Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
2021-10-09XiaXiaoqiLiHongmingLiChunmanMiaoQingLiJingZhuFengHuangQiguYiJianjunZhaoZhong
Xia Xiaoqi; Li Hongming; Li Chunman; Miao Qing; Li Jing;Zhu Feng; Huang Qigu; Yi Jianjun; Zhao Zhong
(1.State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, the College of Material Science and Engineering,Beijing University of Chemical Technology, Beijing 100029;2.Petrochemical Research Institute, PetroChina, Beijing 102206;3.CNPC Key Laboratory of Oil & Gas Storage and Transportation, PetroChina Pipeline R & D Center)
Abstract: Due to the development of the new energy industry, polypropylene with ultra‐high molecular weight plays a crucial role for battery isolation membrane.This work investigated the effect of internal electron donor of Ziegler‐Natta catalyst system on the molecular weight of the obtained polypropylene.The scanning electron microscope (SEM) and Canon camera were used to characterize the surface morphologies of catalyst particles and polymer particles, respectively.Compared with the polypropylene particles featuring a spherical shape, these study results confirmed that the morphology duplication theory from the catalyst particle to the morphology of polymer particle was exhibited.The gel permeation chromatography (GPC) results revealed that the obtained polypropylene has a much higher average molecular weight than those prepared by conventional method.The Fourier transform infrared spectrometry (FT‐IR) and X‐ray photoelectron spectroscopy (XPS) revealed that the carbonyl oxygen atom on ester group was preferentially bound to Mg and Ti, as compared to the ether oxygen atom.The XPS results showed that the ratio of Ti3+/Ti4+ could be changed by internal electron donors.When Ti3+content was nearly 99% in the Ziegler‐Natta catalyst system, isotactic polypropylene with an ultra‐high molecular weight of up to 1.42×106 g/mol was obtained by Cat.3.This result implied that internal electron donor ID3 could reduce the β‐hydride elimination reaction to further increase the molecular weight of the obtained polymer.
Key words: internal electron donor; Ziegler‐Natta catalyst; ultra‐high molecular weight; isotactic polypropylene.
1 Introduction
In industrial applications, molecular weight and molecular weight distribution are very important for the physical properties and subsequent processing of polypropylene[1‐3].Tabatabaei[4]studied the effect of annealing effect on separators of polypropylene mixtures with different molecular weights.The study found that the crystal phase of polypropylene crystals annealed at 140 ℃ is relatively perfect.Scanning electron microscopy (SEM) images showed that as the mass fraction of high molecular weight polypropylene increased, the membrane surface showed much more micropores and more uniform pore size.In addition, with the increase in molecular weight of polypropylene, the gas permeability of battery separators has also improved, and the tensile resistance and puncture resistance of battery separators have also been enhanced.This fact is enough to show how important an increase in molecular weight is for the battery separator materials.Since the beginning of the 20th century, the researchers have done a lot of researches to find catalysts that can polymerize propylene under low pressure conditions.It was not until the early 1950s that K.Ziegler[5]accidentally discovered that a mixture of TiCl4and AlEt3could beused for ethylene polymerization.G.Natta[4]subsequently used a TiCl3/AlEt3two‐component catalyst to catalyze the low‐pressure polymerization of propylene, and successfully prepared a highly isotactic polypropylene.Since then, polypropylene materials have been developed rapidly in the industry.Kaminsky and Sinn[6]discovered a highly active catalytic system consisting of Cp2ZrMe2and [Al(Me)O]n.The application of metallocene catalysts in industry began to develop.Unlike the multi‐active sites distribution of the Ziegler‐Natta catalyst, the metallocene catalyst is a single‐site system and has strong stereoselectivity, so that the obtained polymer has a narrow molecular weight distribution.Because of its high activity, single‐site nature, strong orientation ability and wide adaptability to monomer, the metallocene catalyst has been rapidly developed in recent decades.According to the research and improvements outcome, the catalyst[Me2Si(2‐Me‐4Naph‐Ind)2]ZrCl2of Exxon/Hoechst is considered to be the best isotactic polypropylene catalyst with an isotactic index of over 99% and a molecular weight of over 9×105g/mol.And its polymerization activity is higher than that of the Ziegler‐Natta catalyst[7‐8].Ewen[9]prepared a highly syndiotactic polypropylene with a weight‐average molecular weight of 7.7×105g/mol by using the metallocene catalyst.Miyake[10]synthesized a series of metallocene complexes with C1symmetry for propylene polymerization.It has been found that highly isotactic polypropylene could be obtained by these catalysts, but the molecular weight of the polymers and the activity of these catalysts were suboptimal.It is hard to produce polypropylene with high molecular weight by utilizing metallocene catalyst because of β‐H elimination reaction and chain transfer reaction to MAO.
In the early 1990s, non‐metallocene catalysts were applied in olefin polymerization process.Since they are versatile in structure and easy to design, they can synthesize various metal complexes, and can be used to catalyze the binary and multi‐component copolymerization of propylene and other monomers to obtain olefin copolymers with uniform branch distribution.Dow/Symyx discovered a high‐output screen method and HfⅣ‐pyridyl‐amido‐based catalysts, a new type of single‐site polyolefin polymerization catalysts,which were developed to take part in highly iso‐selective propylene polymerization process with solvent at high temperature[11].However, due to the restrictions of patents and the excessive cost of post transition metal catalysts such as nickel and palladium complexes, the development of non‐metallocene catalysts has been limited in the isotactic propylene polymerization process.In 2016,Yusuke Ota[12]prepared the copolymer of propylene and polar monomer catalyzed by a palladium complex with a phosphine sulfonate ligand, but only got a polymer with a molecular weight of 104g/mol.Li[13]reported a kind of novel amino‐containing isotactic polypropylenes with a high molecular weight of 5.9×105g/mol via a post‐transition metal catalyst system, and obtained a highest molecular weight polypropylene that can be catalyzed by non‐metallocene catalytic system so far.
However, due to the high cost and patent restrictions,the catalyst used in the industrial applications to produce isotactic polypropylene has still been focused on the supported Ziegler‐Natta catalyst[14‐15].Yang[16]reported the synthesis of polyethylene with a weight average molecular weight of higher than 1.5×106g/mol by Ziegler‐Natta catalyst, which was comparable with most commercial ultra‐high molecular weight polyethylene.Since the study found that the addition of internal electron donor (ID) in Ziegler‐Natta catalyst can control the molecular weight and molecular weight distribution of obtained polymer, the researchers have conducted extensive research on the role of the internal electron donor in Ziegler‐Natta catalyst[17‐18].So far, there were many reports on the Ziegler‐Natta catalysts for the synthesis of UHMWPE[19‐24].But there are few reports on the synthesis of ultra‐high molecular weight isotactic polypropylene (UHMWPP) using the Ziegler‐Natta catalysts.Based on the above research, our research group synthesized 9,9‐bis(methyl acetate) fluorene as an internal electron donor for preparation of the supported Ziegler‐Natta catalyst, which was successfully used to prepare UHMWIPP.This research found that the internal electron donor added in the catalyst has a great influence on the molecular weight of the prepared polypropylene.The mechanism about the internal electron donor effect on the molecular weight of polypropylene was investigated.The results showed that the electron‐withdrawing ability of the carbonyl oxygen from the internal electron donorof diester was stronger than that of the ether oxygen on the internal electron donors of diether, so that 9,9‐bis(methyl acetate) fluorene had stronger binding ability to Mg and Ti atoms, and could reduce the electron cloud density around Ti atoms to suppress β‐hydride elimination phenomenon to get higher molecular weight polypropylene.The co‐catalyst loaded on the support together with TiCl4for propylene polymerization was also investigated.This strategy of both internal electron donor and cocatalyst loaded on the support was confirmed to be capable of improving the reaction of Ti4+being reduced to Ti3+, which was favorable for propylene polymerization[25].
2 Experimental
All manipulations of air or water sensitive compounds were carried out under nitrogen atmosphere using the standard Schlenk techniques.
2.1 Materials
Triethylaluminium (2.0 mol/L inn‐hexane), iodomethane and acetyl chloride were purchased from the J&K Chemical Company and used without further purification.n‐Hexane,n‐decane, dimethyl sulfoxide (DMSO),tetrahydrofuran (THF), isooctyl alcohol, and titanium tetrachloride were purchased from the Beijing Chemicals Company.Isooctyl alcohol and DMSO were treated with the activated 5 Å type molecular sieves under nitrogen atmosphere for one week prior to use.n‐Hexane, THF,andn‐decane were refluxed with metallic sodium under nitrogen blanketing for 48 h and distilled before use.Propylene (polymerization grade, with a purity of 99.9%)was obtained from PetroChina and was used after having been passed through activated silica gel, KOH, and a column of activated 5A molecular sieves.Anhydrous MgCl2particles (technical grade) was purchased from the Fushun Taiye Company, Ltd., and was ground to about 10 μm in diameter before use.
2.2 Preparation of internal electron donors
2.2.1 Synthesis of ID1 (9,9-bis(methoxymethyl)fluorene)
The internal electron donor ID1, 9,9‐bis(methoxymethyl)fluorene was synthesized according to the method described in the patent[26].At first, the intermediate compound of 9,9‐bis(hydroxymethyl) fluorine was synthesized (Figure 1).Fluorene (10 g),paraformaldehyde (5 g), dimethyl sulfoxide (50 mL) and sodium ethoxide were added into a 300‐mL Schlenk glass reactor under nitrogen blanketing at room temperature.After the mixture had reacted in an ice‐water bath for 3 hours, hydrochloric acid (1 mL, 35%) was added to terminate the reaction.The obtained crude product was recrystallized at ‐10 ℃ in toluene (40 mL).Finally, a slightly yellow crystal 9,9‐bis(methoxymethyl) fluorene was obtained under reduced pressure distillation with a yield of 81.5%.1H‐NMR (400 MHz, CDCl3, δ) analysis:7.77 (d, 2H, fluorene), 7.61 (d, 2H, fluorene), 7.41 (t, 2H,fluorene), 7.32 (t, 2H, fluorene), 3.99 (s, 4H, CH2), 2.05 (s,2H, OH).ESI‒MSm/zcalculated for [M+H]+.C15H14O2:227.11, found at 227.10.
ID1 (9,9‐bis(methoxymethyl) fluorene) was synthesized(Figure 2).9,9‐Bis(hydroxymethyl) fluorene (4 g),sodium hydride (0.5 g), methyl iodide (3.5 mL) and THF (30 mL) were added into a 200‐mL Schlenk reactor under nitrogen blanketing.The mixture was stirred for 8 hours at room temperature.The solid was filtered off andn‐hexane (30 mL) was added to wash the product for four times.Then the obtained product was recrystallized at ‐10 ℃ in ethanol (40 mL).Finally, a colorless crystal 9,9‐bis(methoxymethyl) fluorene was obtained under reduced pressure distillation with a yield of 74.3%.1H‐NMR (400 MHz, CDCl3,δ) analysis: 7.76 (d, 2H,fluorene), 7.62 (d, 2H, fluorene), 7.40 (t, 2H, fluorene),7.30 (t, 2H, fluorene), 3.94 (s, 6H, CH2OCH*3), 3.28 (s,4H, CH*2OCH3).ESI‒MSm/zcalculated for [M+H]+.C17H18O2: 255.14, found at 255.13.

Figure 1 The synthesis of 9,9-bis (hydroxymethyl fluorene)
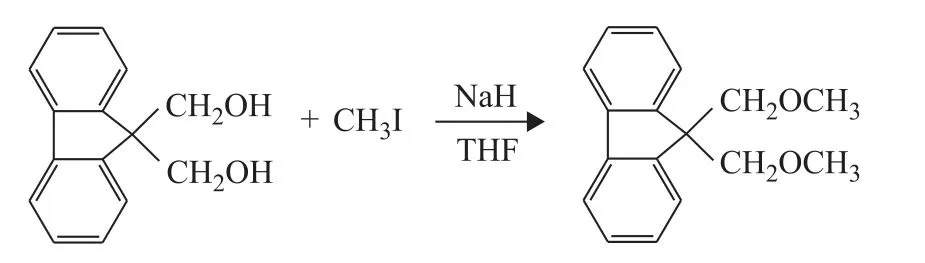
Figure 2 The synthesis of 9,9-bis(methoxymethyl) fluorene
2.2.2 The synthesis of ID2 (9-methoxymethyl-9-(methyl acetate) fluorene)
The route for synthesis of 9‐hydroxymethyl‐9‐(methoxymethyl) fluorene for ID2 is shown in Figure 3.9,9‐Bis(hydroxymethyl) fluorene (4 g), sodium hydride(0.5 g), methyl iodide (1 mL) and tetrahydrofuran (30 mL)were added into a 200‐mL Schlenk glass reactor under nitrogen blanketing at room temperature for 3 hours.The solid was filtered off andn‐hexane (30 mL) was used to wash the product for four times.Then the obtained product was recrystallized at ‐10 ℃ in ethanol (40 mL).Finally,a yellow crystal 9‐hydroxymethyl‐9‐(methoxymethyl)fluorene was obtained after distillation under reduced pressure with a yield of 79.1%.1H‐NMR (400 MHz,CDCl3,δ) analysis: 7.74 (d, 2H, fluorene), 7.62 (d, 2H,fluorene), 7.39 (t, 2H, fluorene), 7.29 (t, 2H, fluorene),3.94 (s, 2H, CH*2OH), 3.69 (s, 2H, CH*2OCH3), 3.38 (s,3H, CH2OCH*3), 2.42 (s, 1H, CH2OH*).ESI‒MSm/zcalculated for [M+H]+.C16H16O2: 241.12, found at 241.13.ID2 (9‐methoxymethyl‐9‐(methyl acetate) fluorene)was synthesized (Figure 4).9‐Hydroxymethyl‐9‐(methoxymethyl) fluorene (1 g), pyridine (0.5 mL), acetyl chloride (0.4 mL) and tetrahydrofuran (20 mL) were added into a 200‐mL Schlenk glass reactor under nitrogen blanketing at room temperature.After the reaction was carried out for 2 hours at room temperature and was continued for 9 hours under THF reflux, the product 9‐methoxymethyl‐9‐(methyl acetate) fluorene was obtained after distillation under reduced pressure with a yield of 54.4%.1H‐NMR (400 MHz, CDCl3,δ) analysis: 7.76 (d, 2H,fluorene), 7.43 (d, 2H, fluorene), 7.38 (t, 2H, fluorene), 7.27(t, 2H, fluorene), 4.66 (s, 2H, CH2O), 3.93 (s, 2H, CH2OCO),2.24 (s, H, CH2OH*), 2.01 (s, 3H, CH3).ESI‒MSm/zcalculated for [M+H]+.C17H16O3: 269.12, found at 269.11.
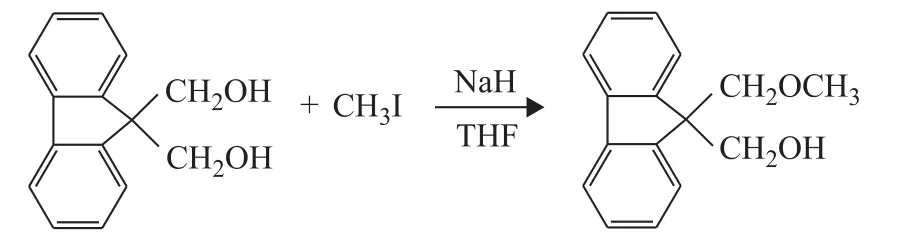
Figure 3 The synthesis of 9-hydroxymethyl-9-(methoxymethyl) fluorene
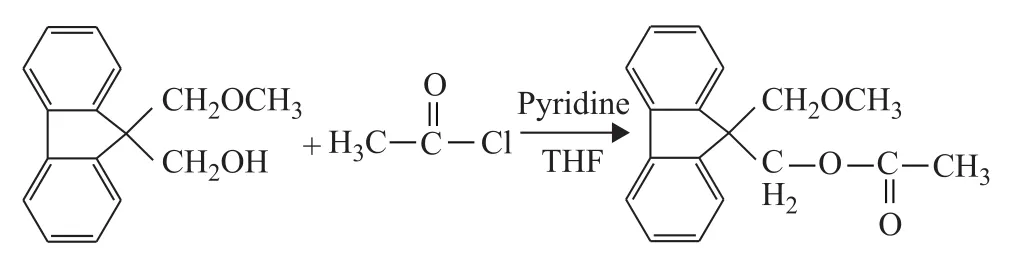
Figure 4 The synthesis of 9-methoxymethyl-9-(methyl acetate) fluorene
2.2.3 The synthesis of ID3 (9,9-bis (methyl acetate)fluorene)
The synthesis of ID3 (9,9‐bis(methyl acetate) fluorene)is shown in Figure 5.9,9‐Bis(hydroxymethyl) fluorene(1 g), pyridine (0.5 mL), acetyl chloride (1 mL) and THF(20 mL) were added into a 200‐mL reactor with nitrogen blanketing at room temperature.After the reaction was carried out for 2 hours at room temperature and was then continued for another 8 hours under THF reflux, the solid was filtered off andn‐hexane (30 mL) was used to wash the product for four times.Then the product 9,9‐bis(methyl acetate) fluorene after being recrystallized at ‐10 ℃ in ethanol (40 mL) was finally obtained after distillation under reduced pressure with a yield of 75.1%.1H‐NMR(400 MHz, CDCl3,δ) analysis: 7.82 (d, 2H, fluorene),7.54 (d, 2H, fluorene), 7.41 (t, 2H, fluorene), 7.35 (t, 2H,fluorene), 4.66 (s, 4H, CH2), 2.01 (s, 6H, CH3).ESI‒MSm/zcalculated for [M+H]+.C19H18O4: 311.13, found at 311.14.

Figure 5 The synthesis of 9,9-bis(methyl acetate) fluorene
2.3 The preparation of catalyst
Anhydrous MgCl2(200 mg),n‐decane (5 mL) and isooctanol (1.5 mL) were sequentially added into a 200‐mL Schlenk glass bottle, which was fully purged with high‐purity nitrogen at room temperature.After the oil bath was heated to 120 ℃, the mixture was stirred until MgCl2solid was completely dissolved to become a homogeneous solution.ID3 (25 mg, in toluene) was added, and then the reaction lasted for 6 h at 120 ℃.After the solution was cooled to ‐25 ℃, TiCl4(20 mL)was added dropwise by a syringe.The temperature was maintained for 4 h after the temperature of the solution was raised to 80 ℃ by stirring.Then the solution was cooled to room temperature, and the mixture was filtered.The residue was washed for 5 times byn‐hexane (40mL×5) and dried under vacuum at 45 ℃.265 mg of brown solid powder were obtained with a Ti content of 3.7%, which was determined by XPS technique.1.5 mL of MAO (10% in toluene) and 0.3 mL of AlEt3(1.5 M in hexane) were added (with Al/Ti = 75 in mol ratio) to mix with 50 mg of solid powder, the mixture was then stirred for 2 hours at 40 ℃.After removing the solvent under vacuum, the product was dried under vacuum at 45 ℃to yield 296 mg of solid Cat.3 (with Al/ Ti = 75 in mol ratio) with a Ti content of 0.63%.
Cat.1 (with Al/Ti = 75 in mol ratio), Cat.2 (with Al/Ti = 75 in mol ratio) and Cat.3 (with Al/Ti = 50 and 100 in mol ratio) were prepared according to the same method mentioned above by adding ID1, ID2, and ID3,respectively.
2.4 Preparation of polypropylene
All polymerization reactions were carried out in a 5‐L autoclave after purging all moisture and oxygen by a high‐vacuum pump.A desired amount of external electron donor, catalyst and hydrogen was added into the reactor at 30 ℃.Then 1500 g of liquid propylene were introduced into the reactor.After the mixture was subjected to pre‐prcessing for 10 min, the reactor was rapidly heated to 70 ℃, which was maintained for 1 h under mechanical agitation.The amount of propylene monomer was excessive in the reactor, so the whole polymerization process was conducted under a pressure of about 3.6 MPa at 70 ℃.The obtained polypropylene product was dried in a vacuum drying box at 60 ℃ and then the product quantity was weighed to calculate the catalytic activity.
3 Results and Discussion
3.1 Propylene polymerization with catalysts
The presence of the internal donor had obviously impacted on the molecular weight of polypropylene.In order to evaluate the performance of catalyst containing different internal donors, the propylene polymerization reaction was carried out under a pressure of 3.6 MPa at 70℃.The polymerization results of propylene evaluated in terms of the isotacticity andTmof polypropylene, and also the catalytic activity are summarized in Table 1.It can be clearly observed that isotacticity andTmof the obtained polymers showed slight change irrespective of the change in hydrogen pressure or the catalysts with different internal donors.After the introduction of a small amount of hydrogen, the catalytic activity increased significantly.But when the amount of hydrogen continued to increase,the catalytic activity was decreased slightly, which was consistent with previous reports[27].With the concentration of hydrogen increasing continuously, the chain transfer reaction to hydrogen became dominant, and the molecular weight of polypropylene was obviously decreased.It also can be clearly observed that the molecular weight of the polypropylene was significantly impacted by those catalysts containing different internal electron donors.
It is found that the polymer prepared by Cat.3 had a highest weight average molecular weight of 1.62×106g/mol without hydrogen uptake during the polymerization reaction (run 7 in Table 1).Even though the hydrogen was charged up to a pressure of 0.1 MPa during the polymerization reaction, the average molecular weight of the obtained polymer was 1.25×106g/mol (run 9 in Table 1).So ID3 was used as the optimal internal electron donor for preparing Cat.3.It can be found from Table 1 that the molecular weight of polypropylene was obviously changed with the Al/Ti mole ratio in catalyst (runs 9, 10 and 11).When the Al/Ti mole ratio was 75:1, a highest molecular weight of polypropylene was obtained.This phenomenon is mainly due to the fact that the addition of an appropriate amount of AlEt3can promote the alkylation of TiCl4and can reduce transformation of Ti4+to Ti3+to promote the formation of catalytic active sites, but when the amount of cocatalyst is excessive, it will poison the active sites and eventually lead to catalyst deactivation[17,28].Cat.3R was also utilized for propylene polymerization by adding a cocatalyst during the polymerization reaction (runs 12‒16 in Table 1).The molecular weight of the polypropylene obtained by adding a cocatalyst during the polymerization reaction was significantly lower than that achieved by cocatalyst loaded on the support.
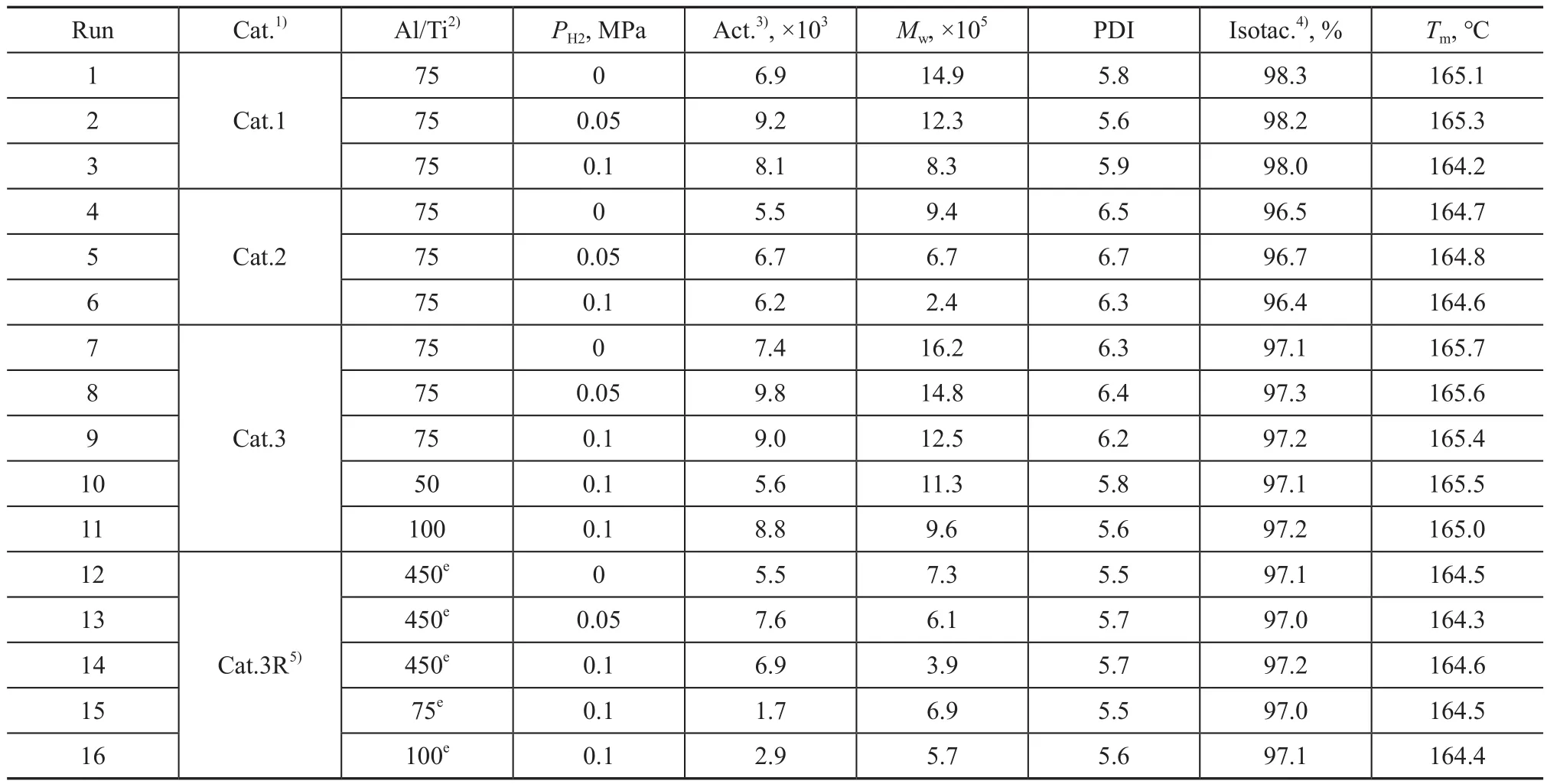
Table 1 Propylene polymerization results
3.2 Morphologies of catalysts and polymer particles
The morphologies related to Cat.1, Cat.2 and Cat.3 were studied by SEM technique, with the results shown in Figure 6.It can be observed from Figure 6 that all the catalysts are quasi‐spherical particles and are distributed uniformlyin a size range of 10 µm ‐20 µm.The spherical supported Ziegler‒Natta catalyst was useful in industries to prepare spherical polypropylene particles[29‐31].The picture of polypropylene particles prepared by the spherical catalyst Cat.3 is shown in Figure 7 (from Cannon), and the better spherical polypropylene particles, which are favorable to processing, are obtained by controlling the morphology and structure of the catalyst particles.
3.3 FT-IR analysis of internal donors and catalysts
The FI‐IR profiles of ID1, ID2 and ID3 are presented in Figure 8.There was only one kind of oxygen atom on the ether group for ID1 (Figure 8a).A wavenumber of 1090 cm‐1in the curve was assigned to the characteristicpeak of C‐O‐C.The stretching frequency of the C‐O‐C ether bond in Cat.1 shifted to a low wavenumber direction and formed a broad peak.The offset wavenumber Δν was 30 cm‐1, which implied that ID1 and magnesium chloride were combined by oxygen in C‐O‐C.

Figure 6 SEM image of catalyst particles

Figure 7 Image of polypropylene particles
There are three different oxygen groups in ID2, namely‐C=O, ‐O‐C=O, and ‐C‐O‐C.In the FT‐IR spectrum(Figure 8b), the wavenumber of 1090 cm‐1was attributed to the characteristic peak of ‐C‐O‐C.The wavenumber of 1275 cm‐1was attributed to the characteristic peak of C‐O bond in ‐O‐C=O, and the wavenumber of 1712 cm‐1was attributed to the characteristic peak of carbonyl group in ‐O‐C=O.The stretching frequency of the ether bond C‐O‐C in Cat.2 moved to a low wavenumber direction and formed a broad peak, whereas the migration of wavenumber Δν was 27 cm‐1.The stretching frequency of the ether bond in ‐O‐C=O of the catalyst did not change,which was still at 1275 cm‐1.The offset wavenumber Δν was 0 cm‐1, indicating that magnesium or titanium was not coordinated with it.The stretching frequency of the carbonyl group in ‐O‐C=O moved to a low wavenumber direction and formed a broad peak.The offset wavenumber Δν was 37 cm‐1.The infrared spectroscopy analysis results showed that the ID2 and magnesium chloride were bound by the oxygen on the carbonyl group in ‐O‐C=O and the oxygen of the ‐C‐O‐C ether group,which was an asymmetric binding mode.
In the FT‐IR spectra of Cat.3 (Figure 8c), the stretching frequency of the =C‐O‐C ether bond was not changed at 1275 cm‐1, indicating that magnesium or titanium was not coordinated with it.The stretching frequency of the ‐O‐C=O carbonyl group in the catalyst shifted to a low wavenumber direction and formed a broad peak with an offset wavenumber Δν of 47 cm‐1.The infrared spectra result indicated that ID3 was bound to magnesium chloride through the oxygen on the carbonyl group.
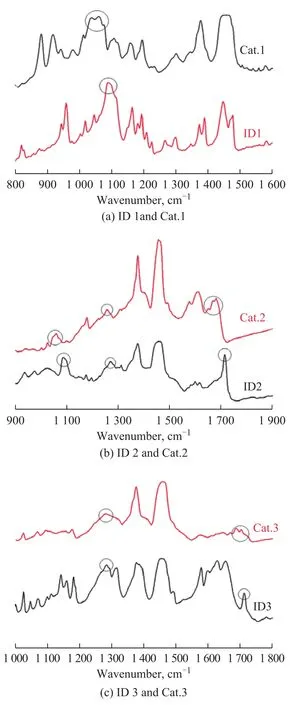
Figure 8 FT-IR spectra of internal electron donors ID 1, ID 2, ID 3 and catalysts
The FT‐IR analysis results revealed that internal electron donors through the oxygen atoms were bound to Ti atom from the catalysts.And the wavenumber offset of the carbonyl oxygen atom was larger than that of the ether oxygen atom, when the carbonyl oxygen atom and the ether oxygen atom were present at the same time.This outcome might be attributed to the stronger electron‐withdrawing ability of the carbonyl oxygen atom, which made it more closely bound to Ti atom from the catalyst.
3.4 XPS analysis of the catalysts
A stable complex was formed by the internal electron donor with titanium tetrachloride and magnesium dichloride through the oxygen from the internal electron donor complexed with titanium and magnesium atoms.The electron cloud was transferred to the metal atoms,which increased the electron cloud density around magnesium and titanium atoms.The result appeared in the XPS spectrum as a decrease in the binding energy of Ti 2p3/2and Mg 2p (Figure 9).Judging from the XPS spectra of ID1 and Cat.1 (Figure 9a), the binding energy of titanium and magnesium in the catalyst was decreased by the difference values of 0.3 eV and 1.41 eV, respectively,indicating that ID1 might coordinate with titanium and magnesium at the same time.The total binding energy change value for ID1 was ΔEID1= 1.71 eV.
Judging from the XPS spectra of ID2 and Cat.2 (Figure 9b), the binding energy of titanium and magnesium in the catalyst was decreased by the difference values of 0.4 eV and 0.58 eV, respectively, indicating that ID2 could coordinate with titanium and magnesium at the same time.The total binding energy change value for ID2 was ΔEID2= 0.98 eV.Compared with ID1, the binding energy of the magnesium atom of ID2 decreased less, which might be due to the weaker binding effect between the internal electron donor ID2 and magnesium chloride.As regrds the XPS spectra of ID3 and Cat.3 (Figure 9c), it can also be found that the binding energy of titanium and magnesium in the catalyst was decreased by the difference values of 0.9 eV and 2.43 eV, respectively, which implied that ID3 might coordinate with titanium and magnesium at the same time.The total binding energy change value for ID3 was ΔEID3= 3.33 eV.The binding energy of titanium and magnesium for Cat.3 decreased more than Cat.1 and Cat.2, implying that ID3 had a stronger binding energy with titanium and magnesium.
The XPS analysis results for these catalysts are shown in Figure 10, and the structure of internal electron donors could impact on the binding energy of titanium and magnesium atoms for these catalyst systems.The order of the total binding energy change value decreased in the following order: ΔEID3> ΔEID1> ΔEID2.The larger the change value of the total binding energy was, the stronger the binding ability would be.

Figure 9 XPS spectra of the catalysts in comparison with the internal electron donors
The Ti 2p XPS spectra collected from catalysts are shown in Figure 10.The binding energy values are in line with the reported values[32].It can be seen from Figure 10 that the ratio of Ti3+/Ti4+in Cat.3 attained a highest value than that of Cat.1 and Cat.2, when ID3 was used to prepare Cat.3, ID1 was used to prepare Cat.1, and ID2 was used to prepare Cat.2, respectively.It is possible that the electron cloud density surrounding Ti atoms was reduced by the reason that the carbonyl oxygen on the ester bond had a stronger electron‐withdrawing ability than the oxygen onthe ether bond did.Therefore the electron cloud density surrounding Ti atoms, which was changed by the internal electron donor added to the Ziegler‐Natta catalyst, could result in a great impact on the catalyst activity and the polymerization behaviors such as molecular weight of polypropylene.

Figure 10 Ti 2p XPS spectra of the catalysts and the internal electron donors
4 Conclusions
The test results showed that ID1, ID2, and ID3 added to these catalysts obviously changed the average molecular weight of the obtained polypropylene.This is possible because the ether oxygen atom and carbonyl oxygen atom can affect the electron density around Ti atom,which can lead to stronger coordinating ability of the active sites loaded on MgCl2particle surface.And it also can be found that the carbonyl oxygen atom on the ester bond has a stronger electron‐withdrawing ability than the oxygen atom on the ether bond.
By means of FT‐IR, XPS and other characterization methods, these results revealed that the internal electron donor could interact with Mg and Ti atoms through oxygen atom.In the case when there were both an ether oxygen atom and a carbonyl oxygen atom, Mg and Ti atoms preferentially were bound to the carbonyl oxygen atom, indicating that the bonding ability of the carbonyl oxygen atom to Mg and Ti atoms was superior to the ether oxygen atom.Judging from the results of XPS analysis, the change values of the total binding energy decreased in the following order: ΔEID3> ΔEID1> ΔEID2.The larger the change value of the total binding energy,the stronger the binding ability of the internal electron donor with Ti and Mg atoms.So the ultra‐high molecular weight polypropylene could be produced by these catalysts prepared by combining internal electron donor and cocatalyst loaded on the support particle surface.And it is also found that with the addition of different internal electron donors the content of trivalent titanium of these catalysts were changed significantly.The result showed that Cat.3 containing a Ti3+content reaching nearly up to 99% for propylene polymerization had exhibited higher catalytic activity, and the ultra‐high molecular weight polymers were obtained.It might be inferred that the internal electron donor ID3 obviously affected the electron cloud density around Ti atom within the catalyst Cat.3 and could attenuate the β‐hydride elimination reaction when the monomers were inserted into the active sites.
Acknowledgements:This study was financially supported by the People’s Republic of China Ministry of Industry and Information Technology (No.gxgh2019‐795), and the National Natural Science Foundation of China (No.U1462102).The authors also appreciate the support provided by the KeyLaboratory of Carbon Fiber and Functional Polymers.
杂志排行
中国炼油与石油化工的其它文章
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Synthesis of Bimodal Mesoporous TiO2 -PTA/BMMS and Its Enhanced Performance in the Photocatalytic Oxidative Desulfurization
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation
