Insights into stem cell therapy for diabetic retinopathy:a bibliometric and visual analysis
2021-08-20XiangJunLiChunYanLiDanBaiYingLeng
Xiang-Jun Li, Chun-Yan Li, Dan Bai, Ying Leng,
Abstract Stem cells have been confirmed to be involved in the occurrence and development of diabetic retinopathy; however, the underlying mechanisms remain unclear. In this study,we used Citespace software to visually analyze 552 articles exploring the stem cell-based treatment of diabetic retinopathy over the past 20 years, which were included in the Web of Science Core Collection. We found the following: (1) a co-citation analysis of the references cited by all 552 articles indicated 15 clusters. In cluster #0, representing the stem cell field, some highly cited landmark studies emerged between 2009-2013. For example, endothelial progenitor cells and diabetic retinopathy gradually received the full attention of scholars, in terms of their relationship and therapeutic prospects. Some researchers also verified the potential of adipose-derived stem cells to differentiate into stable retinal perivascular cells, using a variety of animal models of retinal vascular disease.All of these achievements provided references for the subsequent stem cell research. (2)An analysis of popular keywords among the 552 articles revealed that, during the past 20 years, a relative increase in basic research articles examining stem cells and endothelial progenitor cells for the treatment of diabetic retinopathy was observed. The contents of these articles primarily involved the expression of vascular endothelial growth factor,vascular regeneration, oxidative stress, and inflammatory response. (3) A burst analysis of keywords used in the 552 articles indicated that genetic and cytological research regarding the promotion of angiogenesis was an issue of concern from 2001 to 2012,including several studies addressing the expression of various growth factor genes; from 2014 to 2020, mouse models of diabetic retinopathy were recognized as mature animal models, and the most recent research has focused on macular degeneration, macular edema, neurodegeneration, and inflammatory changes in diabetic animal models. (4)Globally, the current authoritative studies have focused on basic research towards the stem cell treatment of diabetic retinopathy. Existing clinical studies are of low quality and have insufficient evidence levels, and their findings have not yet been widely accepted in clinical practice. Major challenges during stem cell transplantation remain, including stem cell heterogeneity, cell delivery, and the effective homing of stem cells to damaged tissue. However, clinical trials examining potential stem cell-based treatments of diabetic retinopathy, including the use of pluripotent stem cells, retinal pigment epithelial cells,bone marrow mesenchymal stem cells, and endothelial progenitor cells, are currently ongoing, and high-quality clinical evidence is likely to appear in the future, to promote clinical transformation.
Key Words: diabetes; diabetic retinopathy; epithelial cells; macula; progenitor cells; retina;stem cells; visual analysis
Introduction
Existing treatments for diabetic retinopathy primarily include laser photocoagulation, the intravitreal injection of antivascular endothelial growth factor (VEGF) antibodies, and vitrectomy (Wong et al., 2016; Fiori et al., 2018). However,these treatments only aim to delay or prevent this persistent degenerative disease, and few studies have explored the pathogenesis and etiology of this disease. Numerous studies have confirmed that stem cells are involved in the occurrence and development of diabetic retinopathy, and the underlying mechanisms are still being explored (Megaw and Dhillon,2014; Gaddam et al., 2019).
Stem cells have the potential to delay the progression of diabetic retinopathy and to reduce the symptoms of such diseases (Bhattacharya et al., 2017; Kuriyan et al., 2017;Nirwan et al., 2019). In recent years, cell regenerative therapies for diabetic retinopathy have been preliminarily confirmed to be effective in some experimental animal studies, consolidating the preclinical research foundations in this field. The cell types that have been explored for use during regenerative therapy include cell-specific endogenous stem cells, endothelial progenitor cells, embryonic stem cells,induced pluripotent stem cells, and mesenchymal stem cells.Recent studies examining mesenchymal stem cells, endothelial progenitor cells, and adipose stromal cells have shown that cell-based therapies may be viable options for the prevention of neurovascular damage and the promotion of retinal regeneration (Megaw and Dhillon, 2014; Gaddam et al., 2019).In this paper, we visually analyzed the research hotspots related to stem cell use for the treatment of diabetic retinopathy over the past 20 years and the expectations for the future development of cell therapy for similar diseases.
Data and Methods
Retrieval strategy
The first author retrieved all articles regarding the stem cell treatment of diabetic retinopathy indexed in the Web of Science (WOS) Core Collection and ClinicalTrials.gov.
Databases retrieved
WOS core Collection and ClinicalTrials.gov database.
Search strategy
The search terms and strategies used for the WOS database are shown inTable 1.
For the ClinicalTrials.gov database, the search terms used were“Stem Cell, Progenitor Cell, Mother Cell, Diabetic Retinopathy,”and the search strategy was as follows: (Stem Cell OR Progenitor Cell OR Mother Cell) AND Diabetic Retinopathy.
Time range for the literature search
Articles from 2000 to 2020 were included in the search.
Qualification
A full-text search was performed.
Inclusion criteria
WOS Core Collection database: after reading the titles and abstracts, all articles related to stem cell treatment of diabetic retinopathy were selected, irrespective of article styles,including original research articles, meta-analyses, reviews,commentaries, and case reports.
ClinicalTrials.gov database: after reading the titles and study protocol information, clinical trials exploring the stem cell treatment of diabetic retinopathy were selected.
Number of articles included
A total of 552 articles identified in the WOS Core Collection were selected. Nine clinical trials indexed in ClinicalTrials.gov were selected.
The deadline for publications was 2020-04-20.
Literature visual analysis
(1) A total of 552 articles were selected for the generation of a citation analysis report. Citespace 5.2.R1 software was used to visually analyze the references cited by the 552 included articles, as described by Dr. Chao-Mei Chen, a scholar at Drexel University, USA (Chen, 2014; Chen et al., 2014; Chen and Song, 2019).
(2) Research hotspots regarding the use of stem cells for the treatment of diabetic retinopathy were sorted, followed by a keyword co-occurrence analysis.
The keyword co-occurrence analysis generated a visual map of the keywords used by all included articles, which appear as nodes, with the node sizes indicating how often the keywords appear. The keywords used by the 552 articles were selected as required for the co-occurrence analysis, to generate a keyword co-occurrence network map.
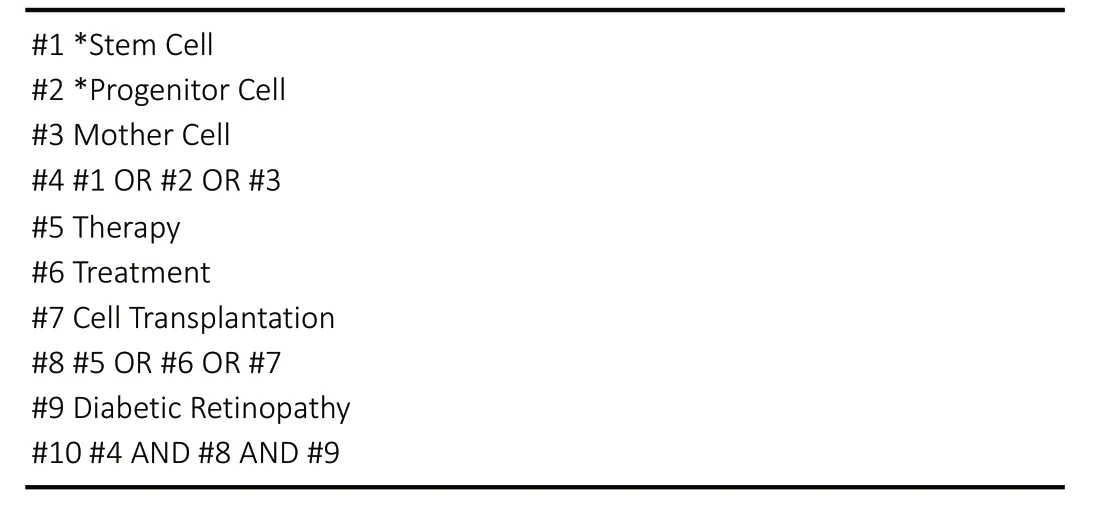
Table 1 |Search strategy for the Web of Science database
(3) Co-citation analysis of the references was performed, to analyze the time-varying upward trend in citations concerning the use of stem cells in the treatment of diabetic retinopathy.Reference co-citation indicates that both literature A and B were referenced by literature C.
The author performed a co-citation analysis of all 17,446 references cited by the 552 included articles to generate a cocitation network map and, then, generated a timeline map of the co-citation network, according to the clusters of the cocited references.
(4) A burst detection of the subject headings was performed,to identify hot words in the field stem cells use in diabetic retinopathy treatment.
A burst term is defined as a keyword that appears with an abrupt change in frequency within the literature, during a specific period, which is also considered to be a hot word in this field at this stage of research.
The burst detection in CiteSpace software was based on the algorithm developed by Goldberg et al. (2002).
(5) Interpretation of the visual map identifications used in the analysis of articles concerning the use of stem cells in the treatment of diabetic retinopathy.
Citation tree rings: In a map drawn using Citespace software,a circular region, termed the citation tree rings, represents the citation history of a paper. The color of the most central citation tree ring indicates the publication year of the paper.Node circles: The size of the radius indicates the number of publications in the author’s co-authored network and the institutional co-authored network and indicates the frequency of keywords that appear in the keyword cooccurrence network.
Links between nodes: A link between nodes indicates the presence of a co-authorship or a co-occurrence relationship. The node colors indicate different years and gradually change from cold colors to warm colors. For example, blue represents earlier years, and yellow represents recent years. Cluster #: A cluster analysis was performed based on the generated map, and each cluster was labeled by citing the title, keywords, and subject headings in the abstract of the cited reference (each label can be divided into several categories, such as #0, #1, #2…).
Results
Citation frequency of the 552 WOS-indexed articles examining stem cell treatment for diabetic retinopathy
(1) From 2000 to 2020, a yearly increase in the citation frequency of articles regarding the stem cell treatment of diabetic retinopathy was observed, with a peak in 2019.Therefore, the interest in research hotspots associated with the stem cell treatment of diabetic retinopathy appears to be gradually increasing each year (Figure 1).
(2) Representative examples of highly cited articles: Four highly cited articles were identified, concerning the therapeutic strategies for the use of pericytes, human embryonic stem cells, hematopoietic stem cells, and endothelial precursor cells during diabetic retinopathy treatment (Grant et al., 2002;Otani et al., 2002; Armulik et al., 2011; Schwartz et al., 2012;Table 2).
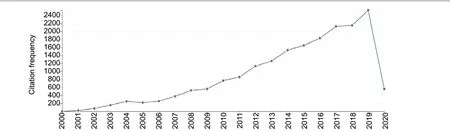
Figure 1 |Trends in the citation frequency of the 552 included articles from 2000 to 2020.From 2000-2019, the citation frequency has increased yearly. Relevant publications indexed in the Web of Science that were published prior to April 20, 2020, are included, but data for the entirety of 2020 is not yet available.
The study performed by Armulik et al. (2011) was the first to introduce the potential application of pericytes that are differentiated into stem cells to the treatment of diabetic retinopathy and was cited 1,086 times. Schwartz et al. (2012)were the first group to describe the subretinal transplantation of retinal pigment epithelium, derived from human embryonic stem cells, for the treatment of retinopathy, and this study was cited 807 times. In this study, the patient’s vision recovered,to some extent, after treatment, and no obvious rejection reaction had occurred within 4 months. Although this study was a one-arm study, with a small sample size and short followup time, the interest in the findings has increased. Two animal experiments reported the effectiveness of hematopoietic stem cells on retinopathy, from different aspects, and were cited 523 and 253 times (Grant et al., 2002; Otani et al., 2002;Table 2).(3) Four types of stem cells have regularly been reported for the treatment of diabetic retinopathy, with increasing incidence over time. Current research on the stem cell treatment of diabetic retinopathy primarily focuses on the treatment of early retinopathy but not on proliferative diabetic retinopathy. Some types of stem cells secrete special growth factors, which, in turn, exert neuroprotective effects on the diabetic retina; other types of stem cells can reduce and repair capillary occlusion, reducing the loss of peripheral cells, and can differentiate into peripheral cells and endothelial cells,protecting blood vessels damaged by diabetic retinopathy.Some of the potential stem cells explored for the treatment of diabetic retinopathy are as follows.
Mesenchymal stem cells: Recent studies have reported that mesenchymal stem cells may represent the optimal therapeutic agent for disease treatment and tissue replacement. The treatment of diabetic retinopathy using mesenchymal stem cells has gradually become a research hotspot in recent years(Gong et al., 2018;Table 2). However, mesenchymal stem cells have not been used in clinical practice to treat diabetic retinopathy, and the optimal approach for the administration of mesenchymal stem cells remains controversial. The effectiveness and safety of mesenchymal stem cells in clinical applications require further evaluation.
Endothelial progenitor cells: Compared with other differentiated and mature endothelial cells, endothelial progenitor cells display a stronger ability to resist oxidative stress (Caballero et al., 2007) (Table 2). A successful therapeutic method that targets retinal angiogenesis is likely to include the elimination of retinal ischemia caused by diabetic retinopathy.
Adipose-derived stem cells: Adipose-derived stem cells can lower blood glucose levels and play a role in neurovascular protection (Elshaer et al., 2018;Table 2). However,whether adipose stem cells indirectly improve the retinal microenvironment by triggering blood glucose reductions or directly play roles in damaged tissues remains unclear.
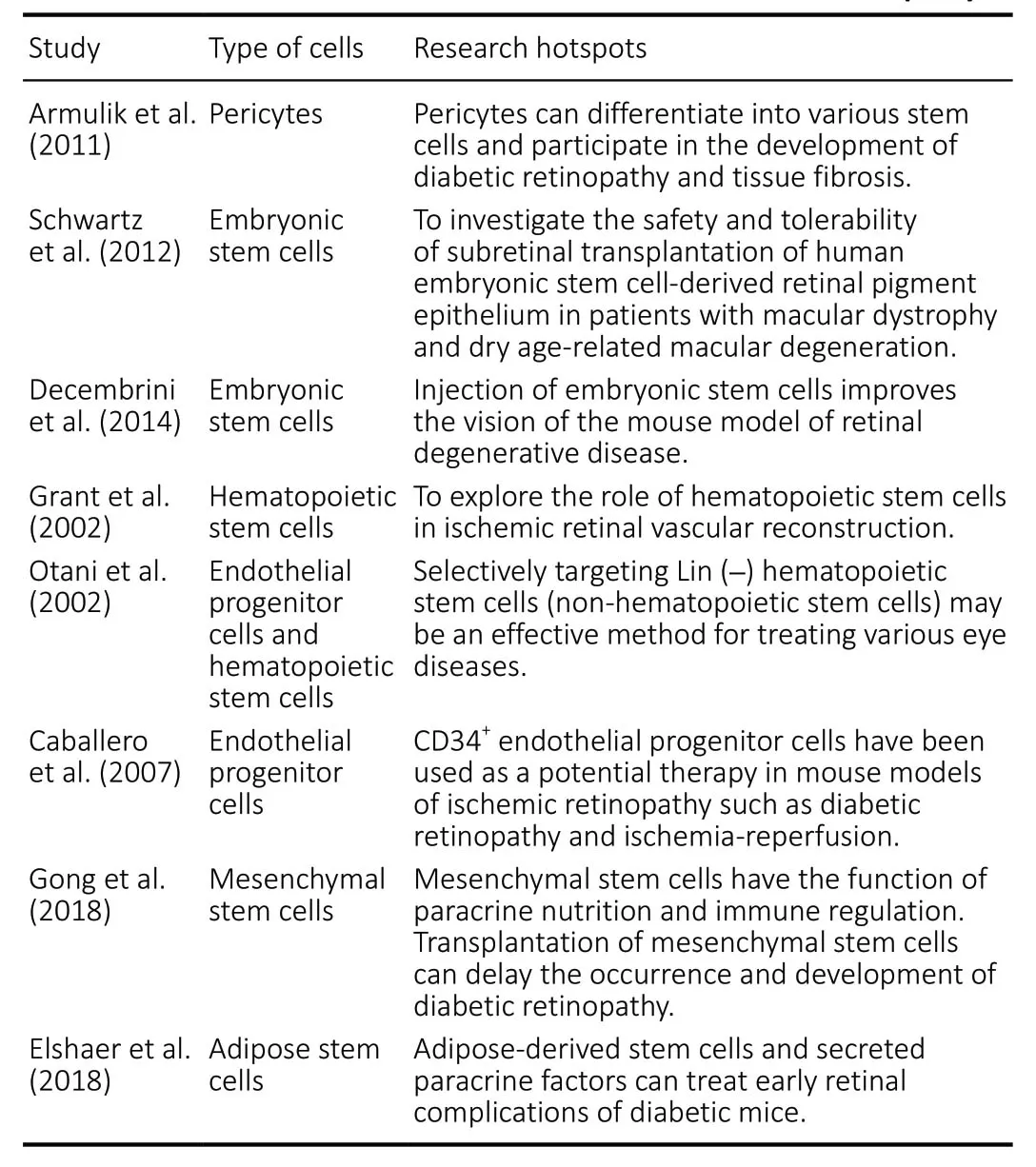
Table 2 |Hotspot analysis of the representative articles, sorted from the 552 included articles regarding stem cell therapy for diabetic retinopathy
Pluripotent stem cells: Pluripotent stem cells have the ability to differentiate into any type of mature cell. Induced pluripotent stem cells are primarily derived from embryonic stem cells.Studies have shown that embryonic stem cells are beneficial for visual acuity improvements in mouse models of retinal degeneration (Decembrini et al., 2014;Table 2). In animal models of diabetes, damage to the tight junctions of the retinal pigment epithelium can destroy the retinal pigment epithelium barrier, indicating that cell replacement therapies using retinal pigment epithelium cells may be important for the treatment of diabetic retinopathy (Xu and Le, 2011).
Co-citation results of the cited references associated with research hotspots in the field of stem cell treatment of diabetic retinopathy
The cited references of the 552 included articles, published from 2000 to 2020, can be divided into 15 cluster categories
A landscape, generated using clusters of co-cited references,presented the following 15 cluster groups, as shown inFigure 2: #0 stem cell, #1 vascular stem cell, #2 laser, #3 health economics, #4 kidney disease, #5 optic nerve, #6 wnt, #7 muller cell, #8 cell death, #9 retinal capillary endothelium,#10 biomaterial, #11 Nrf2, #12 blood-brain barrier, #13 mononuclear phagocytes, and #14 angiotensin.
A timeline view of the cited references in the 552 included articles reveals changes in the citation frequency of classic literature over time
Based on the generated cluster map, a timeline map of cocited references could be obtained, in which the Y-axis is defined as the cluster No. and the X-axis is defined as the publication year. The timeline map showed the time span and research progress of the development and evolution of the 15 identified cluster sub-fields, as shown inFigure 3.
Taking #0 stem cells, inFigure 3,as an example, three highly cited landmark studies emerged from 2009 to 2013, which were the most authoritative studies in the field of stem cell therapy for diabetic retinopathy:
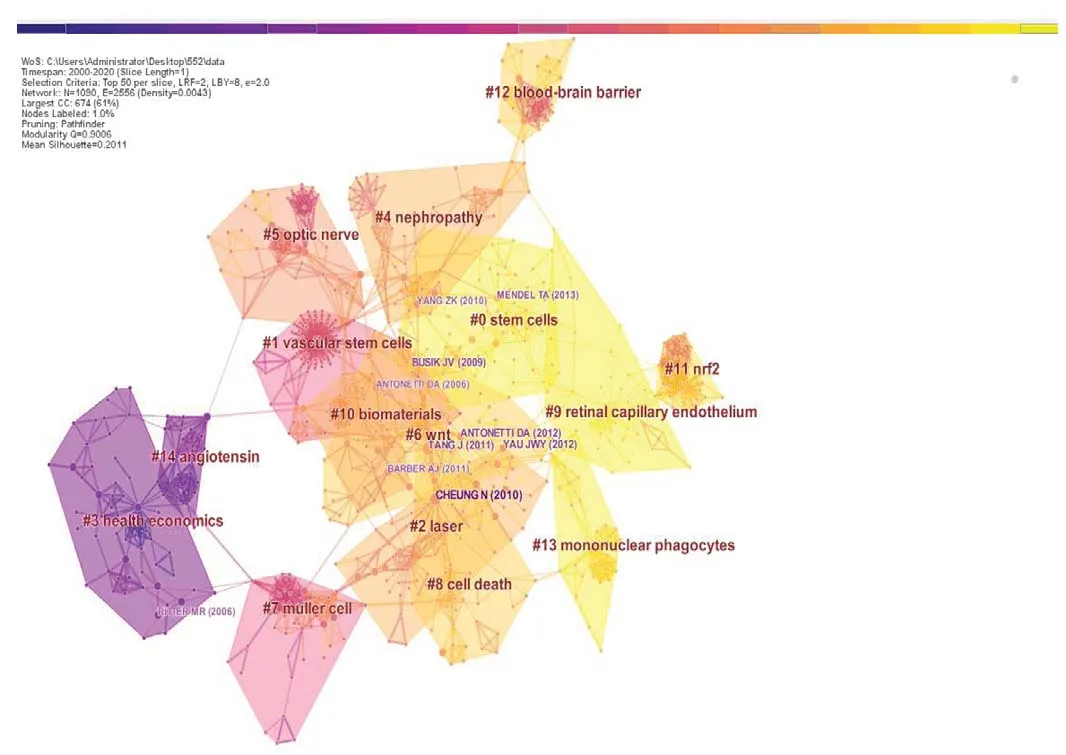
Figure 2 |Reference co-citation network for the 522 included articles(clustered according to index terms).Colors, moving from blue to yellow, indicate the citation time from 2000 to 2020, respectively. Different cluster blocks (# 0-14) are distinguished by different colors. The color of each cluster block indicates the year in which the co-citation relationship first occurred in each cluster. Publications in the blue block were published earlier than those in the green block, and those in the yellow block were published later than those in the green block. Several nodes can be found in each cluster block, and each node represents one reference. The size of each node circle represents the number of times each reference has been cited. The connection lines between nodes indicate the pathways of citation, and the color of each line represents the time of the first citation. Only a few highly co-cited references, such as Cheung et al. (2010)and Busik et al. (2009), are shown, due to the threshold limit.
Busik et al. (2009) reported an animal experiment examining the association between diabetic retinopathy and endothelial progenitor cells and relevant treatment prospects, which has been cited 18 times, including 5 times in 2014 (Figure 4AandB).Robinson et al. (2012) updated several animal models of diabetic retinopathy and introduced relevant cytological features, and this study has been cited 11 times, 5 of which were in 2014 (Figure 4CandD).
Mendel et al. (2013) tested the ability of adipose-derived stem cells to differentiate into pericytes, which stabilize the retinal blood vessels in various animal models of retinal vascular disease. This study has been cited 17 times, including 5 times in 2014, 5 times in 2016, and 5 times in 2018 (Figure 4EandF).
Citation analysis of 10 classic articles, as references cited by the 552 articles
Among the top 10 highly-cited articles, 5 were reviews (Cheung et al., 2010; Barber et al., 2011; Tang et al., 2011; Antonetti et al., 2012; Yau et al., 2012) regarding the pathogenesis, risk factors, and treatment prospects of diabetic retinopathy (Table 3), whereas the other 5 were animal studies that introduced the relationships between diabetic retinopathy and endothelial progenitor cells, derived bone marrow progenitor cells,adherent white blood cells, adipose-derived mesenchymal stem cells, and adipose-derived stem cells (Joussen et al.,2004; Ritter et al., 2006; Busik et al., 2009; Yang et al., 2010;Mendel et al., 2013;Table 3).
Keywords for hotspots regarding the stem cell-based treatment of diabetic retinopathy, over time, based on the burst analysis of high-frequency keywords in the 552 articles
Hot keywords based on the burst analysis of high-frequency keywords in the 552 articles
Among the 552 articles, the top 3 high-frequency keywords were “diabetic retinopathy” (221 times), “endothelial growth factor” (110 times), and “expression” (91 times).“Angiogenesis,” “retina,” “oxidative stress,” “stem cells,”“endothelial progenitor cells,” and “inflammation” ranked 4-10, among which, “stem cells” and “endothelial progenitor cells” appeared 49 times and 42 times, respectively. These findings indicated that relatively more studies were performed to examine the effects of stem cells and endothelial progenitor cells on diabetic retinopathy, involving the expression of vascular endothelial growth factor, vascular regeneration,oxidative stress, and inflammatory reactions (Figure 5).
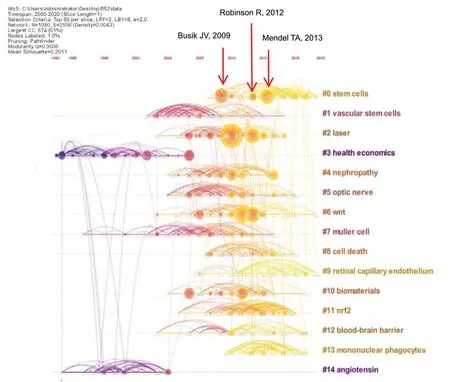
Figure 3 |Timeline view of the citation trends identified in the 552 included articles.The timeline map reveals changes in reference co-citations over time. The circular areas represent citation tree rings, which represent the citation history of each article. The color of the most central area of the citation tree ring represents the year of publication. Each node represents a cited reference. The size of the node indicates the citation frequency. Lines represent relationships between references. The node colors represent different years, which gradually change from cold to warm moving from 2000 to 2020, respectively.Blue color indicates an earlier publication year, and red color indicates the most recent publication year. The longer color line segments indicate largertime spans cited. The cluster labeling on the right shows the research hotspot category associated with each reference. A red annual ring that appears in the node annual rings indicates that the citation frequency increased rapidly or continues to increase rapidly. The distribution of cited time can be judged by the color of each annual ring. On the timeline map, each cluster is arranged in chronological order, and adjacent clusters often correspond to related topics(co-citation between clusters). The flow of knowledge between clusters can also be observed over time (from cool to warm colors).
Two stages for keyword bursting among the 552 articles
As shown inFigure 6, there are two stages for keyword bursting. The first stage: From 2001 to 2012, the primary burst words were neovascularization, cells, endothelial growth factor, central nervous system, gene expression, vitreous fluid,progression, proliferative diabetic retinopathy, endothelial cell,in vivo, and growth factor. “Neovascularization” acted as a burst word from 2001 to 2011, with which continued for 11 years,which indicated that the promotion of neovascularization was the primary research focus for the use of stem cells in the treatment of diabetic retinopathy at this stage, with a focus on genetics and cytology, including the expression characteristics of various growth factors at the mRNA level. The second stage:From 2014 to 2020, the primary hot keywords were mouse model, glaucoma, macular degeneration, diabetic macular edema, neurodegeneration, degeneration, nuclear factor kappa B, and inflammation, each of which had relatively shorter burst times, generally 3-4 years. A mouse model is currently an accepted animal model of diabetic retinopathy. In recent years, studies regarding stem cell therapy in diabetic animals have primarily mostly focused on pathological improvements in macular degeneration, macular edema, neurodegeneration,and inflammation.
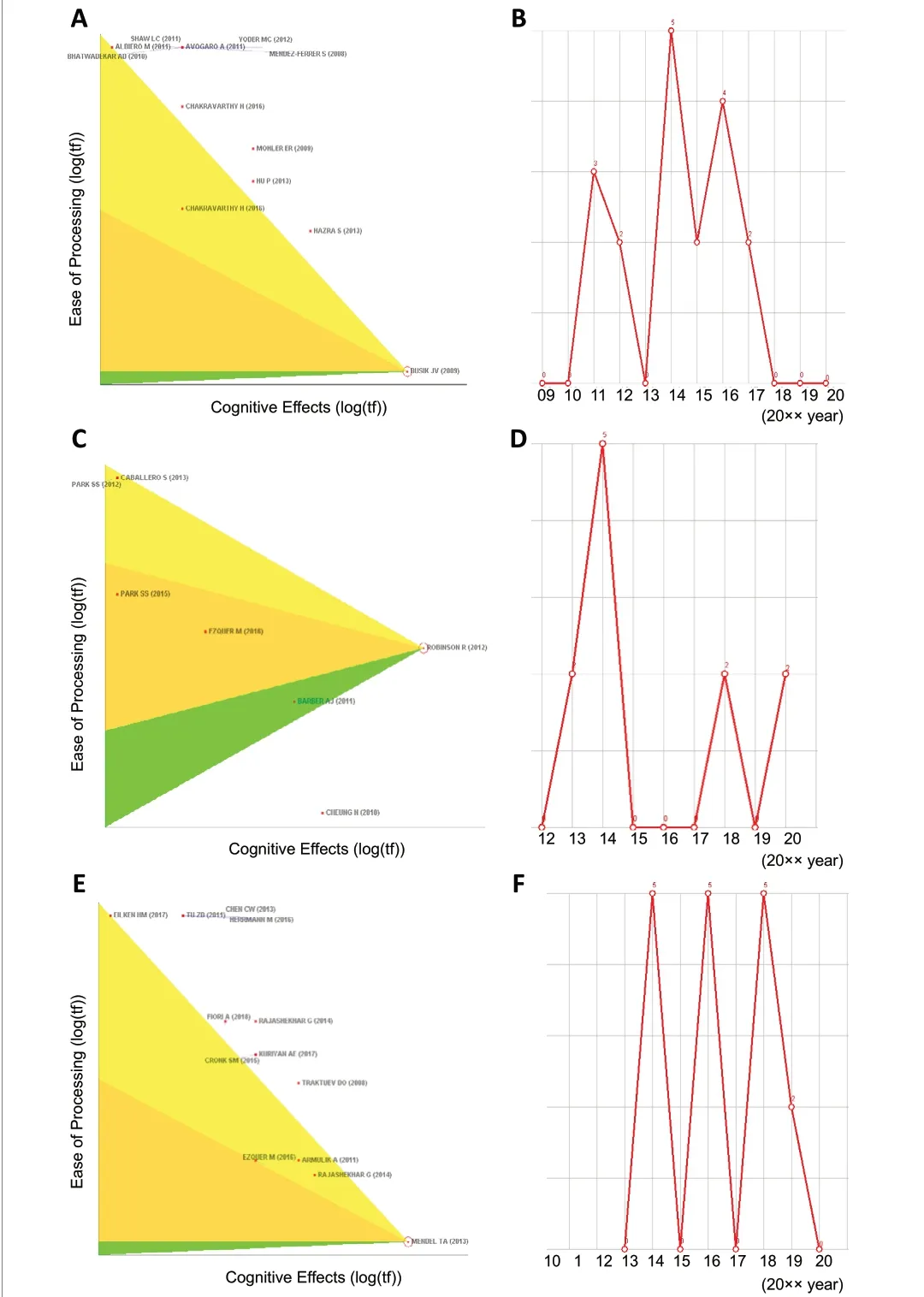
Figure 4 |Co-citation status of three representative articles in the #0 stem cell category in the timeline view.(A, C, and E) The pennant diagrams of Busik et al. (2009), Robinson et al.(2012), and Mendel et al. (2013), respectively, which can be used to view the information for the references directly connected to a node. The figure shows the distribution of other literature that have established citation links with these three representative references. The positions of the references, from top to bottom, demonstrate that the number of citations gradually increases,and the bottom reference has been cited most frequently. (B, D, and F) Thetime trends showing the number of times that Busik et al. (2009), Robinson et al. (2012), and Mendel et al. (2013) are cited, respectively, with the top of the broken line representing the number of citations. All three articles were cited 5 times in 2014, indicating that research on stem cells was relatively concentrated in 2014.
Analysis of clinical research articles on the stem cell-based treatment of diabetic retinopathy
Examples of rare clinical studies from the 552 articles regarding the stem cell-based treatment of diabetic retinopathy
Gu et al. (2018) confirmed the safety and efficacy of intravenous injections of autologous bone marrow mesenchymal stem cells in 17 patients (34 eyes) with diabetic retinopathy; however,no placebo control group was used in this study and the best treatment window and infusion times remain uncertain.
Another clinical study (Liang et al., 2018) observed the safety of subretinal space transplantation using autologous bone marrow mesenchymal stem cells in four patients with proliferative diabetic retinopathy. No complications were observed after cell transplantation, and the transplanted cells played a local anti-inflammatory role. No hyperplasia and circulation improvements were found.
In the clinical trial by Bhattacharya et al. (2017), stem cells derived from fat or bone marrow were directly transplanted into the eye, to repair the damage to the retina caused by age-related macular degeneration and other blinding diseases. Human clinical trials examining the retinal stem cell transplantations of pluripotent stem cells and induced pluripotent stem cells are currently underway. These pluripotent stem cells have the same morphology as photoreceptor cells and retinal pigment epithelial cells (Yoder and Ingram, 2009).
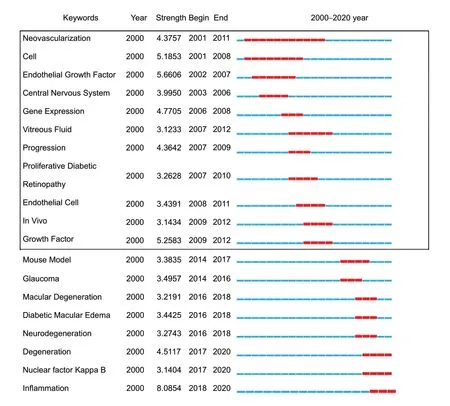
Figure 6 |Time trends of burst keywords included in the 552 articles.Two stages are identified: the range marked by the box indicates the first stage (2000-2009), whereas the second stage is outside the dotted box.“Neovascularization” is the keyword with the highest burst intensity. In the figure, Year represents the earliest year in which the keywords appeared,Strength represents the citation strength, and Begin and End represent thetime when the burst started and ended, respectively.
Analysis of the nine clinical trials examining the stem cell-based treatment of diabetic retinopathy that were registered with ClinicalTrials.gov
Nine clinical trials examining the stem cell-based treatment of diabetic retinopathy were registered on ClinicalTrials.gov, which primarily focused on endothelial progenitor cells (NCT01927315), induced pluripotent stem cells(NCT03403699), hematopoietic stem cells (NCT01972438),embryonic stem cells (NCT02749734 and NCT03046407),autologous CD34+stem cells (NCT03981549), and autologous bone marrow stem cells (NCT03011541, NCT01920867,NCT01736059, and NCT02280135;Table 4).
In an ongoing trial (NCT01736059), the intravitreal injection of CD34+bone marrow mesenchymal stem cells is being used to treat irreversible vision loss caused by retinal degenerative diseases or retinal vascular diseases, including diabetic retinopathy (Table 4).
Another clinical trial (NCT03403699) explored the ability of induced pluripotent stem cells to differentiate into endothelial cells and pericytes in the capillary degeneration region, after diabetic retinopathy (Table 4).
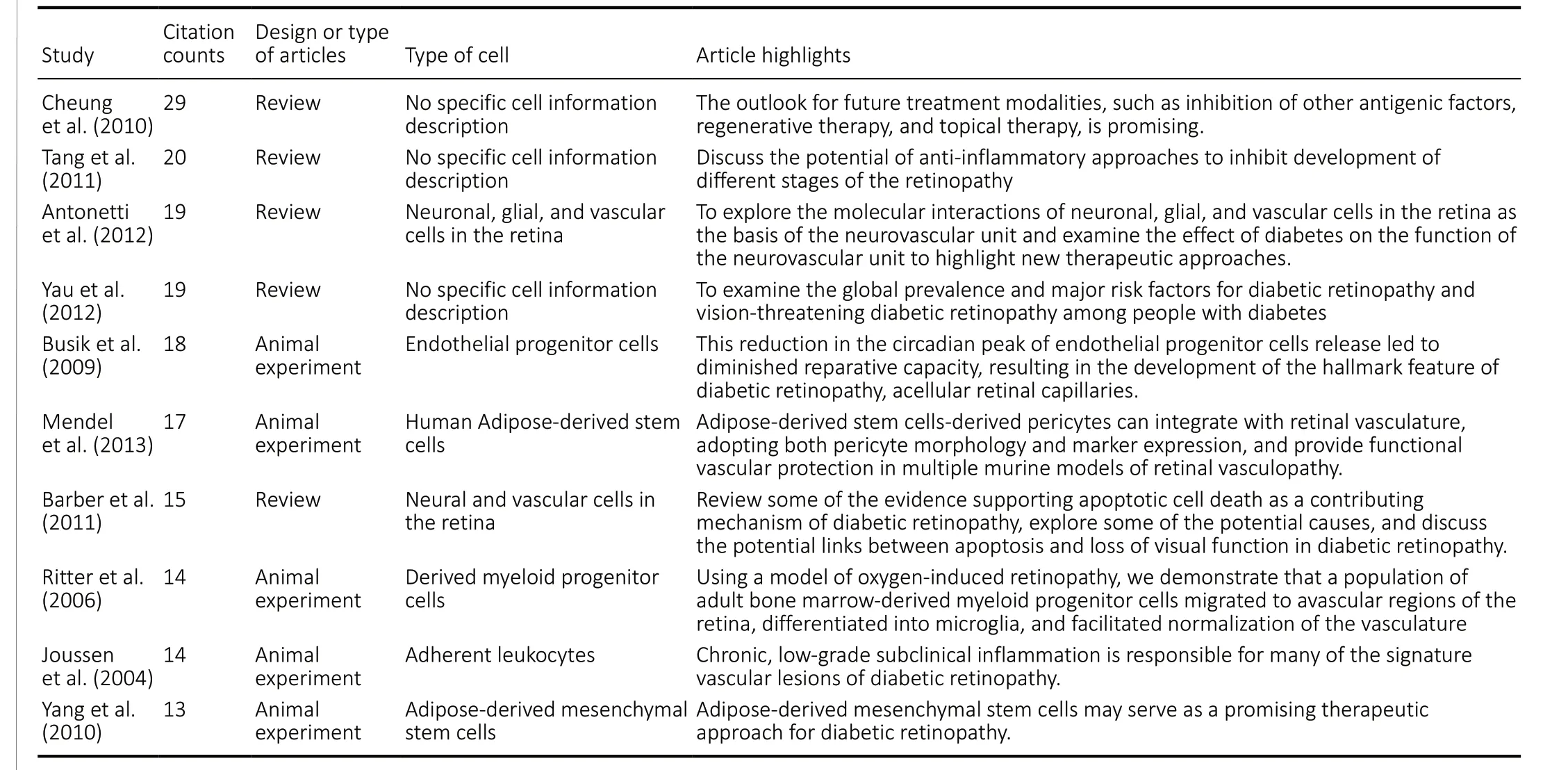
Table 3 |Ten classic articles regarding stem cells and diabetic retinopathy cited by the 552 articles
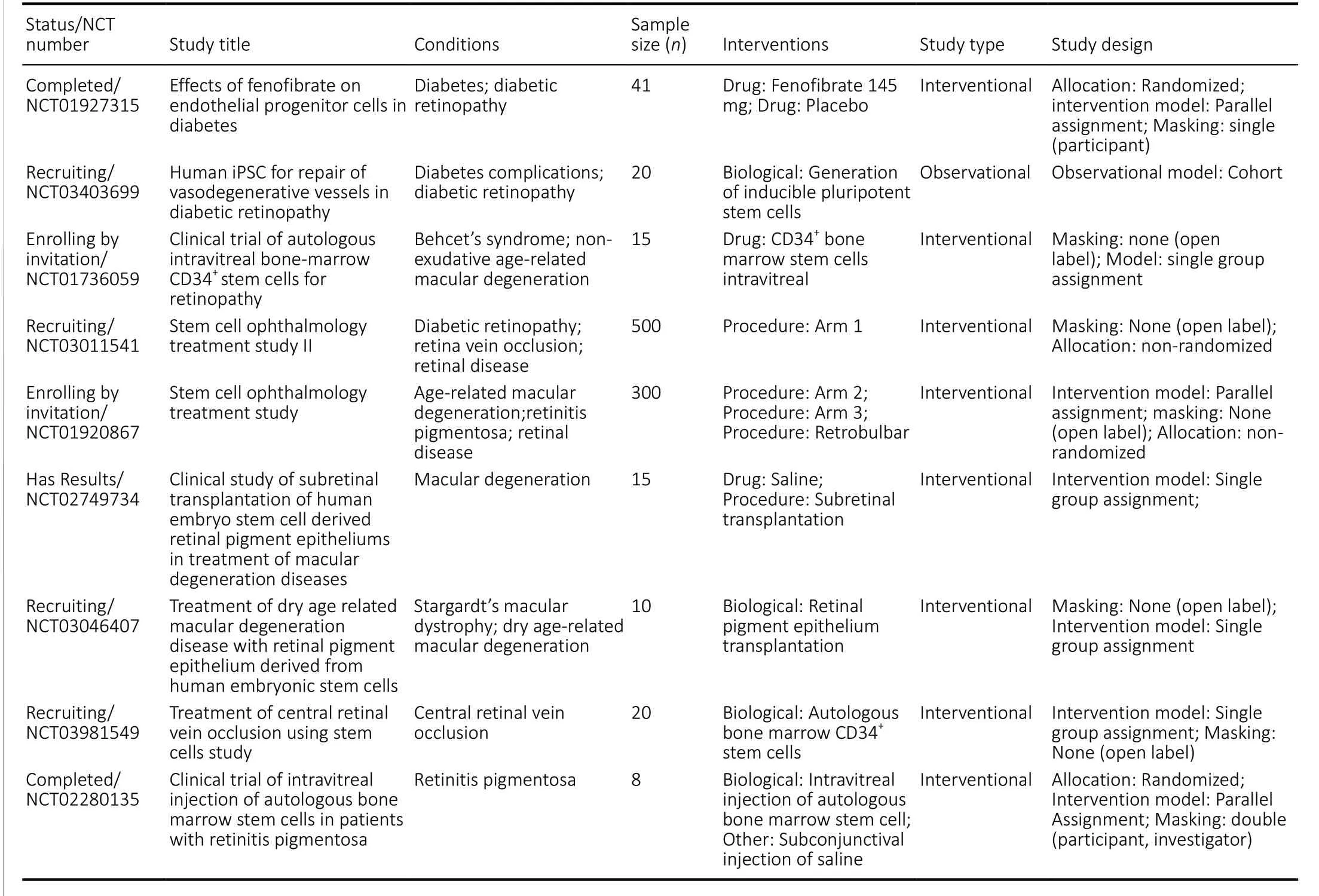
Table 4 |Nine clinical trials exploring the stem cell-based treatment of diabetic retinopathy that were registered with ClinicalTrials.gov
Two prospective non-randomized, controlled trials(NCT03011541, 500 cases included; NCT01920867, 1,000 cases included) investigated the effectiveness of bone marrow mesenchymal stem cell transplantation for retinopathy treatment, including age-related macular degeneration,retinitis pigmentosa, retinal diseases, diabetic retinopathy,retinal vein occlusion, and retinal diseases (Table 4). The results of these trials, which examined relatively large sample sizes, warrant further consideration. The other registered clinical trials included relatively smaller sample sizes and did not present prospective, large sample, randomized, controlled clinical evidence.
Discussion
Current status of research articles on stem cell-based treatments of diabetic retinopathy
Endothelial progenitor cells, mesenchymal stem cells, and hematopoietic stem cells have been found to exert repairing effects on retinal microvascular endothelial cells and pericytes and to inhibit the formation of pathological neovascularization.However, further investigation examining the potential use of other stem cells, such as adult stem cells and induced pluripotent adult stem cells, remains necessary. Currently, few internationally recognized authoritative studies exist examining the stem cell-based treatment of diabetic retinopathy, and most of the published articles are opinion articles, review articles, and animal experiment articles. Most relevant studies remain in the basic research stage, and few representative clinical trials have been documented.
Limitations and challenges
Although tremendous progress has been made in the application of stem cell-based therapies to treat diabetic retinopathy and other diabetic complications, challenges for clinical transformation remain, which must be resolved as soon as possible. Due to the toxic and pathological characteristics of diabetic retinopathy, the affected retina is an unfavorable environment for homing, which represents a primary obstacle for treatment with mesenchymal stem cells.Methods for the exogenous injection of stem cells should be well-clarified to obtain better transplantation results. The limited proliferation and differentiation of certain cell types,such as retinal progenitor cells, represent other obstacles that must be overcome during the development of stem cellbased therapies. Another challenge for the translation of pre-clinical research to applied stem cell-based therapies is the inability of animal models to perfectly represent human conditions. Mismatched clinical endpoints between animal models and humans make predicting the effects of stem cellbased therapies impossible to predict based on existing clinical parameters (Kern et al., 2019). Due to the limitations of the Citespace software, the present article only analyzes the literature indexed in the Web of Science database, whereas other databases, such as PubMed, have not been fully analyzed. Therefore, the 552 articles included in the article only represent the information indexed in the Web of Science and are not yet fully representative of all available information in this field, which is the primary limitation of this article.
Prospects
Stem cell heterogeneity, cell delivery, and the effective homing of stem cells to damaged tissues remain major challenges faced by stem cell transplantations for the treatment of diabetic retinopathy. Some potential clinical trials are ongoing,which primarily focus on the treatment of diabetic retinopathy using pluripotent stem cells, retinal pigment epithelial cells,bone marrow mesenchymal stem cells, and endothelial progenitor cells. Hopefully, high-quality clinical evidence will be obtained in the future.
Author contributions:XJL organized data collection, carried out the analysis,and wrote a first version of this paper. XJL, CYL, DB, and YL supervised data collection and analysis and helped in interpreting the data and writing the paper. XJL and YL designed the study, helped in interpreting the data. All authors approved the final version of this paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the Natural ScienceFoundation of Jilin Province of China, No. 20200201495JC; Jilin Province Health Technology Innovation Project, No. 2017J089. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Information for Authors-Neural Regeneration Research
- Microglia-associated neuroinflammation is a potential therapeutic target for ischemic stroke
- Argon reduces microglial activation and inflammatory cytokine expression in retinal ischemia/reperfusion injury
- Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection
- Nogo-A aggravates oxidative damage in oligodendrocytes
- Deletion of Krüppel-like factor-4 promotes axonal regeneration in mammals