH), superoxide radicals (·O2-) and holes (h+) were detected by adding isopropanol (IPA),p‐benzoquinone (p‐BQ) and disodium ethylenediaminetetraacetate (EDTA‐2Na)to the reaction system, respectively.It is found that in Figure 11 the addition of IPA has a little effect on the degradation results, implying that the ·OH does not play a predominant role for the desulfurization.Whenp‐BQ and EDTA‐2Na were added, the desulfurization rate of the system dropped significantly, indicating thatand h+were the main contributors to the sulfur removal in the catalytic system containing TiO2‐PTA/BMMS photocatalyst.
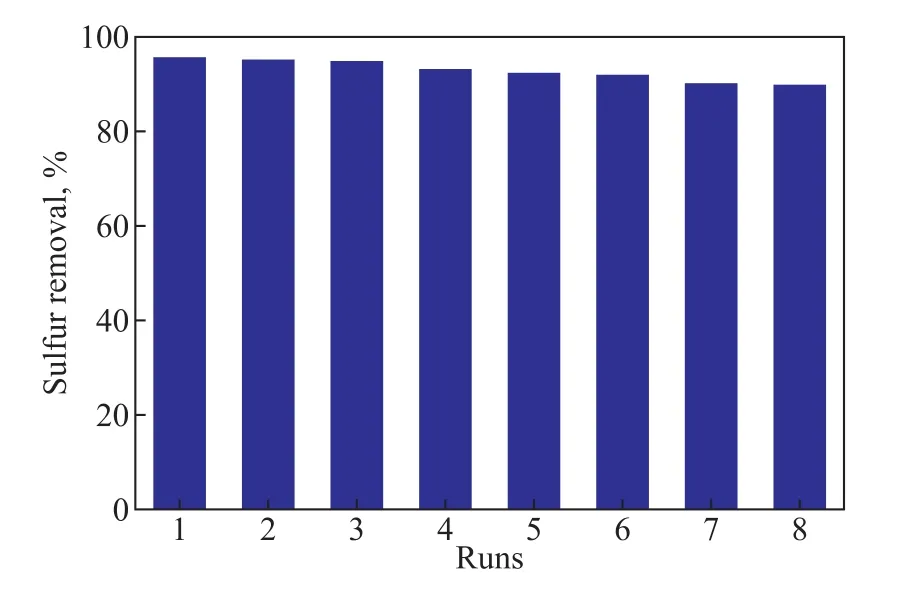
Figure 10 Stability of TiO2-PTA/BMMS
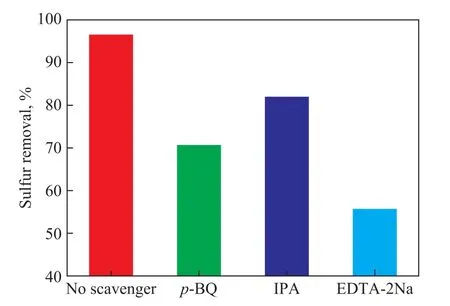
Figure 11 Active species in the presence of TiO2-PTA/BMMS
Under the UV irradiation, the functions of the members in the PODS system were researched, with the results depicted in Table 2.The catalytic function of TiO2‐PTA/BMMS was well verified after making a comparison among systems with different combinations.In this one pot process, CH3OH existed in the system simultaneously at the same time of the reaction and the formed polar sulfur compounds could be extracted in time, which was in favor of the proceeding of the reaction.In such PODS system TiO2‐PTA/BMMS, H2O2and CH3OH had a good synergistic effect and anyone of them was absolutely necessary.
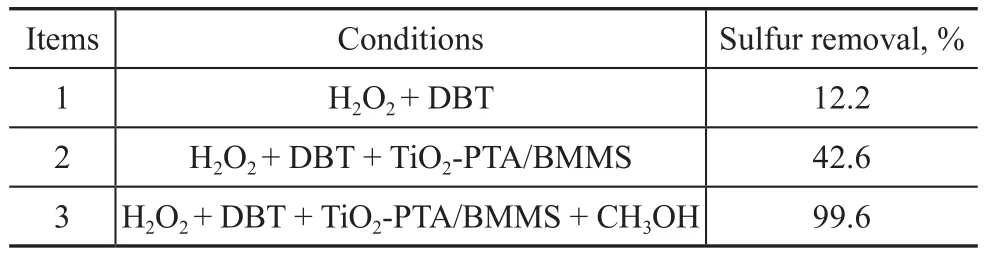
Table 2 Necessity of any composition in the catalytic system
The catalytic mechanism of TiO2‐PTA/BMMS is presented in Figure 12.When the catalyst was irradiated under UV light, the photogenerated electrons and the holes were formed on TiO2, and at the same time PTA could quickly capture electrons into the empty d‐orbital, inhibiting the recombination of them with holes.·OH had a strong oxidation capability to oxidize the DBT adsorbed on the catalyst surface.The ·OH species can originate from the reaction between the transferred photogenerated electrons with H2O2, or can realize the interaction of the holes with the absorbed water and OH-on the catalyst surface[42].Some O2molecules can be also adsorbed on the surface of the catalyst, leading to the formation ofspecies after the reduction of O2on the catalyst.Meanwhile, the presence of H2O2can increase the quantity of O2molecules and consequently improve the concentration of generatedspecies, which can stimulate the oxidation of DBT.At the interface of oil and the methanol, sulfur compounds were oxidized to corresponding sulfones or sulfoxides,and then were separated by methanol extraction, so as to achieve the purpose of deep desulfurization.

Figure 12 Proposed mechanism of photocatalytic oxidation of DBT on TiO2-PTA/BMMS
4 Conclusions
The introduction of TiO2‐PTA does not destroy the bimodal mesoporous structure of BMMS, but on the contrary brings forth the larger specific surface area, pore volume, pore size and the narrower energy gap than TiO2/BMMS.PTA is a good electron carrier to capture the photogenerated electrons produced by TiO2quickly, thus TiO2‐PTA/BMMS catalyst has a lower bandgap width.Therefore, the introduction of PTA into TiO2/BMMS can improve the photocatalytic desulfurization performance of the host catalyst.In the PODS system the synergistic effect of the catalyst, oxidant and the solvent is necessary.The separation of the oxidized sulfur compounds from the model fuel via the extraction of the methanol is very important, and the main intermediate is ·O2-in the process of PODS.
Acknowledgement:This work was supported by the Program for Liaoning Excellent Talents in Universities ‘LNET’(LJQ2015062), the Program for Science and Technology Agency of Liaoning Province (20170540585), the General Scientific Research Project of Liaoning Provincial Department of Education (L2015296, L2016018) and the Science and Technology Planning Project of Fushun (FSKJHT201376).
杂志排行
中国炼油与石油化工的其它文章
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts
