Effect of Zr-TiO2 catalyst on NO emission from coal-burning and its catalytic mechanism
2021-08-15WANGShuqinFUHaoCHENGWeiliangFUYi
WANG Shu-qin,FU Hao,CHENG Wei-liang,FU Yi
(1.Department of Environmental Science and Engineering,North China Electric Power University,Baoding 071003,China;2.Hebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control,North China Electric Power University,Baoding 071003,China;3.Key Laboratory of Regional Energy System Optimization of Ministry of Education,North China Electric Power University,Beijing 10084,China;4.School of Energy Power and Mechanical Engineering,North China Electric Power University,Beijing 10084,China)
Abstract:The effect of desulfurizer,modified TiO2 prepared by different methods on the NO emission from inferior coal burning and the adaptability of coal type were studied by using a tube furnace.The prepared Zr-TiO2 and the char were characterized by XRD,BET,SEM,XPS,TGA,and the mechanism of denitrification was explored.The results show that the addition of desulfurizer can promote NO emission.When the combustion temperature is 850℃,oxygen flow rate is 40 mL/min and the desulfurizer is MgO,the NO emission is the lowest as the catalyst of 5% Zr-TiO2 prepared by impregnation method is added,which is 51.0% lower than that with addition of pure TiO2 and 84.6% lower than that with pure coal under the same conditions.The catalyst 5% Zr-TiO2 can be applied to the coal with sulfur<3% and ash<30%,having a wide application range.The doping of Zr can inhibit the growth of grain,enhance the active component,increase the content of adsorbed oxygen,promote the transformation of the valence states of the elements,accelerate the devolatilization of volatiles,promote the combustion,increase the specific surface area of chars,and enhance the ability of heterogeneous reduction of chars.
Key words:inferior coal;NO emission characteristics;Zr-TiO2;catalysis
As one of the main energy sources in China[1],coal reserves are huge,but more than half of them are of poor quality[2],with a low calorific value,high ash and sulfur content[3].Despite the strong adaptability of coal type and the low pollution of circulating fluidized bed technology,it is difficult to meet the requirements of ultra-low emission.
TiO2is a widely used catalyst[4−7].Due to different preparation methods,TiO2has shown excellent performance in photocatalysis and low-temperature catalysis,etc.However,there are few studies on its catalytic performance at high temperature.This is because TiO2will undergo crystal transformation when the temperature is too high,destroying its original activity.The researches of Wang[8]identified that the efficiency of catalytic desulfurization and denitrification of nano-TiO2at 850℃ was 87.8% and 36.0%,respectively,which was 13.4% and 36.0%higher than that without TiO2.Although the desulfurization rate was high,the denitrification efficiency was far from meeting the requirements of ultra-low emission.Therefore,how to improve the denitrification efficiency while ensuring the desulfurization efficiency is the key to the application of TiO2as a catalyst in coal burning .
Preliminary research results[9,10]proved that changing the preparation method of TiO2and doping different metal elements could achieve a higher denitrification efficiency.Although it meets the requirements of ultra-low emission,it is only suitable for a single type of coal,which has difficulties of popularization and application.Therefore,based on the TiO2catalyst,a Zr doped modified TiO2using the microwave method and impregnation method was prepared when the CaO and MgO were used as a desulfurizer to study the NO release characteristics with several inferior coals under different burning conditions,explore the applicability of coal type under this condition,and further study the denitrification mechanism,so as to provide reference for the clean combustion of inferior coal and the promotion and application of catalysts.
1 Materials and method
1.1 Preparation of desulfurizer and catalyst
CaO,MgO and pure TiO2were chosen as the desulfurizer and the catalyst.Ca(Mg)/S ratio was 2.3 to ensure the desulfurization effect.
Firstly,butyl titanate and anhydrous ethanol were mixed to obtain a solution A.A solution B was gotten by adding proper amount of anhydrous ethanol,zirconium oxychloride solution,glacial acetic acid and deionized water into the drip funnel respectively.Set the parameters at 25℃ and 200 W,then adjusted it to 60℃ and 600 W after dropping B into A,finally a gel was formed.A 1% Zr-TiO2was prepared by microwave through drying,grinding and calcining the gel.
Secondly,a certain amount of Nano titanium dioxide was immersed in a zirconium oxychloride solution,heated at 60℃ in a water bath and stirred for 1 h,then left to rest for 1 h.Dried it to obtain a solid and then ground and calcined the solid,finally a Zr-TiO2was prepared by impregnation.The doping amount of Zr in Zr-TiO2was 1%,5% and 10%,and it was changed according to the concentration of zirconium oxychloride solution.
1.2 The results of proximate and ultimate analysis of the coals
The results of proximate and ultimate analysis of the coals are shown in Table 1 and Table 2.According to the data of ash content,sulfur content and calorific value,all used coals except “h” are of inferior quality.
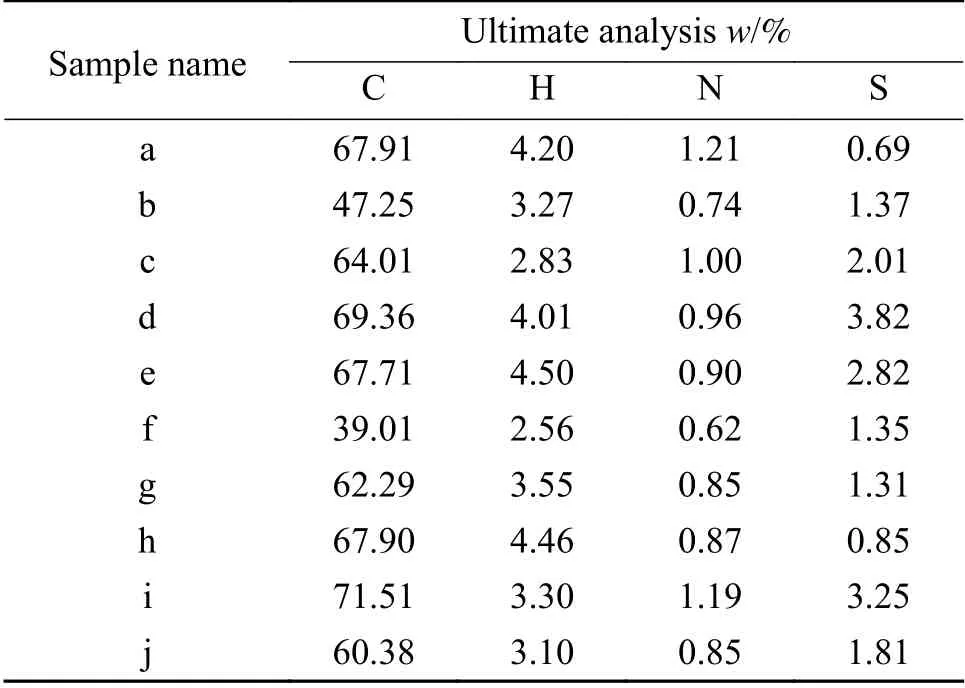
Table 1 Results of ultimate analysis of the coals
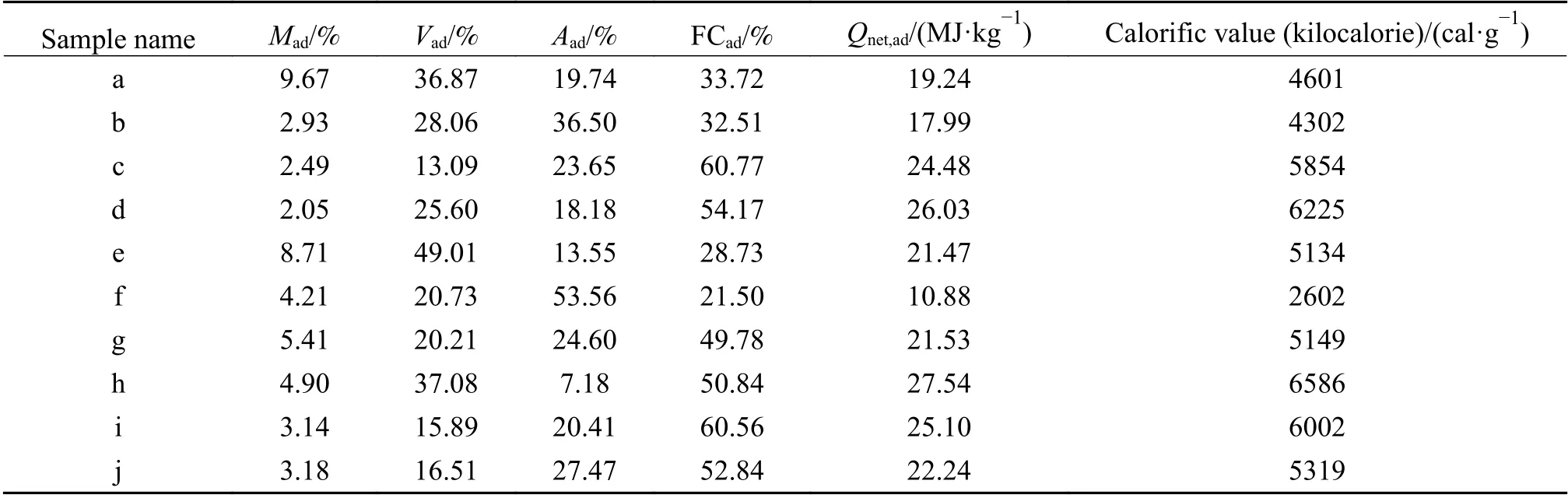
Table 2 Results of proximate analysis of the coals
1.3 Experiment of NO emission in coal combustion
The experiment was performed in a horizontal tubular furnace.The temperature was 850℃ and the oxygen flow rate was controlled at 40 mL/min.The concentration of NO released by combustion of coal samples before and after adding desulfurizer and catalyst was determined by spectrophotometry,and NO2was neglected due to small scale.
1.4 Verification of catalytic mechanism
The char was prepared by adding CaO,MgO and the 5% Zr-TiO2prepared by impregnation into the coal a,b,c,d and e,and then the removal efficiency of NO was measured in 9.13×10−4NO/N2respectively.
1.5 Characterization of samples
The characterization of the catalyst was conducted by X-ray diffractometer Empyrean.An ASAP 2460 specific surface area and porosity analyzer was used to measure the micromorphological parameters of the samples.A Quanta 250 FEG scanning electron microscopy was used to observe the micromorphology of the samples.An X-ray photoelectron spectrometer Kα was used to determine the element valence in the samples.A thermogravimetric analyzer TGA 4000 was used to detect the thermal stability of the samples.
2 Results and discussion
2.1 Influencing factors of NO emission
2.1.1 Effect of desulfurizer
The NO emission concentration for coal samples with and without adding additives are shown in Figure 1.It can be seen from Figure 1(a) that addition of the two desulfurizers increases the NO emission from burning the coal b,c,d and e.This is because the two desulfurizers can catalyze the conversion of volatile N(HCN and NHi) produced to NOx[11],which increases the emission of fuel type NOx.On the other hand,the high sulfur content of the coal b,c,d and e leads to a large amount of SO2formed,inhibiting the coal char heterogeneous catalysis on the reduction of NO due to the competing adsorption.For coal a,the low sulfur content leads to a small amount of SO2formed and the competing adsorption is decreased.In comparison of CaO and MgO,the increase of NO emission with MgO is lower.As shown in Figure 1(b),the addition of 5%Zr-TiO2with an 87.1% reduction on NO emission for coal e obviously decreases the NO emission.
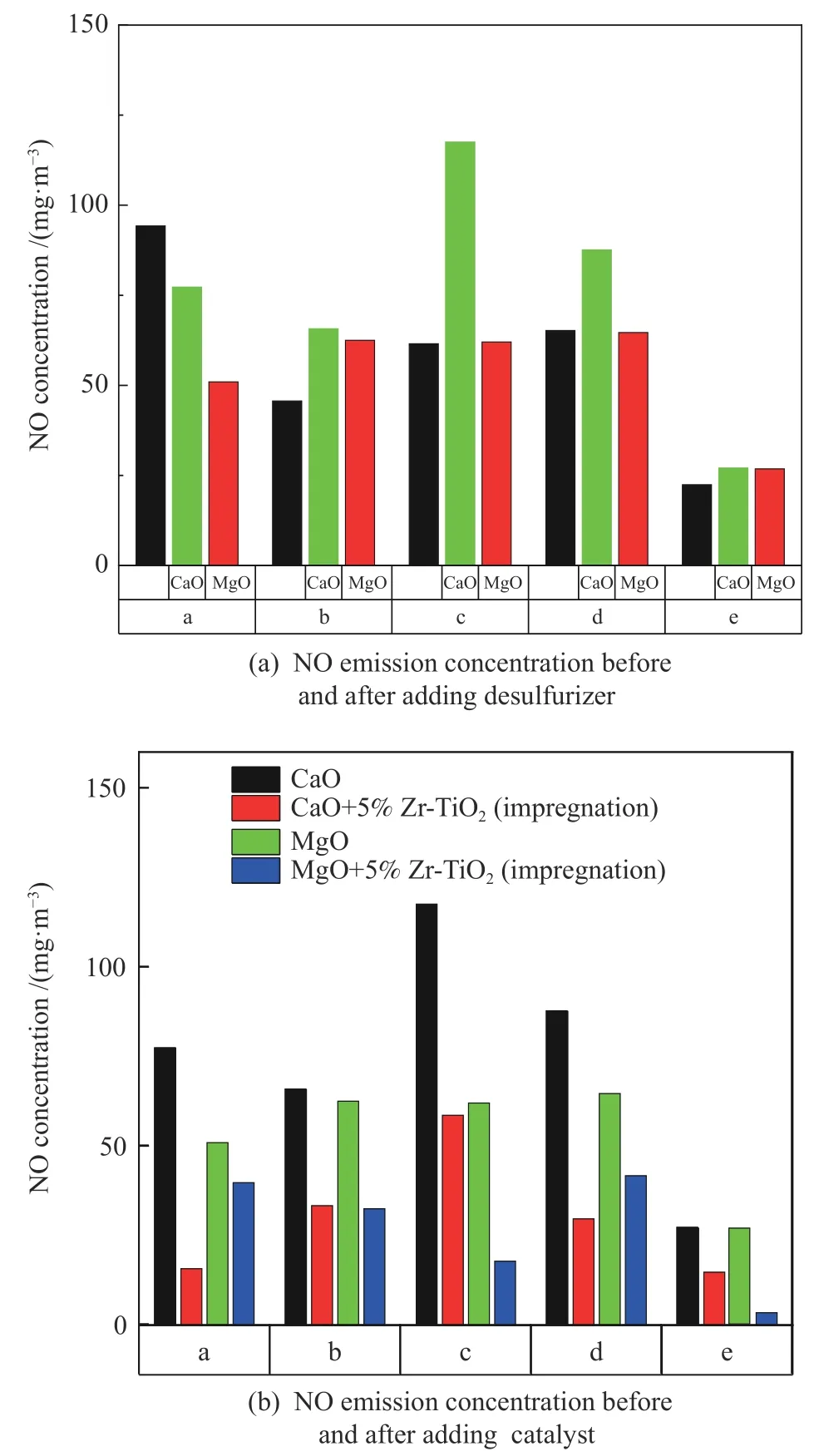
Figure 1 NO emission concentration before and after adding additives
2.1.2 Effect of oxygen flow rate
Under the conditions of 850℃,MgO as the additive and 5% Zr-TiO2being prepared by impregnation method,the NO emission concentration for five coals at an oxygen flow rate of 40 and 60 mL/min are shown in Figure 2.When no additive is added,the NO emission decreases as the oxygen flow rate increases,which is attributed to partial conversion of NO to NO2.However,NO emission increases significantly with the increase of the oxygen flow rate after the addition of additives,since the increase of the oxygen flow rate shortens the contact time between NO and coal char inhibiting the reduction of NO.Therefore,the oxygen flow rate of 40 mL/min is more appropriate under the condition.

Figure 2 Effect of oxygen flow rate
2.1.3 Effect of temperature
The NO emission concentrations for five coal samples at 750,850 and 950℃ with MgO as the desulfurizer and 5% Zr-TiO2being prepared by impregnation method are shown in Figure 3.Except for coal a,the NO emission for other coal samples decreases first and then increases with temperature,so the temperature of 850℃ has the best effect on NO emission reduction.In addition,when the temperature is over 900℃,the sulfur fixation products decompose and affect the sulfur fixation effect.
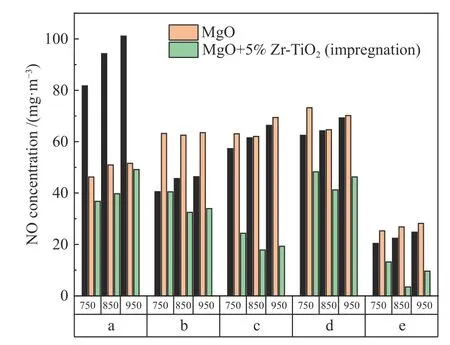
Figure 3 Effect of temperature
2.1.4 Effect of doping amount of Zr by impregnation method
The NO emission concentration when adding Zr-TiO2with different doping amounts prepared by impregnation method and operating at 850℃ and an oxygen flow rate of 40 mL/min with MgO added is shown in Figure 4.Except for coal e,the NO emission concentration for other coal samples decreases after adding pure TiO2.The NO emission concentration for five coal samples decreases with an increase in Zr doping amount,but when the doping amount reaches 5%,further increasing the doping amount has little effect on NO emission,so the doping amount of 5% is the most appropriate.When the doping amount is 5%,the NO concentration can be reduced by 51.0%compared with that with pure TiO2(except for coal e which increases the NO content after adding pure TiO2).
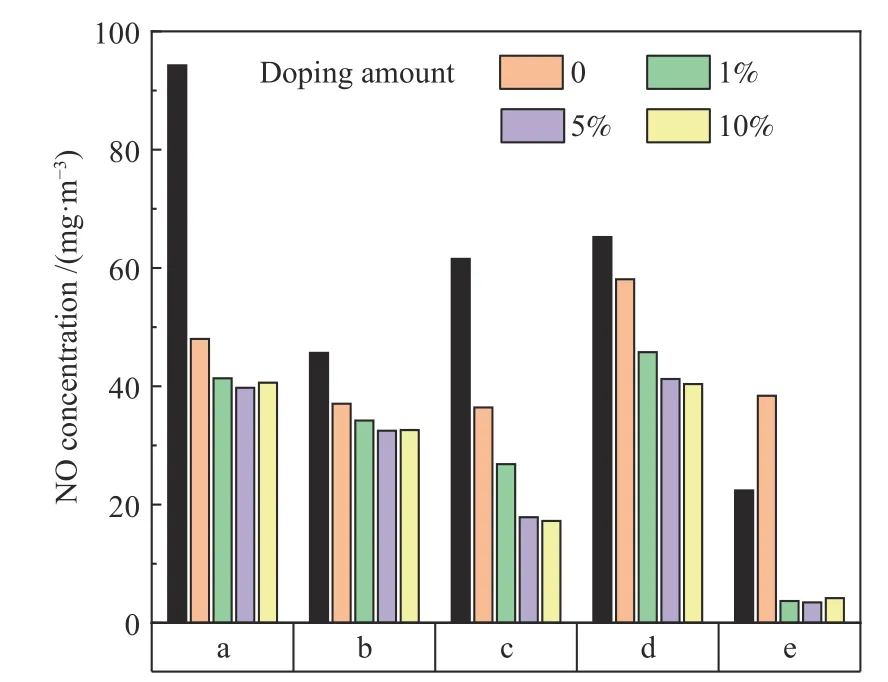
Figure 4 Effect of doping amount of Zr by impregnation method
2.1.5 Optimization of preparation method
The influence of TiO2preparation method including microwave method (1% Zr-TiO2) and impregnation method (5% Zr-TiO2) on the NO emission concentration with two catalysts having the best NO emission reduction effect is shown in Figure 5.It can be seen that the catalyst prepared by impregnation method can reduce NO emission better than that prepared by microwave method.Moreover,the impregnation method is simpler in operation and lower in cost,which makes the preparation method more practical.
2.2 Analysis of coal applicability
When operating at 850℃ and an oxygen flow rate was 40 mL/min,adding MgO and 5% Zr-TiO2prepared by impregnation method,the results of NO removal efficiency for five coals are shown in Figure 6.The preliminary analysis shows that the operating conditions under this experiment are not suitable for combustion of the coal with a high ash content and a high sulfur content,but suitable for the coal with a sulfur content of less than 3% and an ash content of less than 30%.

Figure 5 Effect of preparation methods on NO emission

Figure 6 NO removal efficiency for five coals
In order to test the above analysis results,broaden the use range of catalyst,and guide the removal of nitrogen oxides with pertinence and high efficiency,the other five coals with different sulfur and ash contents were selected,and the NO removal efficiency is shown in Figure 7.The experimental results entirely conform to the analysis of the adaptability of the catalyst for coals.
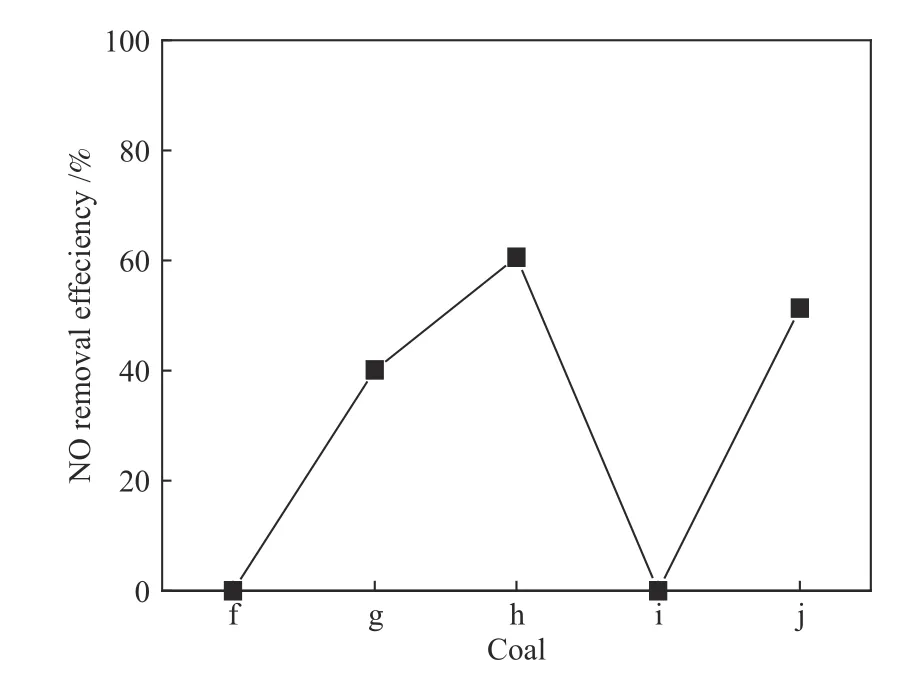
Figure 7 NO removal efficiency for other five coals
2.3 Characterization analysis of the catalyst
2.3.1 XRD analysis
The XRD patterns of pure TiO2and modified Zr-TiO2are shown in Figure 8.According to Scherrer formula,the grain sizes of pure TiO2,1% Zr-TiO2made by microwave,5% Zr-TiO2through impregnation,were 25,34.8,23.7 nm,respectively.The diffraction peaks with a high intensity appear at 25° and belong to anatase,indicating that the prepared TiO2and pure TiO2have high crystallinity[12],but no diffraction peak belonging to Zr is found.This is because the ionic radius of Zr4+is only 0.004 nm larger than that of Ti4+,so in the doping process,Zr4+is easy to replace Ti4+into the TiO2lattice existing in a stereotyped or highly dispersed form[13],the position of diffraction peak of anatase phase shifts slightly to the right,which also indicates that Zr4+enters into the TiO2lattice,and the lattice distortion occurs[14],forming a Zr−O bond.According to the calculations above,compared with the microwave method,the size of Zr-TiO2grains obtained by the impregnation method is smaller,which indicates that the growth of Zr-TiO2grains is inhibited by impregnation.XRD spectra of the 5% Zr-TiO2prepared by impregnation after burning at 850℃ identifies that the crystalline phase structure of the catalyst does not change at high temperature and it is still anatase.
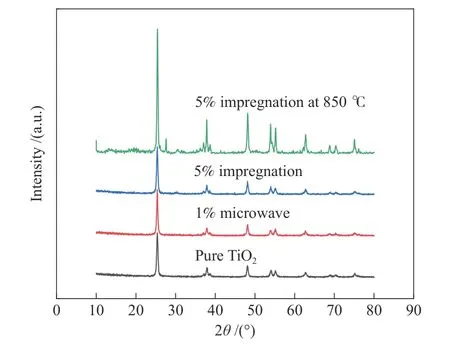
Figure 8 XRD spectra of pure TiO2 and modified TiO2
2.3.2 BET analysis
The specific surface area and pore size parameters of pure TiO2and modified Zr-TiO2are shown in Table 3.Compared with pure TiO2,the modified TiO2has better catalytic performance because of micro morphology parameters.The results show that the micro morphology parameters of Zr-TiO2synthesized by impregnation method are larger than those by microwave method,which is more conducive to the adsorption and reaction of molecules.It is not easy to be blocked by coal ash in the reaction process,so it showed high catalytic performance.Adsorption and desorption curves of catalysts are shown in Figure 9(a) and Figure 9(b),both of them belong to type IV isotherms,and hysteresis loops appear when p/p0is 0.8−1.0 and 0.4−0.8,respectively,indicating that the samples have mesoporous structure.The pore size distribution curve also shows that mesoporous structure is dominant in the two catalysts,and the pore size of Zr-TiO2prepared by impregnation method is smaller,which is consistent with the results in Table 3.

Table 3 BET data of catalysts
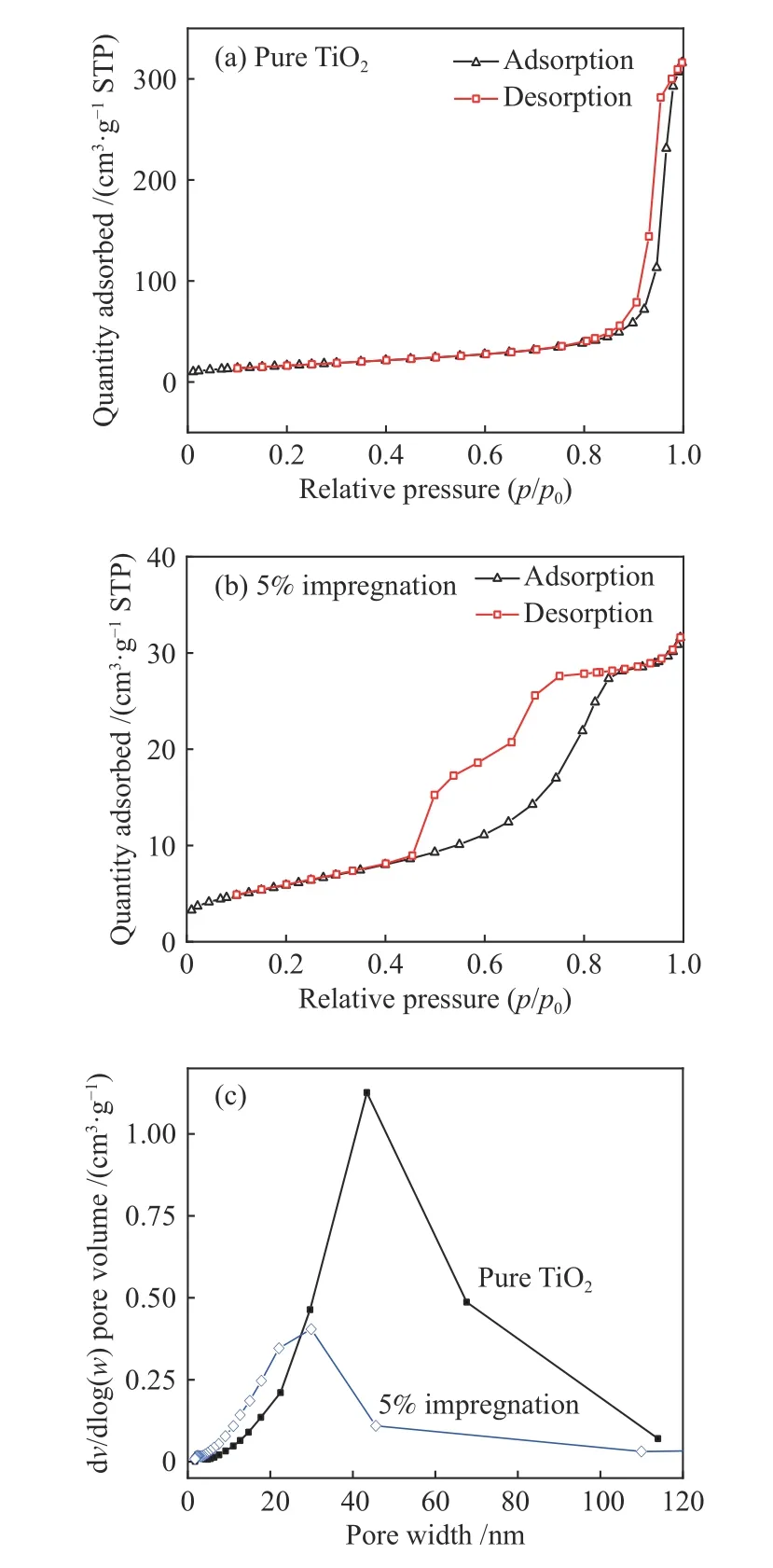
Figure 9 Adsorption and desorption curves ((a) &(b)) of catalysts and pore diameter distribution diagram (c)
2.3.3 SEM analysis
The electron microscope photos of pure TiO2and 5% Zr-TiO2prepared by impregnation method are shown in Figure 10(a) and Figure 10(b),respectively.It can be noted that the former has a local agglomeration phenomenon.The modified Zr-TiO2has better dispersion,smaller particle size and no obvious particle aggregation,which is more conducive to the adsorption and desorption of molecules in the process of catalytic reaction.In the process of coal combustion,it has better fluidity and is not easy to agglomerate,so it can fully catalyze the conversion of NO and reduce the emission of NO,which is consistent with the experimental results of NO emission.
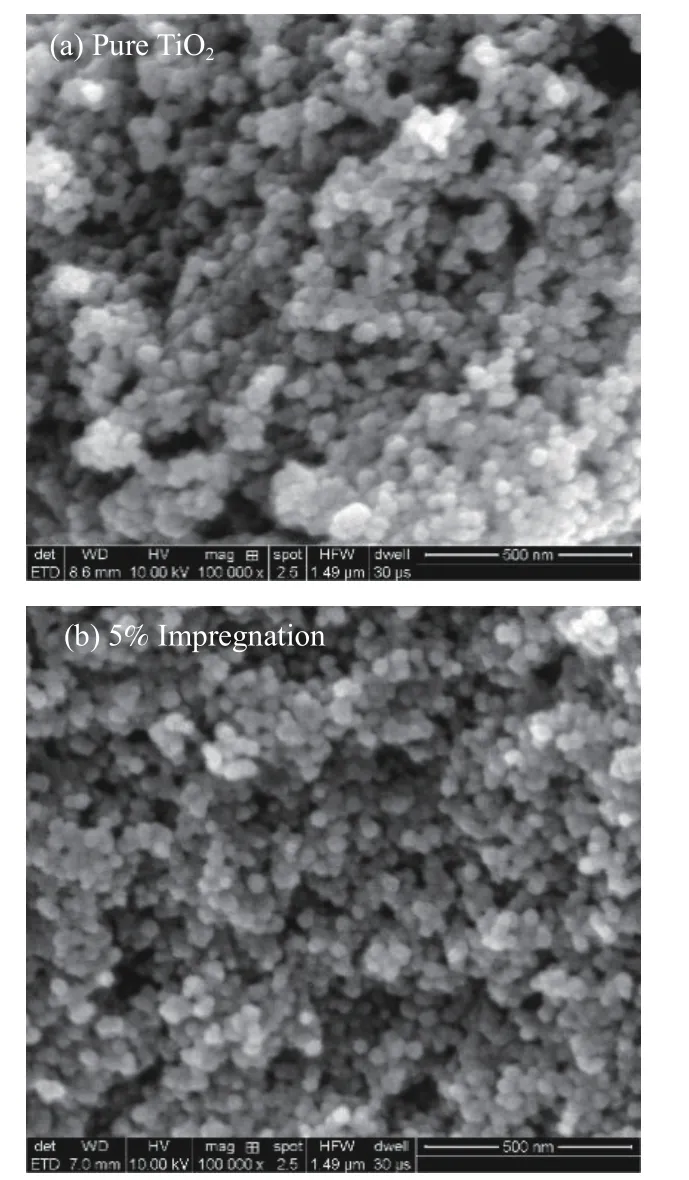
Figure 10 SEM of TiO2
2.3.4 XPS analysis
The high resolution XPS spectra of Zr,Ti and O elements in the pure TiO2,the microwave treated 1%Zr-TiO2and the prepared 5% Zr-TiO2by impregnation are shown in Figure 11.The energy spectra of Zr 3d3/2and 3d5/2are shown in the Figure 11(a).The two energy spectra corresponding to 1% Zr-TiO2prepared by microwave are 180.7 and 182.9 eV,respectively,and those corresponding to 5% Zr-TiO2prepared by impregnation are 181.0 and 183.4 eV,respectively,indicating that the doped Zr is of+4 valence[15].The energy spectrum of 2p1/2and 2p3/2of Ti are shown in Figure 11(b).The characteristic peaks are located at 458.0 and 463.7 eV,indicating that the Ti in the three samples are of+4 valence.The energy spectrum of O 1s is shown in Figure 11(c).Two asymmetric peaks indicate that there are two kinds of bound oxygen,corresponding to adsorbed oxygen and lattice oxygen.The two can transform each other under certain conditions,and lattice oxygen is more stable than adsorbed oxygen.The peaks of lattice oxygen of the modified TiO2obviously are both stronger than that of pure TiO2,identifying that the doping of Zr makes the catalyst more stable,which can promote the process of hole and electron separation[16],and promote the conversion of element valence state.Compared with pure TiO2,Zr doping significantly increases the peak intensity of adsorbed oxygen,so the catalytic performance is better.Zr-TiO2prepared by impregnation has more adsorbed oxygen and less lattice oxygen,which promotes the conversion process of NOxto N2.

Figure 11 High resolution XPS spectrogram of catalyst elements
2.3.5 TGA analysis
The thermogravimetric analysis results of coal d before and after adding additives are shown in Figure 12,according to which the ignition temperature and burnout temperature are calculated and shown in Table 4.The addition of desulfurizer increases the ignition point,and the ignition temperature of coal d with MgO is only 11℃ lower than that with CaO only.The addition of TiO2reduces the ignition point and the burnout temperature.The simultaneous addition of MgO and 5% Zr-TiO2prepared by impregnation can reduce the burnout temperature by 23℃,indicating that the catalyst has a combustion-supporting effect.
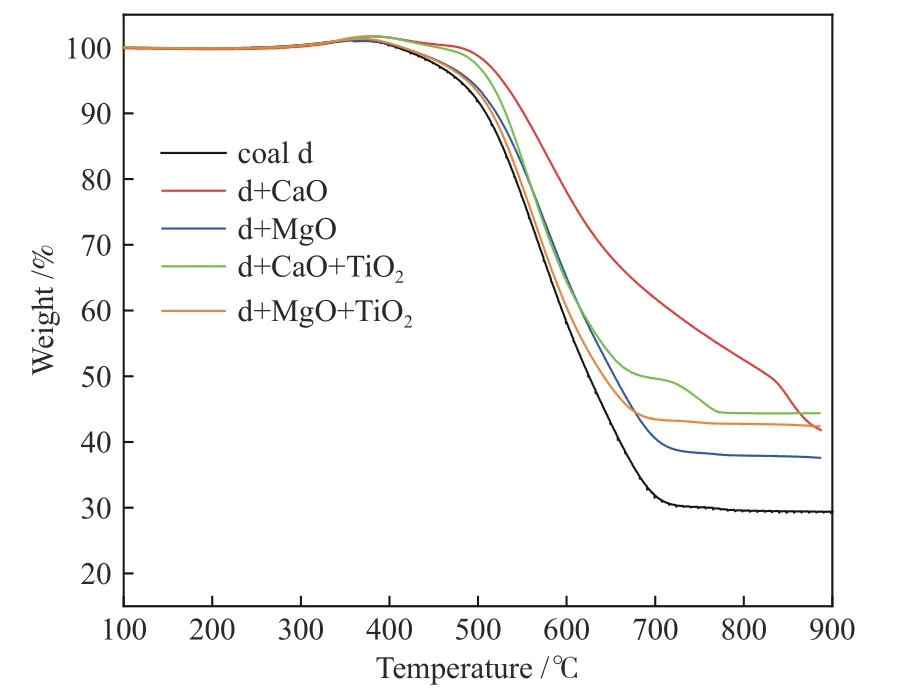
Figure 12 Thermogravimetric analysis of samples
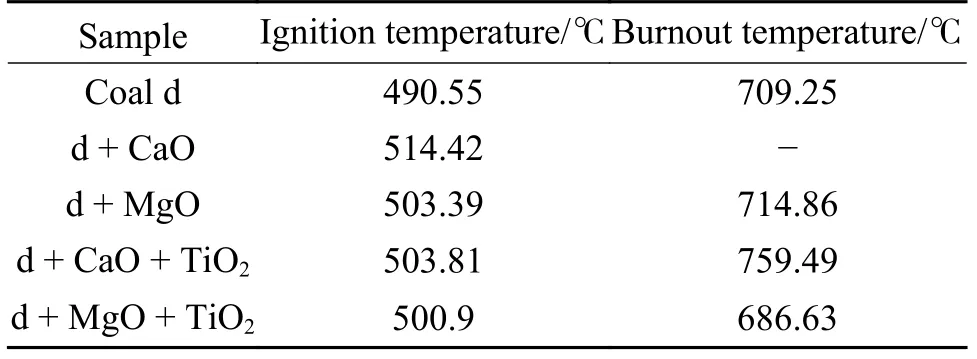
Table 4 Ignition and burnout temperature of samples
2.4 Mechanism analysis of catalytic denitrification
The denitrification result of five kinds of coal char with and without adding additives (MgO and 5% Zr-TiO2prepared by impregnation) at a NO concentration of 9.13×10−4is shown in Table 5.The coal chars have higher denitrification efficiency without adding additives.Except for coal char a,the denitrification efficiency of the other four kinds of coal chars are greatly improved by adding additives.This shows that coal char plays an important role in the denitrification of coal combustion,and the addition of catalyst can further improve the denitrification efficiency.
SEM images of the coal chars are shown in Figure 13 and the BET results are shown in Table 6.It can be seen that the particle size of coal char with adding catalyst is finer.The BET result indicates that because of adding additive the specific surface area is increased by 15 times,the average pore diameter is increased by 6 times and the pore volume is also increased,which makes the coal char be able to accommodate more molecules in the reaction process and more conducive to the reduction of NO.
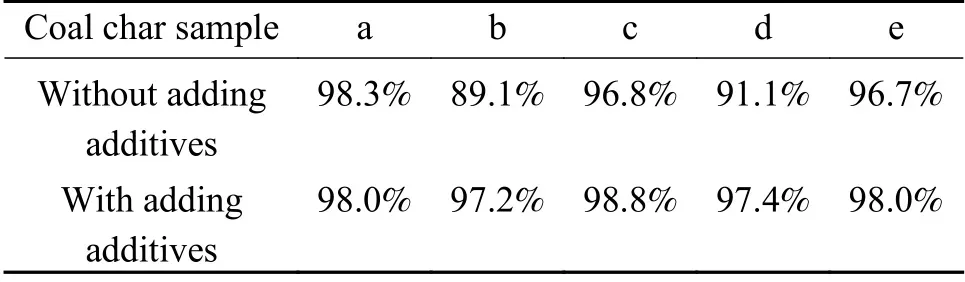
Table 5 NO removal efficiency of coal char before and after adding additives

Figure 13 SEM images of coal chars

Table 6 BET data of coal char
The experimental results are in agreement with previous studies[17]and the mechanism is same as before.On the one hand,during coal combustion,TiO2assists in desulfurizing,reducing the competitive adsorption of SO2,improving the reaction between SO2and desulfurizer,and inhibiting the emission of fuel type NOx.On the other hand,TiO2can improve the NO reduction efficiency by increasing specific surface area,pore diameter and pore volume of coal char,which is significant in the heterogeneous reduction process of coal char.NO is adsorbed on the surface of coal char and reacts with C in the process of coal combustion,TiO2in the catalyst catalyzes the conversion of NHiand NO to reduce the influence of desulfurizer.The doping of Zr enhances the reaction process and reduces the production of NO.The reaction equation is shown in formula (1)−(4),and the mechanism is shown in Figure 14.

Figure 14 Mechanism of catalytic denitrification
Moreover,high volatile matter can improve the ability of heterogeneous reduction of NOx[18],which is also consistent with the results in Figure 4.Zr doping accelerates the reactions (3) and (4),thus accelerating the precipitation rate of volatile matter.Coal is easier to burn to form a coal char with larger specific surface area,providing more adsorption sites for gas molecular reaction and being more conducive to denitrification,which was conclusively proved by the experiments with coal char.
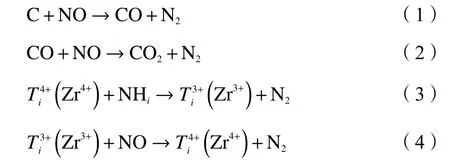
3 Conclusions
Compared with pure TiO2,the modified Zr-TiO2catalyst prepared by microwave method and impregnation method has an obvious inhibitory effect on NO emission.However,the impregnation method is simpler with lower cost,which is not only a good preparation method,but also makes the catalyst performance better.The results show that the addition of desulfurizer can promote the release of NO.When the coal combustion is performed at 850℃ with the addition of desulfurizer MgO and 5% Zr-TiO2prepared by impregnation,the emission concentration of NO is the lowest,with 84.6% lower than that of pure coal combustion,and with 51.0% lower than that adding MgO and the catalyst of pure TiO2.
The characterization of the modified Zr-TiO2shows that the prepared catalyst has high crystallinity,and the doped Zr leads to the lattice distortion and the formation of Zr−O bond.The specific surface area and pore size parameters of the modified Zr-TiO2are improved,and the active components are also enhanced.The addition of Zr inhibits the growth of grains,increases the content of adsorbed oxygen and promotes the valence transformation of elements.In the process of coal combustion,the modified Zr-TiO2can not only assist in desulfurizing and reducing the competitive adsorption of SO2in the high temperature environment,thus reducing the production of fuel type NOx,but also accelerate the release of volatile matter,increase the combustion-supporting effect,and increase the pore structure and specific surface area of coal char formed,and therefore catalyze the heterogeneous reduction reaction of coal char and reduce the release of NO.
Under the experimental conditions with ten kinds of coal,5% Zr-TiO2prepared by impregnation method can effectively reduce NO emission from inferior coal burning,and also has strong adaptability suitable for coals with a sulfur content of less than 3% and an ash content of less than 30%.It will broaden the application range of catalyst and lay a foundation to realize the ultra-low NOxemission from pulverized coal burning.
