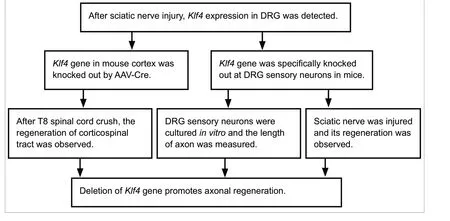
Deletion of Krüppel-like factor-4 promotes axonal regeneration in mammals
2021-08-20JinHuiXuXuZhenQinHaoNanZhangYanXiaMaShiBinQiHongChengZhangJinJinMaXinYaFuJiLeXieSaijilafu
Jin-Hui Xu, Xu-Zhen Qin, Hao-Nan Zhang, Yan-Xia Ma, Shi-Bin Qi,Hong-Cheng Zhang, Jin-Jin Ma, Xin-Ya Fu, Ji-Le Xie, Saijilafu
Abstract Axonal regeneration plays an important role in functional recovery after nervous system damage. However, after axonal injury in mammals,regeneration is often poor. The deletion of Krüppel-like factor-4 (Klf4) has been shown to promote axonal regeneration in retinal ganglion cells. However, the effects of Klf4 deletion on the corticospinal tract and peripheral nervous system are unknown. In this study, using a mouse model of sciatic nerve injury, we show that the expression of Klf4 in dorsal root ganglion sensory neurons was significantly reduced after peripheral axotomy, suggesting that the regeneration of the sciatic nerve is associated with Klf4. In vitro, dorsal root ganglion sensory neurons with Klf4 knockout exhibited significantly enhanced axonal regeneration. Furthermore, the regeneration of the sciatic nerve was enhanced in vivo following Klf4 knockout. Finally, AAV-Cre virus was used to knockout the Klf4 gene in the cortex. The deletion of Klf4 enhanced regeneration of the corticospinal tract in mice with spinal cord injury. Together, our findings suggest that regulating KLF4 activity in neurons is a potential strategy for promoting axonal regeneration and functional recovery after nervous system injury. This study was approved by the Animal Ethics Committee at Soochow University, China (approval No. SUDA20200316A01).
Key Words: axonal regeneration; corticospinal tract; dorsal root ganglion; Klf4; peripheral nervous system; sciatic nerve crush; sensorimotor cortex; spinal cord crushl
Graphical AbstractDeletion of Klf4 gene promotes axonal regeneration after sciatic nerve injury
Introduction
In the mammalian nervous system, the lack of axonal regeneration is the major obstacle to the recovery of neurological function. Experimental approaches to facilitate axonal regrowth in the adult central nervous system include decreasing extracellular inhibitory activity and improving intrinsic growth ability (Moore et al., 2009). Although many molecular mechanisms are involved in the regulation of axonal regeneration, the activation of transcription factors is an important response of neurons to axonal injury, and results in great changes in gene expression (Smith and Skene, 1997;Hanz and Fainzilber, 2006; Michaelevski et al., 2010). These changes are essential for activating regenerative pathways in injured cells (Leppä and Bohmann, 1999; Raivich, 2008). The ensuing changes in the expression of key genes determine whether neurons survive or die after axonal injury (Yang et al., 2007). Early studies demonstrated the presence of transcription-dependent switches that control the ability of adult sensory neurons to grow (Smith and Skene, 1997). A variety of transcription factors in different signaling pathways play a regulatory role in the growth and development of axons (Goldberg et al., 2002a; Raivich et al., 2004; Moore et al., 2009; Ng et al., 2012). Therefore, the regulation of transcription factors is considered a promising method for promoting the regeneration of axons.
Krüppel-like factor-4 (KLF4) is one of 17 members of the KLF family of transcription factors. Most studies on KLF4 have focused on oncology (Kim et al., 2003; Ohnishi et al.,2003; Bianchi et al., 2004; Luo et al., 2004; Ghaleb et al.,2007; Natesampillai et al., 2008; Xu et al., 2008). Apart from regulating cell growth, proliferation and differentiation(Black et al., 2001), KLF4 plays an important role in axonal regeneration (Goldberg et al., 2002b). A study demonstrated that in the nucleus of mammalian neurons, KLF4 inhibits the transcription factors that modify the actin and microtubule cytoskeleton at the growth cone or that transport such growth-enhancing proteins (Subang and Richardson, 2009).
KLF4-deficient retinal ganglion cells show increased axon growthin vitroand after optic nerve injuryin vivo(Moore et al., 2009). However, it remains unclear whetherKlf4gene knockout can promote the regeneration of the corticospinal tract and peripheral nerves. Therefore, in this study, we investigate whether knockout of theKlf4gene promotes the regeneration of corticospinal tract axons and dorsal root ganglion (DRG) sensory neuron axons.
Materials and Methods
Animals
Experimental procedures were approved by the Animal Ethics Committee at Soochow University, China (approval No. SUDA20200316A01). All animals were anesthetized with xylazine (10 mg/kg) and ketamine (100 mg/kg) before surgery.Fifteen 8-10-week-old male Institute of Cancer Research (ICR)mice were used for axotomy. The 4-week-old male and female Advillin-cre mice, the Pirt-cre mice, and the floxedKlf4(Klf4f/f)mice were kindly provided by professor Feng-Quan Zhou and professor Xin-Zhong Dong from The Johns Hopkins University School of Medicine (Baltimore, MD, USA). The Advillin and Pirt promoters are DRG sensory neuron-specific promoters (Lau et al., 2011; Kim et al., 2014) that drive the expression of Cre recombinase in DRG sensory neurons. Mice lacking KLF4 in their DRG sensory neurons were generated by matingKlf4f/fmice with Advillin-cre mice or Pirt-cre mice followed by crossbreeding to produce male and female mice with DRG-specific deletion ofKlf4. Male and femaleKlf4f/fmice were used as controls.
Gene knockout and biotinylated dextran amine labeling
To delete theKlf4gene in the sensorimotor cortex, the adenoassociated virus (AAV)-mediatedin vivoconditional knockout method was employed using a procedure described previously(Liu et al., 2010; Xi et al., 2019). Briefly, 4-week-old femaleKlf4f/fmice were injected with 1.2 µL AAV2/9-CAG-Cre (AAVCre/10082904; Hanbio, Shanghai, China) or AAV2/9-CMV-GFP(AAV-GFP/07031414; Hanbio) into the sensorimotor cortex(Liu et al., 2010) at three points (0.5 mm deep into the cortex;1.5 mm lateral; and 0.5, 0 and -0.5 mm anteroposterior to the bregma) with a 5 µL micro-injector (Hamilton Company,Reno, NV, USA). The injection rate was 1 µL per minute, and the needle was maintained in place for 4 minutes after the injection. After suturing, the mice were placed on a 37°C blanket until fully awake, and spinal cord injury was performed 2 weeks later.
To label the corticospinal tract, we employed Kai Liu’s method(Liu et al., 2010). Briefly, 1.6 µL of biotinylated dextran amine(BDA) (Invitrogen, Carlsbad, CA, USA) (10% in PBS) was injected into four points in the sensorimotor cortex (1.0, 0.5,-0.5 and -1.0 mm anteroposterior to the bregma; 1.5 mm lateral, 0.5 mm deep), as for the virus injection. The mice were then kept alive for 14 days before being killed.
Spinal cord crush
T8 spinal cord crush was carried out 2 weeks after virus injection in femaleKlf4f/fmice, using a procedure essentially as described before (Guth et al., 1985; Fujiki et al., 1996;Inman and Steward, 2003; Liu et al., 2010). In brief, the back was shaved and scrubbed with 75% alcohol. Over the thoracic vertebrae, a 2-cm median incision was made. Laminectomy was performed at T8, and the spinal cord was crushed with No. 5 forceps (RWD Life Technology Co., Ltd., Shenzhen,China) for 1 second, leaving the dura mater intact. At the moment of injury, the mouse twitched once. The muscle and skin were then sutured with 5-0 sutures. The mice were placed on a soft warm blanket at 37°C until totally awake.The mice now exhibited complete paralysis, with their hind legs and abdomen pressed to the ground and their hind toes pointing backwards. Because the bladder could not be emptied spontaneously after spinal cord injury, manual bladder emptying was performed. Male mice were not used in this experiment because of the comparatively long urethra,which is susceptible to urinary tract infection. The bladders were emptied manually twice a day until the mice were able to urinate spontaneously. Corticospinal tracts were labeled with BDA 6 weeks post crush.
Primary culture of sensory neurons
The methods for culturing sensory neurons were described previously (Saijilafu et al., 2013). Briefly, DRGs were isolated fromKlf4f/f,Klf4f/f;Advillin-Cre(Klf4f/f;Avil-Cre) andKlf4f/f;Pirt-Creadult mice and incubated in collagenase I (1.0 mg/mL) at 37°C for 90 minutes. The tissues were then digested with 0.25%trypsin at 37°C for 15 minutes. The digestion was terminated with 10% fetal bovine serum. After the sensory neurons were isolated, 5% fetal bovine serum (diluted with minimal essential medium containing 10 mg/mL streptomycin and 100 IU/mL penicillin) was added. Neurons were plated on coverslips coated with 100.0 µg/mL poly-D-lysine and 10.0µg/mL laminin, and cultured in an incubator (Thermo Fisher Scientific, Waltham, MA, USA) for 3 days at 37°C and 5% CO2.
Western blot assay
DRGs were placed in pre-cooled RIPA lysis buffer (CWBIO,Beijing, China) and kept on ice for 30 minutes to fully lyse.Protein concentration was measured with the bicinchoninic acid assay (Beyotime, Shanghai, China). Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA). The membranes were blocked with 5% skim milk for 1 hour, and then incubated with rabbit anti-KLF4 antibody (1:1000; Abcam,Cambridge, MA, USA) overnight at 4°C. The blots were then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Boston, MA,USA) for 1 hour at room temperature and developed with chemiluminescence reagent (GE Healthcare, Chicago, IL, USA).ImageJ software (National Institutes of Health, Bethesda, MD,USA) was used for protein band quantification. Rabbit anti-GAPDH antibody (1:1000; Cell Signaling Technology) was used as control.
Preparation of sections
The mice were anesthetized with chloral hydrate, and then perfused with 4% paraformaldehyde. The L4-5 DRG, spinal cord and brain tissue were carefully removed and placed in 4% paraformaldehyde at 4°C overnight. Graded dehydration was performed with 15% and 30% sucrose solutions. Spinal cord segments containing the injury site were sliced into serial sagittal sections at a thickness of 25 µm. Brain tissues containing neurons labeled by the cortical BDA injections were sliced into coronal sections in the same manner. DRG tissues for immunofluorescence staining were sliced into 12-µm-thick sections. All sections were stored at -20°C until use.
Immunohistochemical staining
Cultured DRG sensory neurons were fixed with 4%paraformaldehyde for 15 minutes and blocked with 2%bovine serum albumin containing 0.1% Triton X-100 for 1 hour. The tissue was then incubated with primary antibody(neuron-specific class III β-tubulin mouse mAb (Tuj1),1:1000; Biolegend, San Diego, CA, USA) for 1.5 hours at room temperature. The tissue was then incubated with Alexa Fluor®488 goat anti-mouse IgG (1:1000; Life Technologies, Carlsbad,CA, USA) for 1 hour at room temperature, and mounted with Mowiol®(EMD Millipore, Billerica, MA, USA).
For KLF4 labeling, DRG sections were blocked with 10% fetal bovine serum containing 0.2% Triton X-100 for 60 minutes,and then incubated with primary antibody (anti-KLF4, rabbit,1:400; ab129473, Abcam) at 4°C overnight. The sections were then incubated with Alexa Fluor® 594 goat anti-rabbit IgG (1:1000; Life Technologies) at room temperature for 60 minutes. Sections incubated with anti-βIII tubulin antibody(mouse, 1:1000; Biolegend) were processed in the same manner and mounted with Mowiol®.
Immunohistochemistry of brain and spinal cord sections was performed to observe BDA staining of neurons and the corticospinal tract to assess regeneration in mice with klf4 gene knockout in the cortex. Brain and spinal cord sections were incubated with Cy3-conjugated streptavidin (1:500; 016-160-084, Invitrogen) for 3 hours, and then with DAPI for 15 minutes, and mounted with Mowiol®.
Electroporation of DRGsin vivoand sciatic nerve crush
The electroporation procedure was described in detail in our previous study (Xi et al., 2019). Briefly, after anesthesia, the lower back skin was cut approximately 3 cm, and the left L4-5 DRG was fully exposed. A 1.0-µL volume of enhanced green fluorescent protein (EGFP) plasmid (5.0 µg/µL; Gemma Gene,Shanghai, China) was injected into the DRG with a stretched capillary glass tube (Warner Instruments, Hamden, CT,USA). The DRG was then clamped with a custom forceps-like electrode and electroporated with an ECM830 electroporation apparatus (BTX, Holliston, MA, USA) (15 ms pulse, 35 V, and 960 ms interval). After suturing the skin, the mice were fed normally for 2 days. Then, under anesthesia, the skin of the left femur was cut approximately 2 cm to fully expose the sciatic nerve, which was crushed with No. 5 forceps (RWD Life Technology Co., Ltd.). The wound was then closed with 5-0 nylon epineural suture. Then, 3 days later, the sciatic nerve was removed after perfusion with 4% paraformaldehyde and post-fixed in the same solution overnight. The sciatic nerve was then placed on a glass slide and mounted with Mowiol®under a glass coverslip. Using a fluorescence microscope, the length of the EGFP-labeled axon from the tip to the lesion site was manually measured in the whole-mount tissue using AxioVision 4.72 software (Carl Zeiss Micro Imaging, München,Germany). At least 15 axons were measured in each sciatic nerve, and the average length was calculated.
Measurement of axon lengthin vitro
AxioVision 4.72 software was used to manually measure the longest axon length of DRG neurons. The longest axon length greater than twice the cell body diameter was included in the statistical analysis. The axon lengths of 100 DRG neurons were randomly measured for each condition. Average axon length and the longest axon length were obtained from at least three separate experiments.
Axonal counting and quantification
Fluorescence microscopy (Carl Zeiss Micro Imaging) was used to analyze the fibers labeled by BDA in T8-crushed mice.The number of intersections of BDA-labeled fibers with the dorsoventral line was calculated at a specified distance on the caudal side of the lesion. The number of intersections was normalized to the number of corticospinal tract (CST) fibers labeled in front of the injury site, and an average value was obtained. Thus, the numbers of labeled CST axons per section at different distances (fiber number index) were obtained. At least three sections of the main dorsal CST were counted for each mouse.
Statistical analysis
The data are expressed as the mean ± SEM. One-samplet-test or independent samplet-test was used to assess significance using GraphPad Prism 5.0 software (GraphPad Software,San Diego, CA, USA). A value ofP< 0.05 was considered statistically significant.
Results
Production of mice withKlf4gene knockout in DRG sensory neurons
Klf4f/f;Avil-CreandKlf4f/f;Pirt-Cremice had selective knockout of theKlf4gene in DRG sensory neurons (Figure 1A),confirmed by DRG immunohistochemical staining (Figure 1B).In mice withKlf4knockout, the KLF4 protein content in the DRG was very low, as demonstrated by western blot assay,further confirming that the deletion was successful (Figure 1CandD) inKlf4f/f;Avil-Cremice (P= 0.0048) andKlf4f/f;Pirt-Cremice (P= 0.0059).
Peripheral axotomy reduces KLF4 levels in sensory neurons
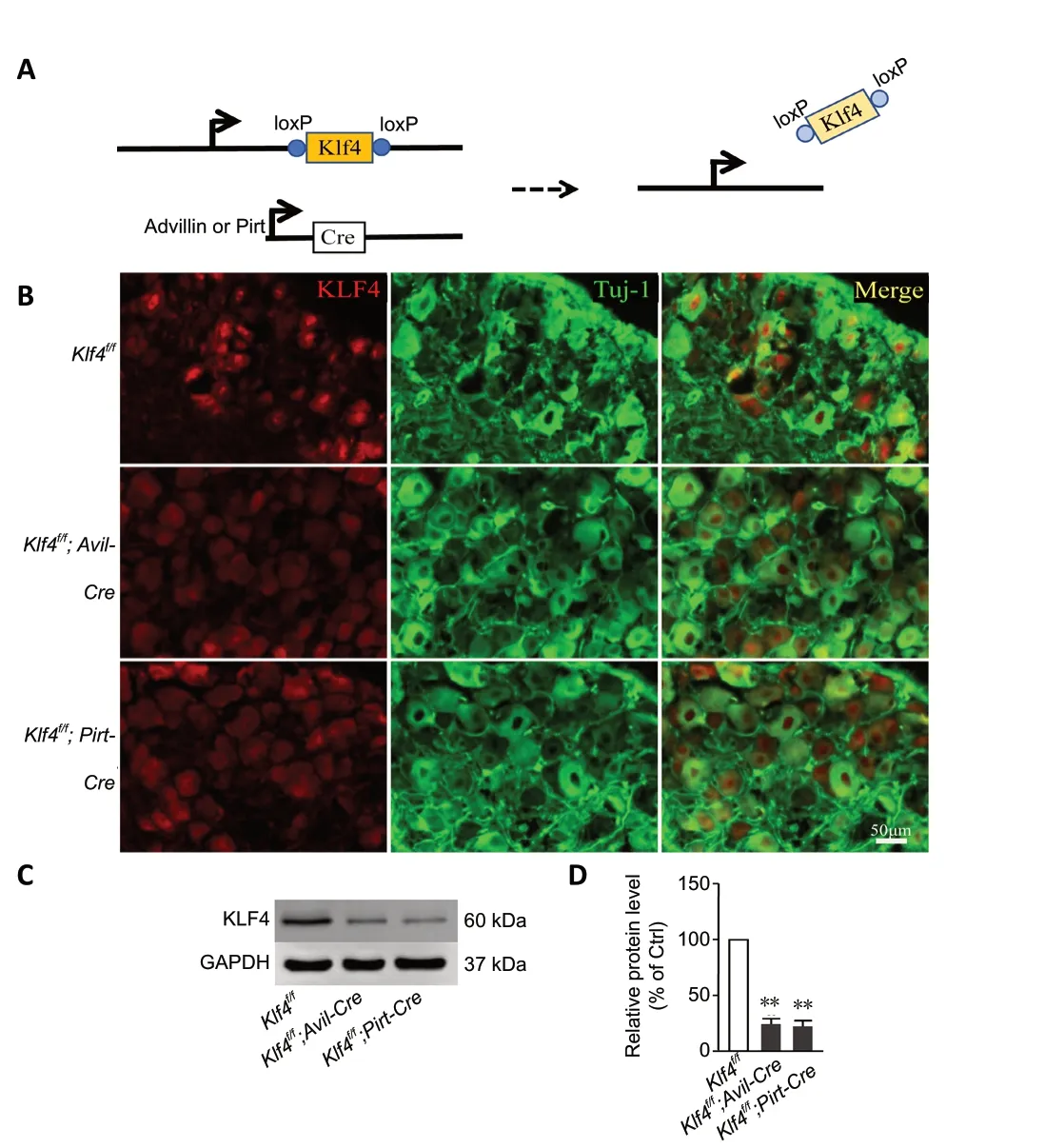
Figure 1 |Successful production of mice with Klf4 gene knockout in the DRG.(A) Schematic of the constructs for conditional knockout of Klf4 using the Cre-loxP system in DRG sensory neurons. (B) Representative images of immunofluorescence staining of DRG tissues in the three different groups of mice. In DRG sections from Klf4f/f mice, KLF4 protein was localized in the nucleus (red). In Klf4f/f;Avil-Cre mice and Klf4f/f;Pirt-Cre mice, KLF4 protein was nearly undetected. Scale bar: 50 µm. (C, D) Western blot assay showing that the expression of KLF4 in the DRG was substantially reduced in Klf4f/f;Avil-Cre and Klf4f/f;Pirt-Cre mice, indicating successful knockout of Klf4. Data are expressed as the mean ± SEM (n = 3). **P < 0.01 (independent sample t-test).DRG: Dorsal root ganglion; KLF4: Krüppel-like factor-4.
It has been shown thatKlf4knockout promotes the regeneration of retinal ganglion cells (Moore et al., 2009).Peripheral nerve axotomy can cause changes in the expression of genes related to axonal regeneration. Therefore, we measured the expression of KLF4 in L4-5 DRG tissue after sciatic nerve crush. Immunofluorescence staining of frozen sections of DRG tissue 7 days after nerve damage showed that the expression of KLF4 in sensory neurons was significantly lower than that in uninjured DRG neurons (Figure 2A).Moreover, western blot assay revealed that KLF4 expression levels decreased after sciatic nerve injury. The protein levels dropped to their lowest level on day 7 (P= 0.0046) and then increased (P= 0.0247) (Figure 2BandC), suggesting that KLF4 may play a potential role in peripheral sensory neuron regeneration.
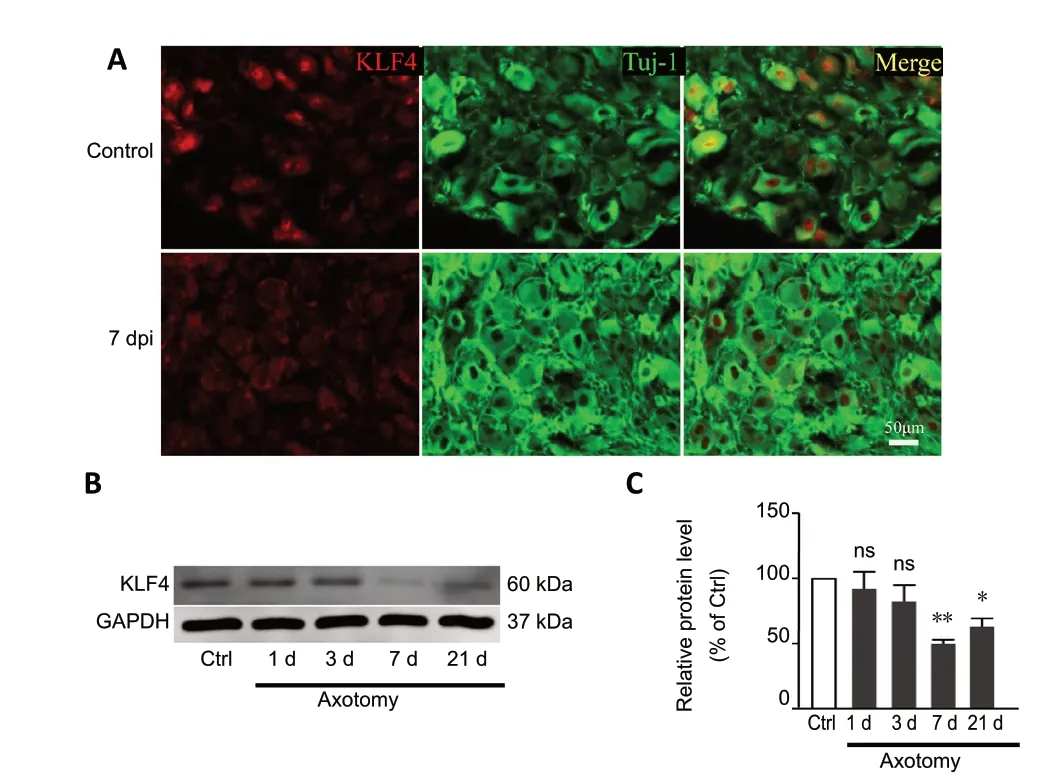
Figure 2 |Peripheral axotomy reduces KLF4 levels of sensory neuron.(A) Immunohistochemical staining showing that KLF4 expression in the DRG is decreased 7 days after sciatic nerve injury, compared with the control group.dpi: Days post injury; green: Tuj1; red: KLF4. Scale bar: 50 µm. (B, C) Western blot assay showing that KLF4 expression is decreased in DRGs 7 days after sciatic nerve crush. Data are expressed as the mean ± SEM (n = 3). *P < 0.05,**P < 0.01, vs. control group (independent sample t-test). Ctrl: Control; KLF4:Krüppel-like factor-4; ns: non significant.
Deletion ofKlf4promotes axonal regeneration of mature peripheral sensory neuronsin vitro
Three different DRG sensory neurons from adult mice were culturedin vitrofor 3 days. The length of regenerating axons was significantly greater in mice withKlf4knockout compared with control (Klf4f/f;Avil-Cre:P= 0.0045;Klf4f/f;Pirt-Cre:P=0.0006) (Figure 3AandB). In mice with Klf4 knockout, the longest axon in each individual experiment was also longer than in the controls (Klf4f/f;Avil-Cre:P= 0.0376;Klf4f/f;Pirt-Cre:P=0.0119) (Figure 3C). Similar results were obtained with deletion driven by the Advillin and Pirt promoters. Together, these results show that Klf4 inhibits axonal regeneration in peripheral sensory neurons.
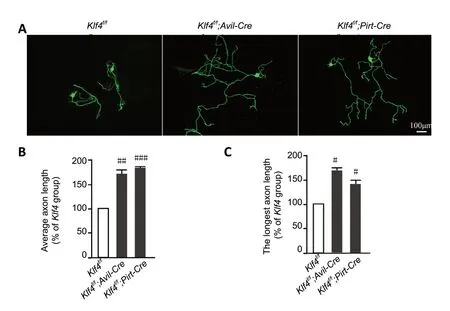
Figure 3 |Deletion of Klf4 promotes axonal regeneration in mature DRG sensory neurons in vitro.(A) Immunofluorescence staining of sensory neurons cultured for 3 days.Deletion of Klf4 promotes axonal regeneration in peripheral sensory neurons in vitro. In Klf4f/f;Avil-Cre and Klf4f/f;Pirt-Cre mice, the regenerated DRG sensory neuron axons were longer than those in Klf4f/fmice. Neurons were stained for Tuj1. Scale bar: 100 µm. (B, C) The average axon length (B) and longest axon length (C) of sensory neurons with Klf4 deletion are greater than control. Data are expressed as the mean ± SEM (n = 3). #P < 0.05, ##P < 0.01, ###P < 0.001,vs. Klf4f/f group (independent sample t-test). DRG: Dorsal root ganglion; KLF4:Krüppel-like factor-4.
Deletion ofKlf4promotes axonal regeneration in DRG sensory neuronsin vivo
To study the regeneration of DRG sensory neurons in mice withKlf4knockoutin vivo, DRG electroporation was used to introduce EGFP into adult mouse L4-5 DRGs. Sciatic crush was performed two days later, and perfusion was performed three days later. Consistent with thein vitroresults,Klf4knockout promoted peripheral nerve regenerationin vivo(Klf4f/f;Avil-Cre:P= 0.0009;Klf4f/f;Pirt-Cre:P= 0.0007) (Figure 4AandB).
Klf4knockout promotes the regeneration of the CST in mice with complete T8 crush
Complete crush of the spinal cord destroys all neural fibers at the injury site and is considered a specific barrier to regeneration (Inman and Steward, 2003). In this model of spinal cord injury, the dura remains intact, and the spinal cord remains connected. At 4 weeks of age, femaleKlf4f/fmice were injected with AAV-Cre or AAV-GFP into the sensorimotor cortex using optimized stereotactic injection, followed by T8 crush 2 weeks later. Two weeks before sacrifice, the CST was labeled with BDA (Figure 5A). Eight weeks after crush, the six AAV-GFP control mice did not show any CST labeling beyond the injury site. In contrast, all six AAV-Cre-injected mice had partial corticospinal axon growth across the injury site (Figure 5CandD). Western blot assay confirmed that the mouse corticalKlf4gene was deleted by AAV-Cre injection (Figure 5B).
Discussion
Our results suggest thatKlf4deletion causes a distinct regenerative response in injured adult corticospinal neurons.This is uncommon in the spinal cord of mammals. The regeneration of mammalian axons is a very complex process.The growth of adult mammalian nervous system axons is often poor, especially in the central nervous system (Xi et al., 2019).Our current study shows thatKlf4knockdown promotes peripheral nerve regeneration, bothin vivoandin vitro. The DRG sensory neuron is located in the periphery, while the cortex is located in the center. The effects ofKlf4deletion on sensory and motor neurons cannot be compared because of the different environments (Liu et al., 2010). A previous study showed thatKlf4knockout promotes axonal regeneration in retinal ganglion cells (Moore et al., 2009).
The regenerative ability of injured neurons is affected by both internal and external factors. Therefore, the deletion ofKlf4coupled with other strategies, such as reducing environmental inhibitors at the site of injury (Houle et al., 2006; García-Alías et al., 2009) and spanning the injury site with grafts (Pearse et al., 2004; Alto et al., 2009), may promote axonal regeneration better after spinal cord lesion.
HowKlf4knockout promote axonal regeneration?Klf4was first identified as a factor associated with growth arrest (Shields et al., 1996). In actively proliferating NIH3T3 cells, KLF4 levels are minimal, but are significantly increased when growth is inhibited by contact inhibition or serum starvation (Zhang et al., 2000). Continuous overexpression ofKlf4blocks the cell cycle in multiple cell lines (Chen et al., 2001; Dang et al.,2003). Indeed, mutations or downregulation are frequently observed in neoplasms (McConnell et al., 2007). Multicellular organisms evolved from single-celled organisms, and singlecelled organisms have an extremely powerful ability to proliferate. Every cell in a multicellular organism has the potential to proliferate, but this ability is inhibited by various factors, includingKlf4. Mature neurons are non-dividing cells.Although knocking outKlf4removes one source of inhibition,other factors that lead to the failure of mature neurons to proliferate still remain, which may be manifested as the enhancement of axonal regeneration ability.
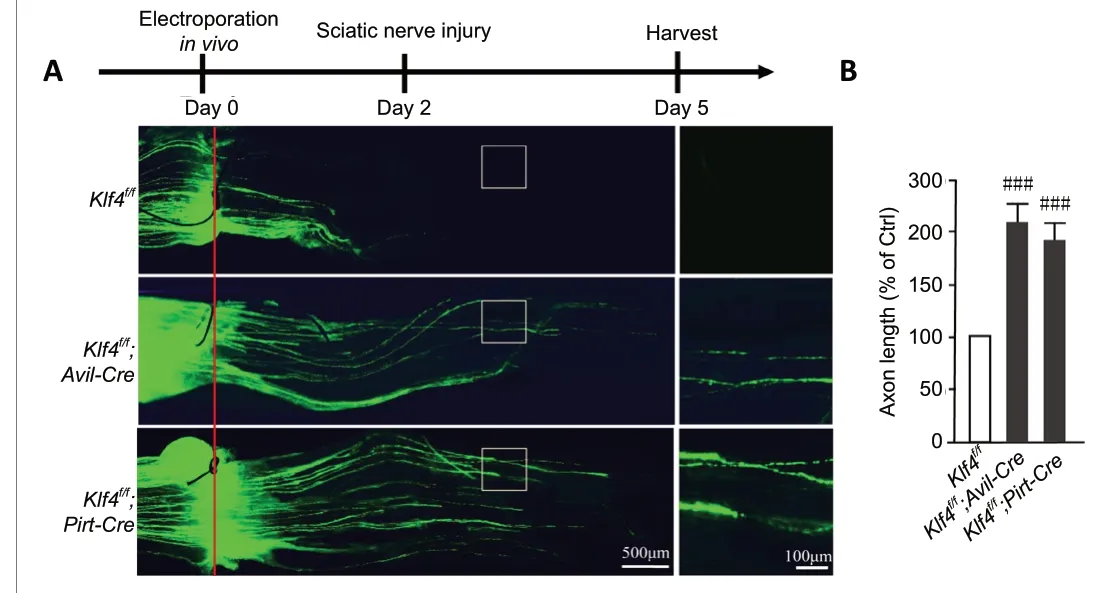
Figure 4 |Deletion of Klf4 promotes axonal regeneration in DRG sensory neurons in vivo.(A) Representative images showing that deletion of Klf4 in DRG neurons significantly enhances axonal regrowth in vivo. In Klf4f/f;Avil-Cre and Klf4f/f;Pirt-Cre mice, the regenerating sciatic nerve was significantly longer compared with the control group. The solid red line indicates the lesion site. Scale bars:500 µm or 100 µm. (B) Measurement of average axon length of regenerated fibers. Deletion of Klf4 in sensory neurons promotes axonal regeneration in vivo. Data are expressed as the mean ± SEM (n = 6; independent sample t-test).###P < 0.001, vs. Klf4f/f group. Ctrl: Control; DRG: dorsal root ganglion; KLF4:Krüppel-like factor-4.
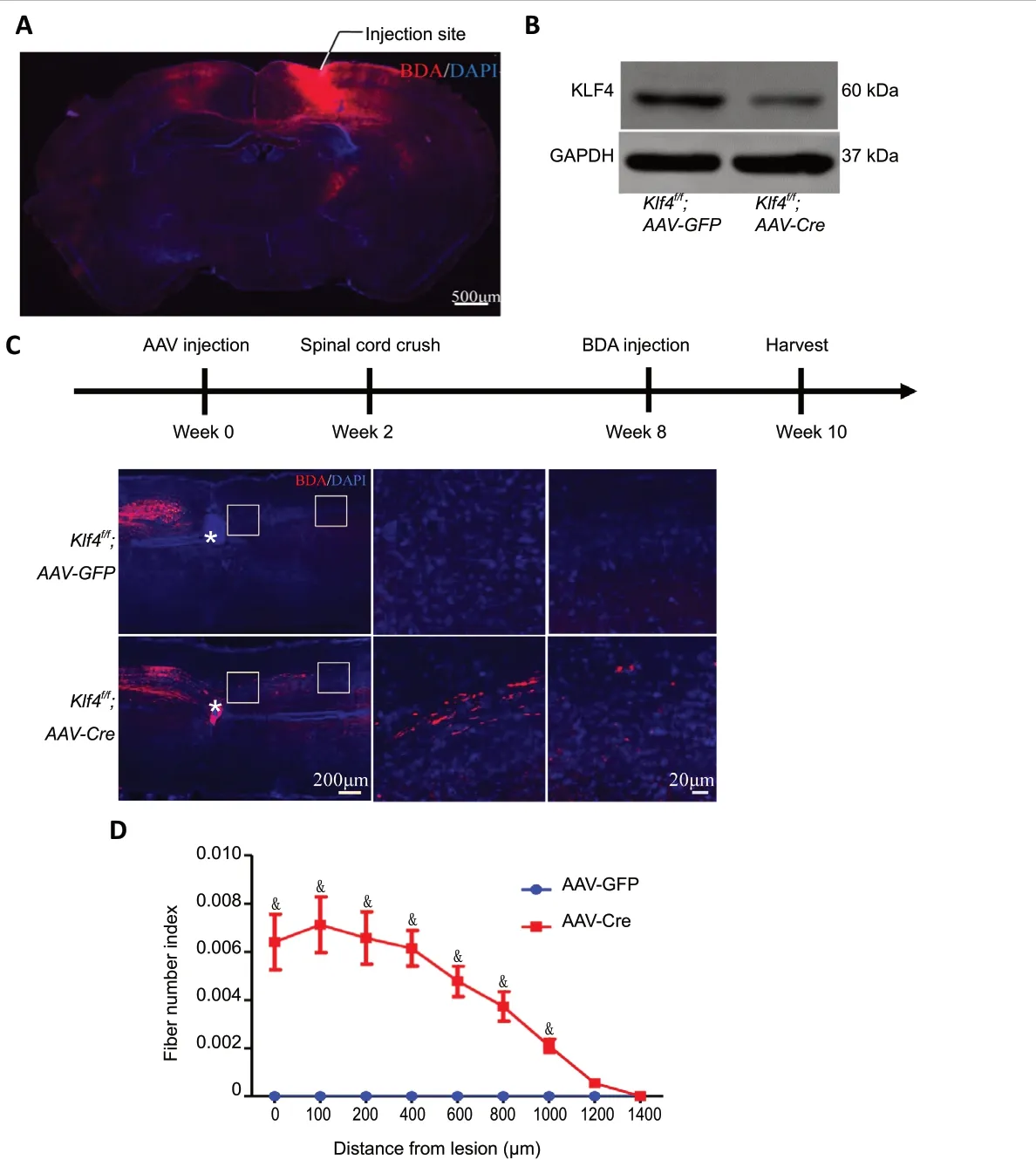
Figure 5 |Klf4 knockout promotes the regeneration of the corticospinal tract (CST) in mice with complete T8 crush.(A) BDA staining of coronal sections of the Klf4f/f mouse brain. The solid line indicates the injection site. BDA (red); DAPI (blue). Scale bar: 500 µm. (B) Images of western blot assay of Klf4f/f mice with AAV injection at 4 weeks of age and sacrificed at 14 weeks of age. (C) Female Klf44f/f mice were injected with AAV at 4 weeks of age and T8 spinal cord crush was performed at 6 weeks of age. BDA was injected into the sensorimotor cortex 6 weeks after injury,and the mice were sacrificed 2 weeks later. Representative images of sagittal sections showing the main dorsal CST. In Klf4f/f;AAV-Cre mice, the regenerated corticospinal tract crosses the site of injury. In Klf4f/f;AAV-GFP mice, the corticospinal tract did not grow beyond the site of injury. The white asterisk indicates the location of the spinal cord injury. BDA (red); DAPI (blue). Scale bars: 200 µm or 20 µm. (D) The number of labeled fibers caudal to the lesion site in the different groups. Data are expressed as the mean ± SEM (one-sample t-test). Six mice were used from each group. Three sections from each mouse were quantified. &P < 0.05, vs. AAV-GFP group. AAV: Adeno-associated virus; DAPI: 4′,6-diamidino-2-phenylindole; KLF4: Krüppel-like factor-4.
The molecular mechanisms behind tumor growth and proliferation may be used to repair damaged neurons (Yang and Yang, 2012). Phosphatase and tensin homolog (PTEN)is a tumor suppressor, and its deletion has been shown to promote axonal regeneration (Park et al., 2008). KLF4 is also a potential tumor suppressor. It has been reported that PTEN directly dephosphorylates KLF4 in vascular smooth muscle cells (He et al., 2015). However, the relationship between PTEN and KLF4 in the nervous system remains unclear.
There are several limitations of this study. KLF4 activators and inhibitors were not used to verify the effect of KLF4 on the regeneration of DRG sensory neurons, because there are no KLF4-specific inhibitors or activators. In addition, in the experiment on corticospinal tract regeneration in mice withKlf4knockout in the sensorimotor cortex, we did not examine whether a similar effect is found in elderly mice. Furthermore,the molecular mechanism by whichKlf4knockout promotes axonal regeneration was not thoroughly investigated. The effect ofKlf4knockout on anterior horn motor neurons in the spinal cord also remains unclear. Axons of motor neurons in the anterior horn of the spinal cord run parallel to DRG sensory neurons in the sciatic nerve, and therefore, the effects ofKlf4knockout on both of these cell types should be examined. Further study is needed to more fully elucidate the role of KLF4 in neural regeneration.
In summary, KLF4 regulates the regeneration of peripheral sensory axonsin vitroandin vivo. KLF4 also regulates the regeneration of the corticospinal tract. Our findings should be of great importance for the treatment of nerve injury.
Acknowledgments:We thank profession Feng-Quan Zhou and profession Xin-Zhou Dong from The Johns Hopkins University School of Medicine,Baltimore, MD, USA for providing the Advillin-cre mice, Pirt-cre mice and floxed Klf4 (Klf4f/f) mice.
Author contributions:Experimental implementation: JHX, XZQ and HNZ;manuscript writing: JHX and Saijilafu. All authors designed the experiment and approved the final version of the paper.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81571189, 81772353 (to Saijilafu).The funder had no roles in the study design, conduction of experiment,data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:All experimental procedures and protocols were approved by the Animal Ethics Committee at Soochow University, China (approval No. SUDA20200316A01). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Hamid Mobasheri, University of Tehran, Iran;Hedong Li, Pennsylvania State University, USA.
Additional file:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Information for Authors-Neural Regeneration Research
- Microglia-associated neuroinflammation is a potential therapeutic target for ischemic stroke
- Argon reduces microglial activation and inflammatory cytokine expression in retinal ischemia/reperfusion injury
- Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection
- Nogo-A aggravates oxidative damage in oligodendrocytes
- Insights into stem cell therapy for diabetic retinopathy:a bibliometric and visual analysis