Inhibition of mTOR signaling by rapamycin protects photoreceptors from degeneration in rd1 mice
2021-08-16Jia-LiangYang,Tong-DanZou,FangYang等
Retinitis pigmentosa (RP) is an inherited retinal degenerative disease that begins with defective rod photoreceptor function,followed by impaired cone function,and complete blindness in its late stage.To date,however,there is no effective treatment for RP.By carrying a nonsense mutation in thePde6bgene,rd1mice display elevated cGMP in conjunction with higher intracellular Ca2+in their rod photoreceptors,resulting in fast retinal degeneration.Ca2+has been linked to activation of the mammalian target of rapamycin (mTOR) pathway.The mTOR pathway integrates extracellular and intracellular signals to sense the supply of nutrients and plays a central role in regulating protein and lipid synthesis as well as apoptosis and autophagy.In the present study,we showed that mTOR and phosphorylated mTOR (p-mTOR,activated form of mTOR)are up-regulated inrd1photoreceptors at postnatal day 10(P10),a pre-degenerative stage.Moreover,the downstream effectors of mTOR,such as pS6K and S6K,are also increased,suggesting activation of the mTOR signaling pathway.Intravitreal administration of rapamycin,a negative regulator of mTOR,inhibits the mTOR pathway inrd1photoreceptors.Consequently,the progression of retinal degeneration is slower and retinal function is enhanced,possibly mediated by activation of autophagy in the photoreceptors. Taken together,these results highlight rapamycin as a potential therapeutic avenue for retinal degeneration.
RP is a devastating retinal disease that begins with impaired functions of the rod photoreceptors and subsequently affects the cone photoreceptors,ending with complete blindness in the late stage (Dias et al.,2018;Hartong et al.,2006).Over 70 genes are known to be associated with RP (Dicarlo et al.,2018),highlighting the complexity of this disease. To understand the underlying mechanism of RP and explore potential treatment strategies,numerous engineered mouse models have been generated (Collin et al.,2020).Additionally,dozens of inbred mouse lines harboring spontaneous mutations are a great resource for the study of retinal degeneration.Therd1mouse model,with a spontaneous nonsense mutation inPde6b,was developed as early as 1924(Keeler,1924,1966) and is still widely used in studies on retinal degeneration (Pittler & Baehr,1991;Pittler et al.,1993).
Pde6bencodes the beta subunit of rod PDE6.As a key enzyme in the phototransduction cascade in rod photoreceptors,PDE6 catalyzes the hydrolysis of cGMP to GMP.Moreover,PDE6 is required for maintaining cGMP homeostasis in dark-adapted photoreceptors (Doshi et al.,1985;Ferrendelli & Cohen,1976),essentially in two ways:(1) non-stimulating stochastic activation of PDE6 can hydrolyze cGMP by thermal activation (Rieke & Baylor,1996)and (2) non-catalytic cGMP binding domains within alpha and beta subunits sequester a significant fraction of free cGMP(Gillespie & Beavo,1989).Inrd1mice,thePde6bloss-offunction mutation results in elevated levels of free cGMP in the rod photoreceptors.Increased cGMP concentration causes more cGMP-gated channels to open,allowing an influx of calcium into the photoreceptors (Arshavsky et al.,2002).Excess calcium is toxic to photoreceptor cells and induces the rapid degeneration of rod photoreceptors.At three weeks after birth,nearly all rod photoreceptors in therd1retina have degenerated (Farber,1995).The mechanism behind this rapid degeneration is not fully understood.
Calcium ion is a messenger that mediates multiple intracellular processes.Elevated calcium released from the lysosome can activate the mTOR signaling pathway,which is mediated by calmodulin (Li et al.,2016).mTOR,a conserved serine/threonine protein kinase,is the central component shared by two protein complexes:mTORC1 and mTORC2(Loewith et al.,2002;Reinke et al.,2004).mTORC1 consists of the regulatory-associated protein of mTOR (Raptor) (Hara et al.,2002;Kim et al.,2002),whereas mTORC2 includes the rapamycin-insensitive companion of mTOR (Rictor) (Jacinto et al.,2004;Sarbassov et al.,2004).mTORC1 is a hub that integrates extracellular and intracellular signaling.It plays a central role in promoting cell proliferation,protein synthesis(Beretta et al.,1996;Chung et al.,1992),and lipid synthesis(Düvel et al.,2010),and in inhibiting autophagy (Dubouloz et al.,2005). mTOR is the target of rapamycin,an immunosuppressant and anti-tumor drug (Douros & Suffness,1981;Sehgal & Bansbach,1993).Binding of rapamycin to mTOR negatively regulates mTORC1,but not mTORC2(Loewith et al.,2002).The mTOR signaling pathway also modulates the apoptotic effect induced by oxidative stress in cultured cells (Majumder et al.,2004).A prior study showed that the mTOR signaling pathway is activated in an RP mouse model carrying a missense mutation in thePde6bgene(H620Q) (Tsang et al.,2014).However,the role that the mTOR signaling pathway plays in retinal degeneration is yet to be elucidated.
To test whether the mTOR signaling pathway is activated inrd1photoreceptors,retinal sections fromrd1mice were immunostained with antibodies against mTOR and p-mTOR,two central components of the mTOR pathway.As shown in Supplementary Figure S1A,B,significantly more mTOR and p-mTOR,an activated form of mTOR,were detected in the P10rd1photoreceptors,suggesting that the mTOR signaling pathway was activated in therd1mouse photoreceptors.This is consistent with previous research showing activation of mTOR in another mouse model with a missense mutation inPde6b(Tsang et al.,2014).One of the major targets downstream of activated mTOR is the ribosomal protein S6 kinase (S6K),which can be phosphorylated by mTOR.Hence,as expected,significantly more phosphorylated S6K (p-S6K),the activated form of S6K,was present in therd1photoreceptors (Supplementary Figure S1C).Additionally,S6K was up-regulated in therd1photoreceptors(Supplementary Figure S1D).These results suggest more active protein synthesis inrd1photoreceptors.However,immunoblotting examination of the key phototransduction proteins in the rod photoreceptors showed that the expression of rhodopsin,guanylate cyclase 2 (GC2),and transducin α(Tα) were not increased in therd1retinas (Supplementary Figure S1E).Instead,their expression was unaltered (Tα) or markedly reduced (rhodopsin and GC2) in therd1photoreceptors,suggesting a more active protein turnover and complicated regulatory mechanism governing protein expression in photoreceptors.
The retina is one of the most metabolically active tissues in the body.Photoreceptors consume a substantial amount of energy to maintain the dark current in the dark-adapted state.Activation of the mTOR signaling pathwayrd1photoreceptors may place an extra burden on the cells,which could contribute to the fast death of photoreceptors in therd1retina.To explore the role of the mTOR signaling pathway in photoreceptor viability in therd1retina,we inhibited the mTOR pathway using rapamycin.We mixed rapamycin with lipid emulsion,which can slow the release of rapamycin and prolong its presence (Hippalgaonkar et al.,2010;Sakaeda & Hirano,1995).We administered an intravitreal injection of rapamycin inrd1mice at P10,a time point before retinal degeneration is evident.Approximately one day (20 h) later,the expression of mTOR was attenuated in a dose-dependent manner (Supplementary Figure S2A–C).Moreover,mTOR phosphorylation was significantly reduced.These results indicate that rapamycin can effectively inhibit the mTOR signaling pathway by reducing mTOR expression and mTOR phosphorylation.Although mTOR and p-mTOR were reduced,p-S6K,and S6K (downstream target of mTOR) was not affected in therd1photoreceptors after rapamycin treatment(Supplementary Figure S2A–C),implying that phosphorylation of S6K in therd1photoreceptors is dominated by a yet-to-beidentified pathway.
To test whether inhibition of the mTOR signaling pathway by rapamycin protects therd1retinal photoreceptors,a TUNEL assay was carried out on the retinal sections.As shown in Figure 1A,B,rapamycin exhibited a dose-dependent protective effect on therd1photoreceptors.The rapamycintreated retinas had fewer TUNEL-positive cells,with rapamycin at 25 μmol/L being the most effective.Western blot analysis using cleaved caspase 3 confirmed reduced apoptotic activities in the rapamycin-treated retinas(Figure 1C,D).Thus,rapamycin effectively protectedrd1retinal photoreceptors from apoptosis.
To test whether rapamycin can slow the progression ofrd1retinal degeneration,retinas were analyzed 6 days after intravitreal administration of a single dose of rapamycin.In the vehicle-treatedrd1retinas,only 1–2 rows of nuclei remained in the outer nuclear layer (ONL) across the entirerd1retina(Figure 1E),indicating fast degeneration.In contrast,those retinas treated with rapamycin had significantly more rows of nuclei in the ONL (Figure 1E).Remarkably,retinas treated with 25 μmol/L or 50 μmol/L rapamycin had 4–6 rows of nuclei,indicating that one dose of rapamycin was sufficient to slow retinal degeneration.Relative to the vehicle control,the ONL thickness of all rapamycin-treated retinas was consistently greater across the entire retina (Figure 1F).
Additionally,the protective effect of rapamycin on rod photoreceptors also partially rescued cone photoreceptor function.As shown in Figure 1G,the photopic response was almost undetectable in the vehicle-treated and 10 μmol/L rapamycin-treated mice,indicating that cone cells lose their function after the rods are nearly completely degenerated by P16.In contrast,the 25 μmol/L and 50 μmol/L rapamycintreated mice exhibited sizable responses with significantly higher amplitudes.Thus,rapamycin is a potent drug that can inhibit retinal degeneration and preserve the function of cone photoreceptors.
To gain insight into the protective mechanism of rapamycin,phototransduction protein expression in rapamycin-treatedrd1retinas was analyzed by immunoblotting.As shown in Supplementary Figure S3A,B,rapamycin did not change the expression of rhodopsin or transducin.Hence,the protective effects of rapamycin on photoreceptors does not appear to be mediated by phototransduction proteins.Next,we examined the expression of autophagy pathway-related proteins,including LC3B and SQSTM1,which are two markers of autophagy flux.Autophagy plays a vital role in photoreceptor survival by inhibiting apoptosis (Besirli et al.,2011).Furthermore,mTOR is a negative regulator of autophagy.Thus,inhibition of the mTOR pathway by rapamycin may reactivate autophagy to exerts its protective functions.As shown in Supplementary Figure S3C–E,in the presence of hydroxychloroquine (HCQ),which inhibits autophagosomelysosome fusion and prevents degradation of components associated with autophagosomes,rapamycin significantly increased the ratio of LC3B-II to LC3B-I and reduced the expression of SQSTM1 in a dose-dependent manner,suggesting that rapamycin promotes autophagy flux inrd1photoreceptors.
To verify that the suppression of retinal degeneration by inhibition of the mTOR pathway is mediated by autophagy activation,we treatedrd1mice with Torin 1,a more potent inhibitor of the mTOR pathway.As shown in Supplementary Figure S4A,B,Torin 1 robustly inhibited the expression and phosphorylation of mTOR at a concentration of 10 μmol/L.Torin 1 also inhibited photoreceptor apoptosis and slowed the progression of retinal degeneration inrd1retinas(Supplementary Figure S4C–F). Interestingly,similar to rapamycin,Torin 1 also increased the LC3B-II/LC3B-I ratio and reduced SQSTM1 expression in a dose-dependent manner,demonstrating the activation of autophagy by Torin 1 inrd1retinas (Supplementary Figure S4G–I).Furthermore,the autophagy inhibitor HCQ abolished the rescue effects of rapamycin and Torin 1 onrd1photoreceptors (Supplementary Figure 5A,B). Therefore,the enhanced survival of photoreceptors inrd1retinas by rapamycin or Torin 1 treatment is likely mediated by activation of the autophagy pathway.
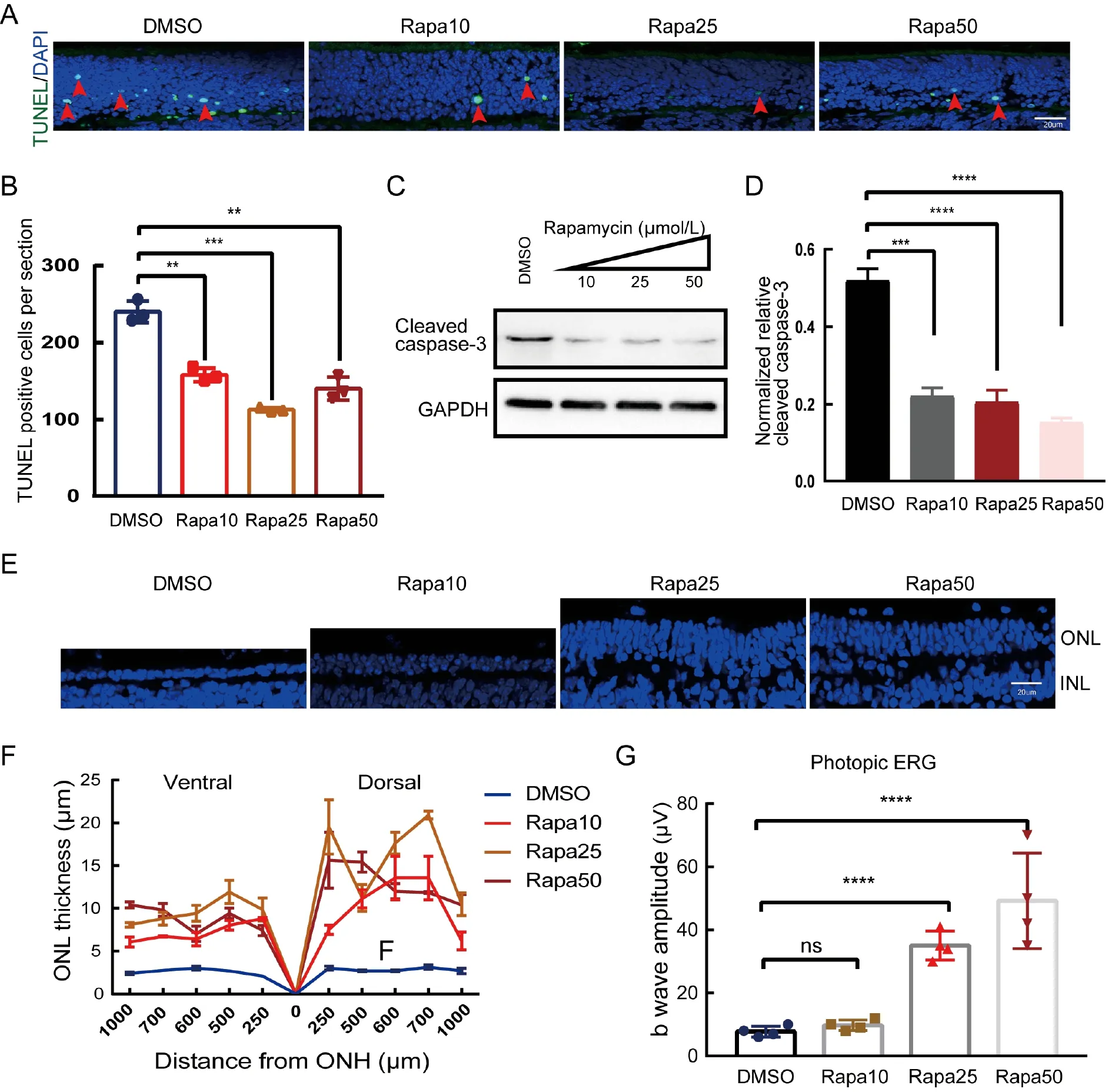
Figure 1 Inhibition of retinal degeneration and preservation of cone function in rd1 mice treated with rapamycin
In this study,we showed that the mTOR signaling pathway is activated in photoreceptors along with retinal degeneration in therd1retinas.Furthermore,inhibition of the pathway by rapamycin or Torin 1 treatment protects the photoreceptors from apoptosis,indicating that the mTOR signaling pathway plays a vital role in retinal degeneration.
mTOR is the master regulator of metabolism.The mTOR signaling pathway is activated when nutrients are adequate and cells engage in metabolic activities in favor of anabolism.There are several potential reasons why the mTOR pathway is activated inrd1photoreceptors.First,mTOR activation may occur due to the increased level of calcium.Notably,thePde6bmutation causes an influx of calcium ions inrd1photoreceptors,and it has been reported that high levels of calcium parallel mTOR signaling (Tsang et al.,2014).Second,the outer segments in therd1photoreceptors are underdeveloped and much shorter than normal.The outer segments of rod photoreceptors are comprised of hundreds of membranous discs composed of lipids and proteins required for photoreceptors to function.Shorter outer segments mean that the cells save considerable material,suggesting an abundance of nutrients available for cell utilization,which is sufficient to activate the mTOR signaling pathway.
The mTOR pathway negatively regulates autophagy.Autophagy is a mechanism used by cells to recycle intracellular components for the synthesis of macromolecules.Inrd1photoreceptors,autophagy is inhibited in conjunction with active mTOR.This implies the presence of an ample supply of nutrients in therd1photoreceptors.However,inhibition of autophagy in therd1photoreceptors leads to an accumulation of damaged organelles and macromolecules within the cells,which may partially contribute to photoreceptor death.The evidence presented in this study shows that inhibition of mTOR signaling by rapamycin and Torin 1 activates the autophagy pathway in the retina.In turn,activated autophagy may protect the photoreceptors by removing damaged macromolecules and relieving energy stress.Moreover,proper regulation of autophagy by mTOR is also important for the health of retinal ganglion cell loss(Madrakhimov et al.,2021). Thus,the rescue of photoreceptors by rapamycin or Torin 1 is possibly mediated by autophagy activation.This finding is supported by previous research,which reported inhibition of FAS-induced photoreceptor apoptosis by autophagy activation (Besirli et al.,2011).
Overactivation of mTOR is also cytotoxic to other cells in the retina.For example,conditional knockout ofTsc1,a negative regulator of the mTORC1 pathway,in retinal pigment epithelial cells causes hyperactivation of mTORC1,leading to cell degeneration (Go et al.,2020;Huang et al.,2019).Intriguingly,however,an earlier study suggested that conditional knockout ofTsc1in thePde6bmutant retina slows retinal degeneration progression (Zhang et al.,2016).The protective effects of TSC1 deficiency are presumably mediated by activation of the mTOR signaling pathway.Another study also showed that overexpression of S6K,a downstream component of the mTOR signaling pathway,promotes the survival of both rod and cone photoreceptors(Lin et al.,2018).These results differ from our findings.One possible interpretation for this discrepancy is that the mTOR pathway assumes multiple roles in photoreceptors.A previous report showed thatTsc1is involved in regulating cell cycle progression during retinal development (Choi et al.,2018).Conditional knockout ofTsc1inhibits the progress of retinal progenitor cells,thus retarding retinal development (Choi et al.,2018).This may also slow the degenerative process through a different mechanism.
In summary,we showed that the mTOR pathway is activated inrd1photoreceptors.Treatment ofrd1mice using rapamycin effectively inhibits mTOR activation and suppresses mTOR expression,leading to slower photoreceptor degeneration.Thus,rapamycin is a potential therapeutic drug for treating retinal degeneration caused by defective PDE6 function.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
J.L.Y,T.D.Z.,and F.Y performed the experiments and analyzed the data.Z.L.Y.and H.B.Z.analyzed the data and supervised the project.H.B.Z.conceived the project,designed the experiments,and wrote the manuscript.All authors read and approved the final version of the manuscript.
杂志排行
Zoological Research的其它文章
- Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways
- Genome and population evolution and environmental adaptation of Glyptosternon maculatum on theQinghai-Tibet Plateau
- 3DPhenoFish:Application for two-and threedimensional fish morphological phenotype extraction from point cloud analysis
- A new snake species of the genus Gonyosoma Wagler,1828 (Serpentes:Colubridae) from Hainan Island,China
- A bright future for the tree shrew in neuroscience research:Summary from the inaugural Tree Shrew Users Meeting
- PINK1 gene mutation by pair truncated sgRNA/Cas9-D10A in cynomolgus monkeys
