Age Structure of Two Species of Odorous Frogs (Odorrana margaretae andOdorrana grahami)
2021-09-27LianjuYUANMaojunZHONGandWenboLIAO
Lianju YUAN ,Maojun ZHONG and Wenbo LIAO,2,3*
1Key Laboratory of Southwest China Wildlife Resources Conservation (Ministry of Education),China West Normal University,Nanchong 637009,Sichuan,China
2Key Laboratory of Artificial Propagation and Utilization in Anurans of Nanchong City,China West Normal University,Nanchong 637009,Sichuan,China
3Institute of Eco-adaptation in Amphibians and Reptiles,China West Normal University,Nanchong 637009,Sichuan,China
Abstract Variation in age structure and body size benefits are identified to understand the evolution of life history.Here,we estimated the age structure and body size of two species of odorous frogs (Odorrana margaretae and Odorrana grahami) by using skeletochronology.The ages at sexual maturityofO.grahami andO.margaretae in both sexes were 1 and 2 years,respectively.For both sexes,the maximum age observed in O.margaretae was six years.ForO.grahami, the maximum age observed in males and females were 4 and 5 years,respectively.Males and females did not differ in mean age in the two species.The average body size of both species considerably differed between sexes,with females being larger than males.The body size of females was also larger than that of males when the effect of age was removed.We also found positive correlations between body size and age within each sex in O.margaretae,but only for female inO.grahami.The female-biased sexual size dimorphism of the two species suggested that fecundity selection for larger female size may increase the reproductive output.
Keywords age structure,body size,odorous frogs,skeletochronology
1.Introduction
The global population declines of amphibians have highlighted the lack of knowledge in population dynamics (Green,2003).The population dynamics are underpinned by mortality rates which are also affected by life-history traits (e.g.,growth rates,age at sexual maturity and longevity;Wells,2007).The knowledge of individual lifespan and population age structure facilitates the identified factors that affect the mortality of certain life-history stages (Bieket al.
,2002) and develop appropriate conservation strategies for anurans (Wells,2007).Hence,identifying the patterns of age structure may help us to understand life-history traits in organisms (Zhonget al.
,2018).The sexual size dimorphism (SSD),which is the difference in body size between males and females,is widespread (Wells,2007;Adamset al.
,2020),and females are the larger sex in 90%of anuran species (Shine,1979).Previous studies indicated that the body size of individuals differs significantly between sexes,and the body size of females is larger than that of males in most anurans (Leclair and Laurin,1996;Liao and Lu,2010;Liaoet al.
,2011;Liao and Chen,2012).This pattern has been successfully output favours large body size in females,thereby leading to female-biased SSD (De Lisle and Rowe,2013;Liao,2013;Liaoet al.
,2015).Skeletochronology represents a reliable method for determining age structure in anurans on the basis of presence of the lines of arrested growth (LAGs) recorded in the crosssections of phalanges (Castanet and Smirina,1990).Over the past 30 years,this method has been used in estimating the age of anurans from different areas (temperate:Hemelaar and Van Gelder,1980;Guarino and Erismis,2008;Kyriakopoulou-Sklavounouet al.
,2008;subtropical:Morrisonet al.
,2004;Liao and Lu,2011;Liaoet al.
,2011;Zhanget al.
,2021;tropical:Khonsueet al.
,2000;Kumbar and Pancharatna,2001) without killing frogs.In the present study,we investigate the age structure and body size of two species of odorous frogs (i.e.,Odorrana margaretae
andOdorrana grahami
) inhabiting the ravine streams in China by using skeletochronology.The green odorous frog(O.margaretae
) is a species distributed in Hengduan Mountains where it is restricted to a narrow range of elevations,i.e.,500-1650 m (Fei and Ye,2001).The disk-fingered odorous frog (O.grahami
) is one of the most widely distributed species in China with elevations elevation ranging from 1720 to 3200 m (Fei and Ye,2001).The two species have similarities in habitat types,diet and activity time (Feiet al.
,2010).However,their population size has declined in recent years due to human activity.Although altitudinal variations in body size ofO.grahami
have been reported by Liet al.
(2013),information about the age structure data of the two species during the breeding period is unavailable.Our aims are to compare age structure,body size,and the relationship between age and body size between sexes in the two species,where these demographic parameters are poorly known,and provide conservation suggestion on the basis of the age structure and body size of two species.2.Materials and Methods
2.1.Study area
The population ofO.margaretae
was located in a stream of Wawushan National Park in Ya’an City(29.64°N,103.09°E),at an altitude of 1105 m a.s.l.in the Hengduan Mountains of Western China.The population ofO.grahami
was located in a stream of Midu County in Dali City (25.12°N,100.61°E) at an altitude of 1550 m a.s.l.in the Hengduan Mountains of Western China.The two study areas have a strong seasonal variation in climate.The monthly average temperature ranges from 3 °C to 22 °C in Wawushan National Park and from 11 °C to 22.5 °C in Midu County.2.2.Sampling methods
All frogs were captured in ravine streams from late June to early August 2018 during their reproduction.We collected 43O.margaretae
individuals in June 2018 and 43O.grahami
individuals in August 2018.We determined their sexes and sexual maturity by observing the secondary sexual characteristics (i.e.,nuptial pads in males and evidently swollen belly due to egg masses in females).We used a calliper with accuracy up to the nearest 0.1 mm to measure body size (snout-vent length,[SVL]) within each individual.We then clipped the third phalanx of the longest toe of the right hind limb,and stored them in 4% neutral buffered formalin for skeletochronology.2.3.Skeletochronology
Following the methods used for determining the age of each frog (Liao and Lu,2010),we removed the skin and muscle tissues of each digit and used water to wash the remaining bones for 2 h.The digits were decalcified in 5% nitric acid for 48 h,washed in running tap water overnight and stained with Ehrlich’s haematoxylin for 100 min.Subsequently,we dehydrated the stained phalanges of each digit through successive ethanol stages of 70%,80%,95%,and 100% for approximately 1 h at each concentration.Phalanges were processed for paraffin embedding in small blocks (50 mm × 15 mm × 10 mm;L × W × H).We used a rotary microtome to obtain thin cross-sections (13 μm) of the mid-shaft diaphysis,selected the phalanx with the smallest medullar cavity,and then mounted them onto glass slides.We used a light microscope to observe the sections and the Motic BA300 digital camera mounted on the Moticam2006 light microscope at 400 × magnifications to photograph the best section.LAGs were identified by two persons (M.J.Zhong and L.J.Yuan),who were equally experienced in the technique and identification criteria and agreed on the final age estimation.The number of LAGs corresponded to the age of individuals due to distinct temperature cycles experienced through the year (Morrisonet al.
,2004).We considered the surface of the bone as an additional LAG based the specimens collected during the breeding seasons after emergence from hibernation(Liao and Lu,2010).We used the presence of the Kastschenko Line (KL,the interface between the endosteal and periosteal zones;Rozenblut and Ogielska,2005) to confirm the endosteal resorption of LAGs for the study species by following the protocol by Castanet and Smirina (1990).2.4.Statistical analysis
We performed all statistical tests by using the SPSS 22.0 software (Statistical Product and Service Solutions Company,Chicago).We used parametric tests when the data met assumptions of the tests (i.e.,normality of the distribution and homogeneity of the variance) and used non-parametric tests when assumptions were not met.We first identified the age structure between sexes by using the Chi-square test and then used the Mann-WhitneyU
-test to compare differences in body size and age between males and females.We conducted ANCOVAs and treated SVL as a dependent variable,and age as covariate to test significant difference in body size between sexes when correcting the age effects.Finally,we used general linear models and treated SVL as a dependent variable,and age and sex as fixed factors to assess the effect of interaction between age and sex on SVL.All values given were shown as mean ± SD,and the level of significance wasP
< 0.05.3.Results
3.1.Skeletochronology
A total of 80 individuals were aged successfully by skeletochronology.We obtained 32 males and 9 females inO.margaretae
and 18 males and 21 females inO.grahami
(Table 1).Six individuals lacked LAGs were excluded from the analysis.Extremely closely spaced hematoxylinophilic lines (so-called double LAGs) were found in twoO.margaretae
females.We observed false LAGs (i.e.,LAGs that were faint and did not form a complete ring in the bone section) in 1 male and 1 female inO.grahami
.However,we did not find problems in estimating their age.Moreover,we found that the endosteal resorption of the innermost LAG occurred in 7.5% of males and 8.5% of females in two species based on the occurrence of KLs(Figure 1).
Table 1 Body size and age of two species of odorous frogs (Odorrana margaretae and Odorrana grahami) in southwestern China.Values in descending order are mean ± SD and sample size.
3.2.Age at sexual maturity and longevity
The age at sexual maturity was estimated to be two years in males and females inO.margaretae.
ForO.grahami
,the minimal age of males and females at sexual maturity was 1 year.The lifespan of individuals inO.margaretae
was estimated to be at least six years in males and females,whereas the lifespan of males and females inO.grahami
did not exceed 4 and 5 years,respectively(Figure 2).Males and females did not differ in age inO.margaretae
(Mann-WhitneyU
-test:Z
=0.469,n
=32,n
=9,P
=0.639) andO.grahami
(Z
=0.320,n
=18,n
=21,P
=0.749).InO.margaretae
,the average age of adult males and females were 3.88 ± 1.21(range=2-6) and 3.90 ± 1.24 (range=2-6) years,respectively.InO.grahami
,the average age of males and females were 2.57 ± 1.40(range=1-4) and 2.39 ± 0.98 (range=1-5) years,respectively.3.3.Age structure
The adult age ofO.margaretae
ranged from 2 years to 6 years in males and females (Figure 2).Age distributions differed significantly between sexes (Chi-square test:χ
=12.902,P
< 0.001).The adult age ofO.grahami
ranged from 1 year to 4 years in males and from 1 year to 5 years in females (Figure 2).Age distributions did not differ significantly between sexes (χ
=0.231,P
=0.631).The predominant age class of males and females was 4 years (34.19%) inO.margaretae
and 3 years (30.8%) inO.grahami
.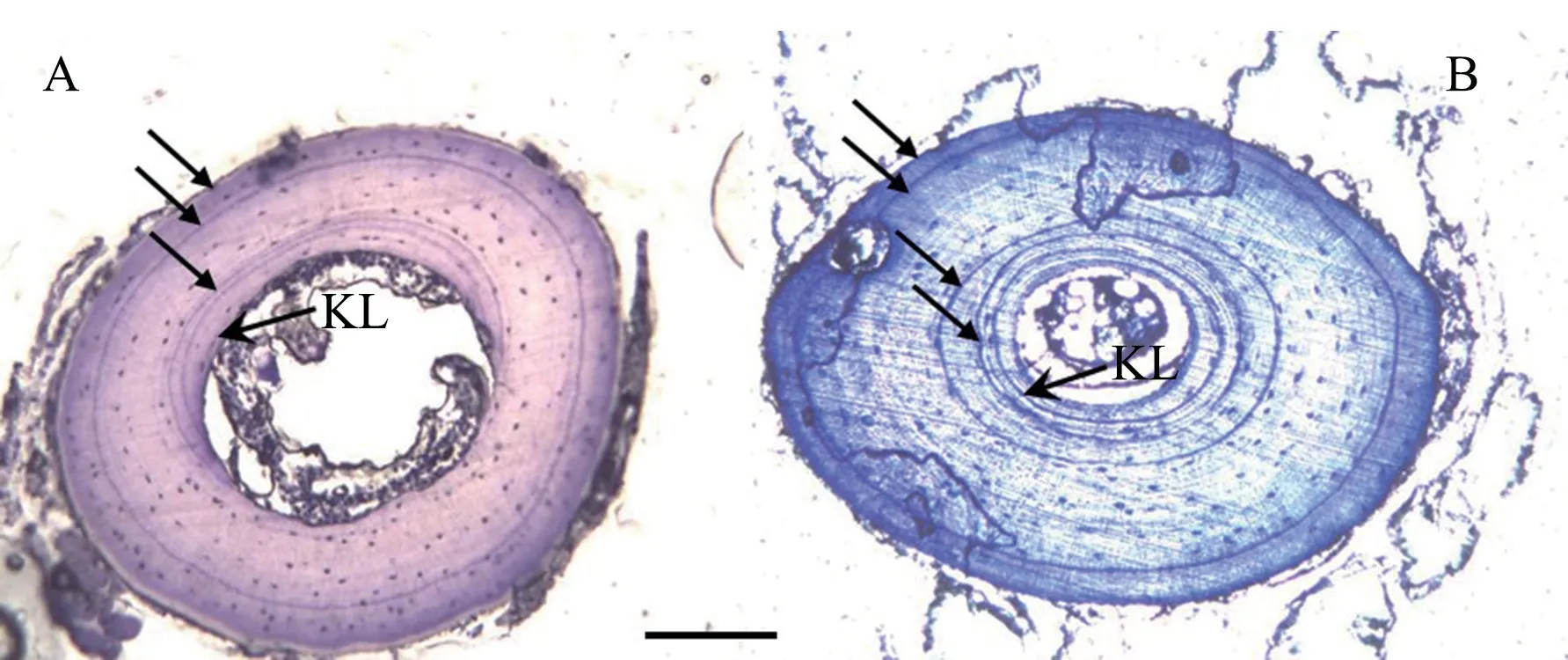
Figure 1 Two hematoxylin-stained cross sections of the phalangeal bone (A:a 3-yrs old male Odorrana margaretae;B:a 4-yrs old female Odorrana grahami) in southwestern China.Arrows indicate the arrested growth lines (LAG).KL represents Kastschenko Line,the interface between the endosteal and periosteal zones.Scale bar:100 μm.
3.4.Body size
The average SVL differed significantly between males and females inO.margaretae
(Mann-WhitneyU
-test:Z
=23.116,n
=32,n
=9,P
< 0.001) andO.grahami
(Z
=2.338,n
=18,n
=21,P
=0.019).When correcting the age effect,the differences in body size between sexes remained significant inO.margaretae
(ANCOVA:F
=220.676,P
< 0.001) andO.grahami
(F
=6.275,P
< 0.019).A significant effect of interaction between age and sex on body size inO.margaretae
(F
=763.382,P
< 0.001)revealed that the relationship between age and size differed between males and females.ForO.grahami,
no significant effect of interaction between age and sex on body size(F
=1.369,P
=0.250) was observed,showing that the relationship between age and size did not differ between sexes.
The average SVL (Mann-WhitneyU
-test:females,Z
=10.868,n
=9,n
=21,P
< 0.001;males,Z
=18.901,n
=32,n
=18,P
< 0.001) and average age (females,Z
=16.430,n
=9,n
=21,P
<0.001;males,Z
=21.519,n
=32,n
=18,P
< 0.001) ofO.margaretae
females and males were larger than those ofO.grahami
females and males.3.5.Relationship between age and body size
Regression equations revealed significant relationships between SVL and age within each sex inO.margaretae
(females:y
=11.303x
+43.823,F
=7.534,r
=0.518,P
=0.029;males:y
=2.693x
+64.554,F
=9.425,r
=0.239,P
=0.005) and in femaleO.grahami
(y
=9.003x
+49.084,r
=0.249,F
=13.181,P
=0.002),but not in maleO.grahami
(y
=4.180x
+49.528,r
=0.097,F
=2.008,P
=0.176).A large overlap of SVL amongst the estimated age classes occurred within each sex,showing that age cannot be predicted from body size (Figure 3).
Figure 2 Adult age structure (male:close bars;female:open bars) of (A) Odorrana margaretae and (B) Odorrana grahami in southwestern China.

Figure 3 The relationship between age and body size of (A)Odorrana margaretae and (B) Odorrana grahami in southwestern China (female:close circle,full line;male:open circle,dotted line).
4.Discussion
We investigate forage structure and body size in
O.
margaretae
andO.grahami
.We provide clear evidence that females have larger body size than males when correcting for the age effect,but no significant difference is observed in the average age between males and females in the two species. We also find that body size displays a positive correlation with age within each sex in two species except maleO.grahami
.Our results suggest that the relatively short lifespan in both species is likely to result in increasing mortality rates,thereby decreasing the population size.The use of phalangeal growth marks under low environmental temperature during the hibernating period depresses physiological functions of the frogs and can assess individual age in most anurans (Morrisonet al.
,2003;Laiet al.
,2005;Huanget al.
,2014;Yuet al.
,2018).The formation of LAGs inO.margaretae
is affected by inactivity for a long period (Liet al.
,2013).In this study,the hibernation in the two species begins in mid-October,and the inactive period lasts until early May,which produces the clear LAG formation for all frogs.The accuracy of the age determination in the frogs is often affected by double and false lines (Hemelaar and van Gelder,1980).Here,we find 2 individuals with double lines and 2 individuals with false lines which do not affect age estimation and data analysis.As critical transitions in life-history traits,the age at sexual maturity and longevity remarkably display the influences of individuals’ fitness (Charlesworth,1980;Xionget al.
,2020).ForO.margaretae,
the age atsexual maturity of males and females arise after the second hibernation due to the low temperature experienced at high altitude.Similar to some anurans (Estebanet al.
,1996;Guarinoet al.
,2003;Liao and Lu,2010;Liao and Lu,2012),the age at sexual maturity inO.grahami
in both sexes was 1 year,suggesting that individuals possibly spend time in reproduction due to high temperature at low altitude.We find that the maximum ages recorded are 6 years in males and females forO.margaretae
,4 years inO.grahami
males,and 5 years inO.grahami
females.
Relatively shortlongevity for both species suggests that high mortality rates are likely to linked to human activity in studied populations.SSD may be consequences of differences in most key lifehistory traits between sexes (e.g.,age structure and growth rate before the attainment of sexual maturity;Monnet and Cherry,2002;Zhang and Lu,2013).Sex differences in age at maturity do not seem to explain differences in SSD in the studied species due to the same age at maturity in males and females.ForRana limnocharis,
the non-significant interaction between sex and age on body size shows that age-SVL relationships between sexes do not differ in slope,and males and females have similar growth patterns,which do not affect SSD (Liaoet al.
,2011).We find that SSD inO.grahami
is not affected by growth rate due to the non-significant effect of interaction between age and sex on body size.By contrast,the growth rate inO.margaretae
is a major factor affecting SSD because the interaction between age and sex on body size differs significantly between sexes.The SSD is regarded as an outcome of sexual and natural selection (Fairbairn,1997).We find that independent from age,females are larger than males in the two species,suggesting that natural selection on larger body size in females favours the improved fecundity or offspring size (Trivers,1974).Sexual selection can drive the evolution of SSD,in which increased male body size may promote male mating success (Andersson,1994).However,we do not provide the evidence that male body size is positively associated with male mating success in the two species.Finally,differences in mortality rates (e.g.due to predation) between sexes may lead to differences in the average age of adults and may affect variation in SSD (Liaoet al.
,2015).Body size changes with altitude and/or latitude in most anurans,with individuals living in high-altitude and/or latitude having larger body size than those living in low altitude and/or latitude (Miaudet al.
,1999;Luet al.
,2006;Matthews and Miaud,2007;Chen and Lu,2011).A previous study on body size variation across fourO.grahami
populations has suggested that in disagreement with Bergmann’s rule,body size does not change along the 830 m altitudinal gradients (Liet al.
,2013).We do not find increases in body size in the 1550-m population with altitude when we analyze data on life history across fiveO.grahami
populations.In most anurans,age displays positive correlation with body size within each sex (Estebanet al.
,1996;Ryser,1996;Kyriakopoulou-Sklavounouet al.
,2008;Liao and Lu,2011).We find that body size is positively correlated with age for both sexes inO.margaretae
.However,in other anurans a positive correlation may be true for only one sex (Gibbons and McCarthy,1984;Leclair and Castanet,1987;Cherry and Francillon,1992).ForO.grahami
,SVL displays a significant correlation with age in females but not in males.In combination with life-history traits,data on age structure in anurans may provide important insights into demography within and amongst populations,which have an important implication for the decisions of conservting species.The age structure collected onO.
margaretae
andO.grahami
is being used to develop stage-structured population models to estimate population dynamics and test for the causes of population decline in the two species.Acknowledgments
We thank Jianping Yu and Tao Tang for assistance with filed work.Financial support was provided by the National Natural Sciences Foundation of China (Nos.31772451,31970393) and the Science and Technology Youth Innovation Team of Sichuan Province(2019JDTD0012).All experiments involving the live animals were approved by the Animal Ethics Committee of China West Normal University (18002).杂志排行
Asian Herpetological Research的其它文章
- A New Species of Cyrtodactylus (Squamata:Gekkonidae) from the Karst Forests of Daweishan National Nature Reserve,Yunnan,China
- Disordered Translocation is Hastening Local Extinction of the Chinese Giant Salamander
- Molecular Evolution of Visual Opsin Genes during the Behavioral Shifts between Different Photic Environments in Geckos
- Effects of Life Histories on Genome Size Variation in Squamata
- Complete Mitochondrial Genome of the Steppe Ribbon Racer (Psammophis lineolatus):The First Representative from the Snake Family Psammophiidae and its Phylogenetic Implications
- Transcriptome Sequencing Provides Evidence of Genetic Assimilation in a Toad-Headed Lizard at High Altitude
