Aging is associated with cardiac autonomic nerve fiber depletion and reduced cardiac and circulating BDNF levels
2021-08-14AndreaEliaAlessandroCannavoGiuseppinaGambinoMariaCiminiNicolaFerraraRajKishoreNazarenoPaolocciGiuseppeRengo
Andrea Elia, Alessandro Cannavo, Giuseppina Gambino, Maria Cimini, Nicola Ferrara,2,Raj Kishore, Nazareno Paolocci, Giuseppe Rengo,2
1. Department of Translational Medical Sciences, Federico II University of Naples Italy; 2. Istituti Clinici Scientifici ICSMaugeri, Telese Terme (BN), Italy; 3. Center for Translational Medicine, Department of Pharmacology, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania, USA; 4. Division of Cardiology, Johns Hopkins University Medical Institutions, Baltimore, MD, USA; 5. Department of Biomedical Sciences, University of Padova, Italy
ABSTRACT Background Aging is a multifactorial process associated with an impairment of autonomic nervous system (ANS) function. Progressive ANS remodeling includes upregulation of expression of circulating catecholamines and depletion of cardiac autonomic nerve fibers, and it is responsible, in part, for the increased susceptibility to cardiac diseases observed in elderly subjects. Neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), are involved in synaptogenesis and neurite outgrowth processes, supporting neuronal cell differentiation and maturation. However, whether and how these factors and their downstream signaling are involved in cardiac aging remains unclear. Here, we tested whether, in the aged heart,the overall extent of autonomic fibers is reduced, owing to lower production of trophic factors such as BDNF and NGF.Methods In vivo, we used young (age: 3 months; n = 10) and old (age: 24 months; n = 11) male Fisher rats, whereas, we used human neuroblastoma (SH-SY5Y) cells in vitro.Results Compared to the young rats, old rats displayed a marked reduction in the overall ANS fiber density, affecting both sympathetic and cholinergic compartments, as indicated by dopamine β-hydroxylase (dβh) and vesicular acetylcholine transporter(VaChT) immunohistochemical staining. In addition, a marked downregulation of GAP-43 and BDNF protein was observed in the left ventricular lysates of old rats compared to those of young rats. Interestingly, we did not find any significant difference in cardiac NGF levels between the young and old groups. To further explore the impact of aging on ANS fibers, we treated SH-SY5Y cells in vitro with serum obtained from young and old rats. Sera from both groups induced a remarkable increase in neuronal sprouting, as evidenced by a crystal violet assay. However, this effect was blunted in cells cultured with old rat serum and was accompanied by a marked reduction in GAP-43 and BDNF protein levels.Conclusions Our data indicate that physiological aging is associated with an impairment of ANS structure and function and that reduced BDNF levels are responsible, at least in part, for these phenomena.
Aging is a complex multifactorial physiological process that is typically associated with structural and functional changes in biological, physiological, and behavioral aspects.[1]However, these age-related changes are not considered signs of any pathology. Senescence results in a progressive reduction in the functional reserve of several organs and systems that increase the susceptibility to chronic diseases, disability, and frailty.[2,3]Indeed, aging represents a significant risk factor for developing many chronic disorders in humans.[4]Among these, cardiovascular diseases represent the leading cause of death, hospitalization,and disability worldwide, globally driving elevated healthcare costs.[5,6]Thus, there is an urgent need for novel strategies and therapeutic approaches to reduce this social and economic burden.
Notably, the aging heart undergoes several structural and functional modifications that include cardiac chamber dilation with left ventricular (LV)wall stiffness and maladaptive hypertrophic remodeling with consequential diastolic dysfunction.[7]Moreover, aging features sympathetic nervous system (SNS) derangement, as indicated by increased circulating catecholamine levels and cardiac β-adrenergic receptor (βAR) signaling dysfunction.[8]These modifications have also been observed in the postischemic failing heart, which is sympathetically denervated[9]and depleted of catecholamines,[10,11]and have been recognized to worsen cardiac function and increase mortality. Therefore, it is plausible that physiological aging increases SNS outflow and reduces cardiac sympathetic nerve fibers,which may account for the elevated cardiovascular mortality observed in the elderly population.[12,13]However, whether and how aging affects the cardiac autonomic nervous system (ANS) remains to be examined in depth. Previous studies have assessed ANS status in the aged heart by measuring norepinephrine levels and nerve fibers or by metaiodobenzyl guanidine iodine 123 (123I-MIBG) uptake in humans.[14]MIBG is a norepinephrine analog and one of the most commonly used tracers for singlephoton emission tomography (SPECT) and positron emission tomography (PET) that allows the visualization of presynaptic sympathetic nerve function because it has a high affinity for presynaptic norepinephrine uptake-1 (NET).[15,16]However, the mechanisms involved in these age-related alterations of cardiac autonomic fibers remain elusive.
Whether constitutive (nerve growth factor, NGF)or on-demand (brain-derived neurotrophic factor,BDNF) neurotrophic factors are pivotal for neuronal growth, differentiation, and connectivity is still under debate.[17,18]Moreover, while early studies attested to the relevance of NGF and BDNF for heart structural maturation,[19]there is now rapidly accumulating evidence showing that BDNF, for example,modulates myocardial mechanical function directly under normal and disease conditions. Simultaneously, reducing the circulating levels of BDNF contributes to the progression of cardiac disease, particularly that of ischemic etiology.[20−22]However, to the best of our knowledge, there are no reports thus far examining whether physiological aging (“eugeria”) has an impact on the extent of cardiac autonomic innervation and functional integrity and whether the biological profile of neurotrophins,such as BDNF and NGF, is altered during eugeria and thus contributing to or resulting from cardiac autonomic fiber impoverishment. The present study was designed to fill this gap, and to this end, we monitored the status of cardiac autonomic fibers and BDNF/NGF levels in young and old rats. For these purposes, rats are very convenient because old animals are relatively easy to obtain and due to the extensive work on BDNF/NGF previously done in this species, particularly concerning heart structure and function.[21,23–25]
MATERIALS AND METHODS
Animal Model and Experimental Design
All experiments and animal protocols were performed following the Institutional Animal Care and Use Committee of University Federico II of Naples,Italy guidelines. Three-month-old (young group,n=10) and 24-month-old (old group,n= 11) male Fisher rats were purchased from Jackson Research Laboratory (Bar Harbor, ME) and were allowed to acclimatize for ten days under standard conditions proposed by the local animal care committee.
Histology
Cardiac samples were fixed overnight in 4% paraformaldehyde (Sigma, St. Louis, MO, USA) at room temperature. After dehydration, the specimens were embedded in paraffin and cut into 5-μm-thick sections using a Microm HM 325 (Thermo Scientific).Interstitial fibrosis was evaluated in cardiac sections stained with 1% Sirius Red in picric acid solution (Sigma-Aldrich, St. Louis, Missouri), and images were acquired with a BA410 microscope (Motic®).[26]Cardiac fibrosis density was measured using ImageJ software (NIH, version 1.30) and expressed as a percentage of fibrosis.
To assess vessel density, 5-μm-thick cardiac sections were allowed to react overnight with a biotinylated lectin antibody fromBandeiraea simplicifolia(Sigma) conjugated with a Tyramide Signal Amplification (TSA) Biotin System kit (Perkin Elmer Life Sciences, MA) following the manufacturer’s instructions. Then, sections were rinsed in PBS, incubated with horseradish peroxidase-conjugated streptavidin (1: 5 000; Dako), and detected with 3,3-diaminobenzidine (DAB). Images were acquired and analyzed with a BA410 microscope (Motic®).[27]For each sample, five fields were randomly selected, and vessel density was quantified. The quantification was expressed as the number of capillaries per area (capillaries/mm2).
To measure the extent of cardiac hypertrophy, 5-μm-thick cardiac sections from each experimental group were stained with 5 μg/mL wheat germ agglutinin (WGA, #W834, Thermo Fisher Scientific,Waltham, MA, United States), as previously described.[28]Sections were rehydrated, washed with PBS, permeabilized, and incubated at 37°C for 20 min. Next, sections were washed and mounted with Fluoroshield with 4′,6-diamidino-2-phenylindole(DAPI) (Sigma-Aldrich, St. Louis, Missouri). Images were acquired and analyzed with a ZOE™Fluorescent Cell Imager (Bio-Rad). Five fields were randomly selected for each sample, and a total of 50 cardiomyocytes per section were analyzed (10 cells/area). Then, the mean cardiomyocyte diameter(μm) was measured.
Nerve Fibers Density
Cardiac specimens were fixed overnight in cold Zamboni solution (2% paraformaldehyde and picric acid) at 4°C, cryoprotected with 20% sucrose in PBS for 24 h at 4°C and sectioned (20-μm-thick sections)using a freezing sliding microtome (Leica 2000R,Germany). Free-floating sections were stained via indirect immunofluorescence with mouse monoclonal antibodies against dopamine β-hydroxylase(DβH; 39 600 506, Bio-Rad 1:150) to mark adrenergic nerve fibers and with goat polyclonal antibodies against the vesicular acetylcholine transporter(VaChT; H-V007, Phoenix Pharmaceuticals. Inc.,1:400) to stain parasympathetic nerve endings. Most of the cardiac nerve fibers showed a regular arrangement along cardiomyocytes.[29,30]Cardiac nerve fibers were calculated using a square grid with 24 probes (area 1 cm2per each) overlapping to digital images acquired by non-laser confocal microscopy (Apotome confocal system; Zeiss,Oberkochen, Germany) with a 20 X objective. For each sample, three sections were chosen, and five fields per section were randomly selected. Cardiac autonomic nerve fiber density was quantified and expressed as the mean number of fibers per area(fibers/mm2).
Immunoblotting
Cells (SH-SY5Y) and cardiac samples were homogenized in RIPA lysis buffer solution (cOmplete-Roche, USA) supplemented with a proteinase and phosphatase inhibitor tablet (PhosSTOP-Roche,USA). Next, the lysates were sonicated and centrifuged for 10 min at 4°C at 13,000 r/min, and the insoluble debris was removed. Total proteins were then quantified via a dye-binding protein assay kit(Bio-Rad) and detected with an iMark microplate reader (Bio-Rad) at a wavelength of 750 nm. Equal protein concentrations (20–40 μg) were separated using 4%−20% SDS-PAGE and detected by Western-Blot analysis. Total lysates were used to investigate the protein levels of BDNF (ANT-010; Alomone labs; 1: 1 000), GAP-43 (Millipore AB552; 1: 1 000),NGF (sc-548, H-20; Santa Cruz Biotechnology; 1: 1 000),Collagen III-A1 (COL3A1; sc8780, S-17; Santa Cruz Biotechnology; 1: 1 000) and GAPDH (sc-32 233,6C5; Santa Cruz Biotechnology; 1: 2000). Protein bands were visualized using enhanced chemiluminescence (ECL; Millipore) according to the manufacturer's instructions and were quantified by densitometric analysis (ChemiDoc-XRS system software,Bio-Rad, USA).[31]
ELISA
Serum NGF and BDNF levels were assessed using a commercial kit (biosensis®-BEK-2 227). At the end of the study period, 1 mL of blood was collected. Blood samples were centrifuged at 1 500 r/min at 15°C for 15 min. ELISA was performed using 25 μL of serum from each sample. The plate was then read at 450 nm by an iMark microplate reader (Bio-Rad)for protein level quantification.
Cell culture and treatment conditions
Human neuroblastoma cells (SH-SY5Y from ATCC-CRL-2266) were cultured in 10 cm2dishes and incubated for five days in a humidified environment with 5% CO2at 37°C in DMEM/F12 medium (Lonza Ltd. Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P/S, Lonza). For the experiments with rat serum, cells were serum-starved for 24 h and then stimulated with 4% serum from young or old rats for 24 h.
Neurite outgrowth analysis in vitro
Adherent cells were fixed with 4% paraformaldehyde for 10 min, washed with PBS, and stained with a 0.1% crystal violet solution (Sigma) for 10 min.Images were acquired with a BA410 microscope(Motic®) for analysis. From each sample, five areas(10−30 cells per area) were randomly selected. SHSY5Y neurite length was measured using ImageJ software (NIH, version 1.30).[32]
Statistical Analysis
Allin vitroandin vivoexperiments were performed in a blinded manner. Data were expressed as means ± SE. Statistical significance was determined by exact tests (Student’st-test or the Mann-WhitneyUtest). All data were examined using GraphPad Prism software version 5 (GraphPad Software, La Jolla, California). The accepted statistical significance level was set atP< 0.05.
RESULTS
Aging Fueled Myocardial Interstitial Fibrosis While Negatively Impacting Vascularization and Innervation
To study the effects of aging on autonomic innervation, we used old Fisher rats (24 months old;Supplementary Figure 1). Young rats (3 months old)were used as controls.[33,34]As expected, aged rat myocardium presented increased interstitial fibrosis(Figure 1A), associated with augmented expression of collagen, type III alpha-1 (COL3A1, Figure 1B),compared to young hearts. Furthermore, old hearts showed impaired cardiac vascularization (Figure 1C),as demonstrated by lectin staining, and a significant increase in cardiomyocyte hypertrophy, as assessed by the wheat germ agglutinin (WGA) assay(Figure 1D), compared to the myocardium of young rats. Next, we analyzed cardiac sympathetic and cholinergic nerve fiber density via DβH and vesicular acetylcholine transporter (VaChT) staining in cardiac sections from the two experimental groups.Notably, marked impairment in DβH-positive(DβH+) and VaChT+fibers was observed in old rats compared to young rats (Figure 1E & F). Thus, we confirmed the hypothesis that aging is associated with altered ANS function.
Aging Reduced the Circulating Levels of BDNF but not NGF and was Coupled to Marked Impairment of Autonomic Fiber Regeneration
Next, we investigated whether aging is accompanied by altered levels of secreted and cardiac neurotrophins such as BDNF and NGF. As shown in Figure 2A&B, we observed a drastic reduction in circulating and cardiac BDNF protein levels in serum from old rats compared to young rat serum. Intriguingly, aging did not affect NGF levels, either systemically or at the cardiac level (Figure 2C&D).Furthermore, we analyzed myocardial expression levels of growth-associated protein 43 (GAP-43) as a marker of nerve fiber regeneration. Interestingly, a significant reduction in GAP-43 levels was observed in old hearts compared to young hearts (Figure 2E).
Aging Impaired Nerve Fiber Outgrowth, an Effect Due to Deficient BDNF Production
Ourin vivodata indicated that aging was accompanied by a sizable depletion of autonomic nerve fibers within the myocardial tissue. To further investigate whether and how aging affects neuronal cell branching formation, we stimulated human neuroblastoma (SH-SY5Y) cells with serum obtained from young and old rats for 24 h. The effects of serum stimulation on GAP-43, BDNF, and NGF protein expression were assessed. Of note, stimulation with old rat serum resulted in a significant reduction in GAP-43 and BDNF protein expression compared to expression levels following stimulation with young rat serum (Figure 3A-C). In contrast, no differences in terms of NGF protein levels were observed between young and old serum stimulation groups (Figure 3D). Finally, we analyzed the effects of serum on neuronal outgrowth. Notably, we observed a marked impairment in neuronal sprouting in cells stimulated with old rat serum compared to those treated with young rat serum(Figure 3E). In aggregate, these findings support the notion that aging is liable for altered nerve fiber outgrowth and is associated with reduced BDNF production.
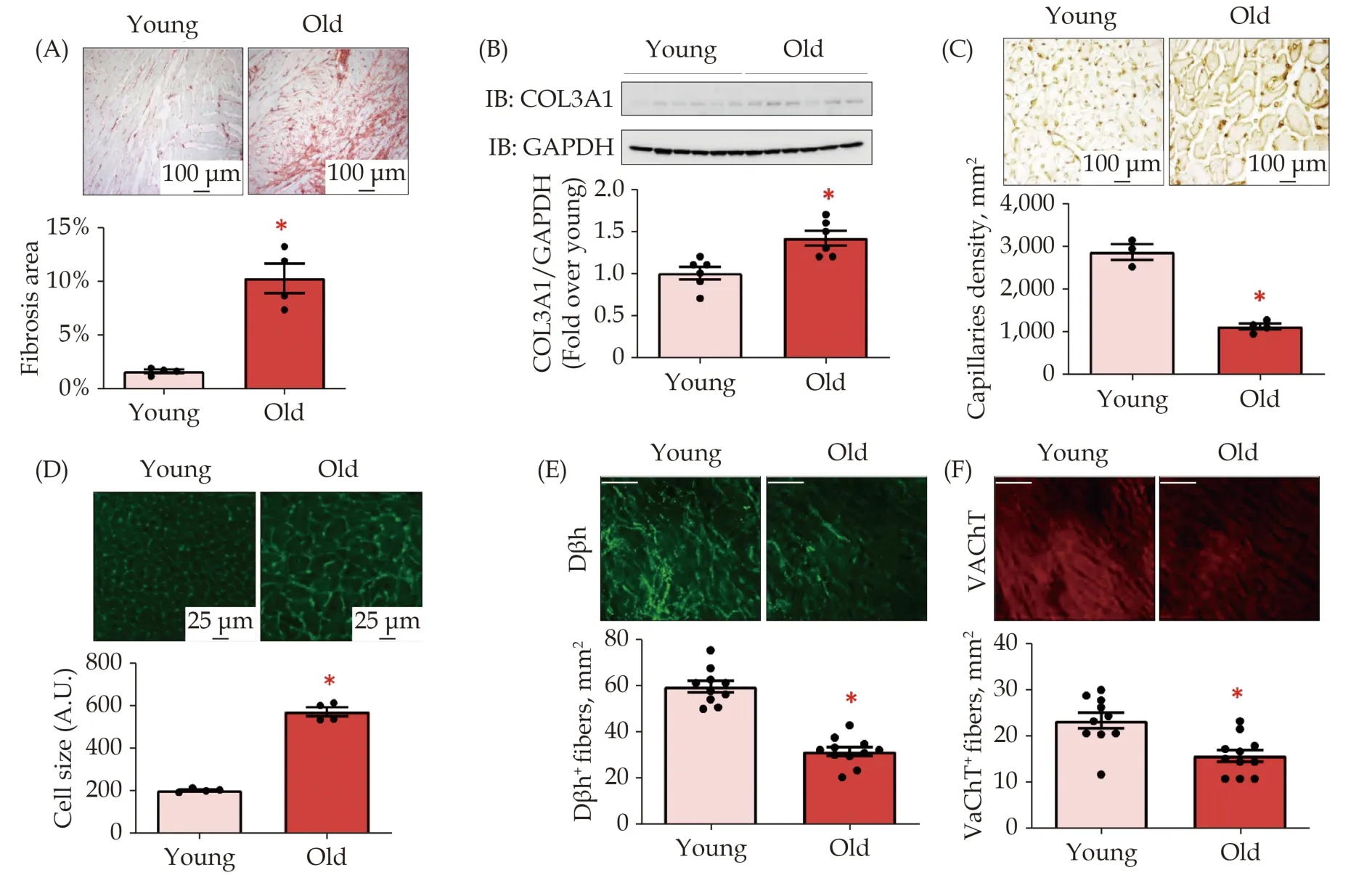
Figure 1 Impact of aging of cardiac fibrosis, hypertrophy, vascularization, and ANS. (A): Representative images (upper panels) and quantitative data (lower panels) showing percentage (%) of cardiac fibrosis in LV sections from young and old rats, assessed via Picro-Sirius red staining (scale bar 100 μm); (B): representative immunoblots (upper panels) and densitometric quantitative analysis (lower panels) showing levels of COL3A1 in total cardiac lysates of young and old rats. GAPDH levels were used as loading control; (C): representative images (upper panels; scale bar 100 μm) and quantitative analysis (dot plots; lower panels) showing capillaries density in left ventricle sections of each experimental group, detected by Lectin Bandeiraea simplicifolia I (BS-I) staining; (D): representative images (upper panels, scale bar 25 μm) and quantitative data (lower panels) showing myocyte cell size measured via wheat germ agglutinin (WGA) staining in left ventricle sections from young and old rats; and (E-F): confocal digital images (upper panels; scale bar 100 μm) showing (E) cardiac adrenergic nerve fibers, labeled with anti-dopamine beta-hydroxylase (Dβh; in green), and (F) cardiac cholinergic nerve endings, labeled with anti-vesicular acetylcholine transporter (VAChT; in red) in left ventricle sections from young and old rats. *P < 0.05 vs. Young. Data are shown as a mean ± SE. ANS: autonomic nervous system; LV: left ventricle.
DISCUSSION
The autonomic nervous system helps the body cope with changes occurring in the internal milieu and external environment by modulating visceral functions. Age increases the incidence of cardiovascular diseases, and this increase is, at least in part,due to the consequence of ANS structural and functional derangement. Autonomic fiber remodeling is manifested as an increase in sympathetic outflow with a consequent persistent elevation in circulating catecholamine levels, which is followed by cardiac βAR expression desensitization/dysfunction and downregulation.[35]
However, with increasing age, persistently elevated sympathetic discharge can lead to an exhaustion of the sympathetic response, along with a decline in the parasympathetic response.[36]Another prominent feature of this scenario is the decline in the expression/activity of the norepinephrine transporter (NET) in sympathetic nerves that is liable for impaired reuptake of norepinephrine from the neuroeffector junction,[37,38]thus contributing to catecholamine spillover. Concerning the eventual alterations imparted by the aging process on the extent of parasympathetic nervous system (PNS)fibers, studies have shown that both the density and function of the M2 receptor decline with age, leading to a decrease in cardiac parasympathetic activity and thus accounting for diminished baroreflex activity with age.[39–41]Furthermore, it is well known that the vagal component of heart rate variability(HRV) decreases with age. At the same time, HR appears to be less sensitive to pharmacological blockade of muscarinic acetylcholine receptors, thus denoting an overall decline in PNS function.[42,43]Altogether, this evidence helps explain the increased cardiovascular morbidity and mortality seen in the elderly population.[14,35]However, whether and how age affects neural growth and cellular neural function in the aging myocardium remains poorly understood. Previous studies have examined the effect of aging on intracardiac ganglia, suggesting changes in the total neuronal number and density[44]and in axonal organization,[45]leading to perturbations in cardiac electrical genesis and propagation.Nevertheless, to date, no studies have examined the status of cardiac autonomic fibers in the aged myocardium. Since cardiac aging has been well studied in rats and found to recapitulate many features of the changes occurring in aged human hearts (i.e.,continuous loss of myocytes in the ventricles, increased myocyte cell volume, interstitial fibrosis,and impaired vascularization), we monitored the progressive deterioration of heart structure in young and old rats. As expected, we found a substantial loss of viable myocardium replaced by the deposition of extracellular matrix, promoted by activated fibroblasts. Myocyte loss contributes to the cardiac aging process with harmful and adverse effects, thus promoting maladaptive ventricular remodeling.[34,46]We also observed an age-related reduction in the vascularization of the myocardium,according to previous reports.[47,48]In turn, this impaired revascularization of the myocardium accounts, at least in part, for the onset and maintenance of maladaptive cardiac hypertrophy (Figure 1D),as we also previously reported.[49]
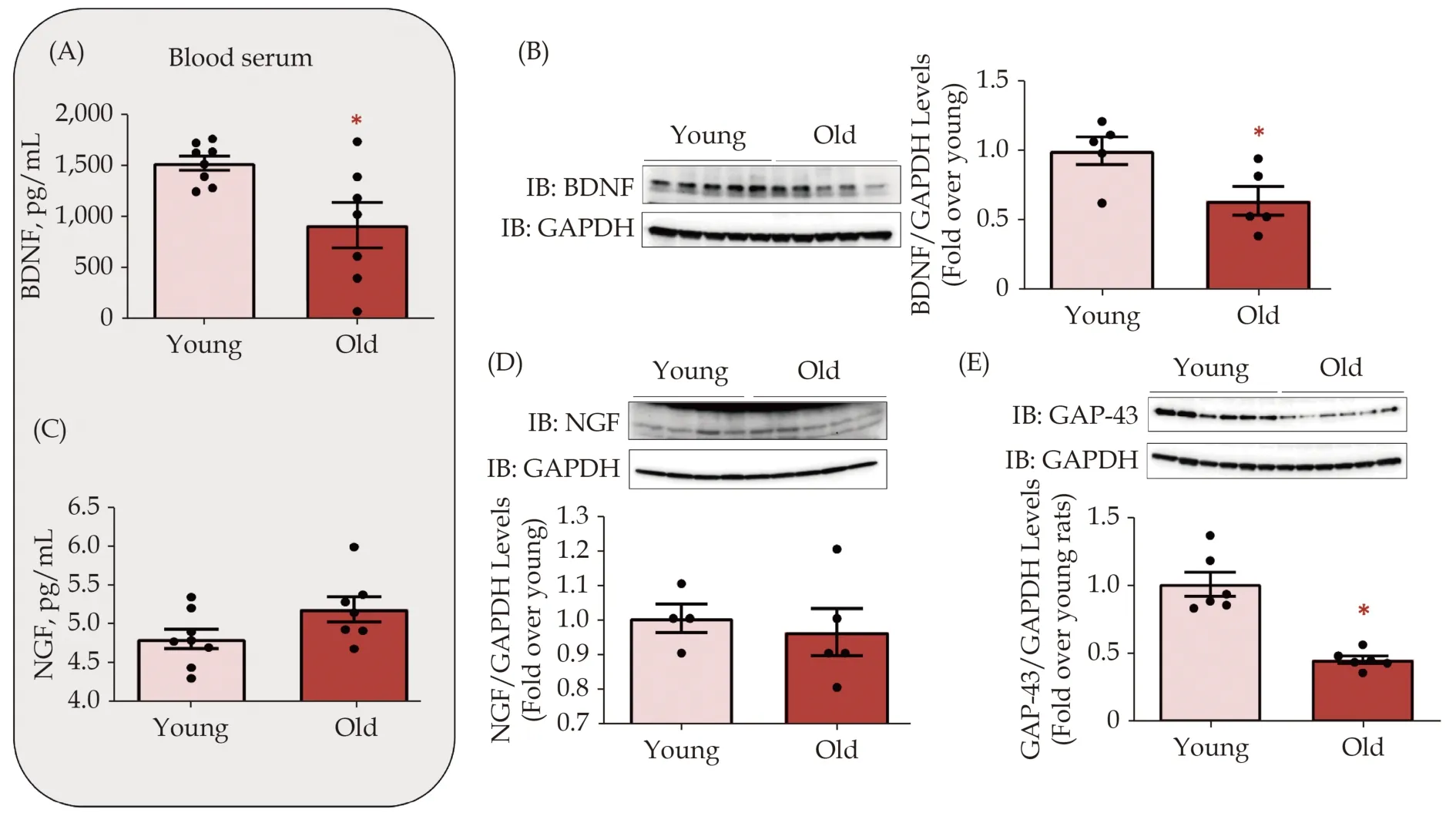
Figure 2 Impact of cardiac senescence on neurotrophic factors synthesis and release. Figure 2. Impact of cardiac senescence on neurotrophic factors synthesis and release. (A): Dot plots showing BDNF blood serum levels (pg/mL), assessed by ELISA assay, in the blood serum from young and old rats. Data are presented as a mean ± SE. *P < 0.05 vs. young serum; (B): representative immunoblots(left panels) and densitometric quantitative analysis (right panels) showing protein levels of BDNF in total cardiac lysates from young and old rats. GAPDH levels were used as loading control; *P < 0.05 vs. young. Data are shown as a mean ± SE; (C): dot plots showing NGF blood serum levels (pg/mL), assessed by ELISA assay, in the blood serum from young and old rats; and (D-E): representative immunoblots (upper panels) and quantitative data (lower panels) showing levels of NGF (D) and GAP-43 (E) in total cardiac lysates from young and old rats. GAPDH levels were used as loading control. *P < 0.05. Data are shown as a mean ± SE. BDNF: brain-derived neurotrophic factor; NGF: nerve growth factor.

Figure 3 Effects of old rat serum on neurotrophic factors and neuronal function in vitro in SH-SY5Y cells. (A-C): Representative immunoblots (A, left panels) and densitometric quantitative analysis (right panels) showing BDNF (B) and GAP-43 (C) in total protein lysates from human neuroblastoma cells (SH-SY5Y) stimulated with blood serum from young and old rats for 24 h. GAPDH levels were used as loading control; *P < 0.05 vs. young. Data are presented as a mean ± SE; (D): representative immunoblots (upper panels)and quantitative data (lower panels) showing levels of NGF in total SH-SY5Y cells lysates stimulated with blood serum harvested from young and old rats for 24 h. GAPDH levels were used as loading control; and (E) representative images (left panels) and quantitative data (right panels) showing the effects of Young and Old blood serum on neuronal sprouting (left panels, red arrows), assessed by crystal violet staining (scale bar 100 μm). Data are shown as a mean ± SE; *P < 0.05 vs. Young. BDNF: brain-derived neurotrophic factor;NGF: nerve growth factor.
Notably, the well-recognized physiological features of these aging hearts were accompanied by a marked reduction in autonomic nerve fiber density of both sympathetic and cholinergic compartments.These data confirm findings previously obtained by our group, showing an independent age-related effect on cardiac SNS innervation.[14]Altogether, this evidence also suggests that normal aging and heart failure share some salient features when cardiac innervation is concerned. Next, we assessed the impact of the aging process on neurotrophic factors such as BDNF and NGF, which govern cardiac development and cardiac function, under basal and disease conditions.[19]Loss of BDNF is implicated in age-related synaptic deterioration, preventing cognitive decline and cerebral atrophy. Of note, cognitive function gradually decreases with age, likely owing to cellular/metabolic changes resulting in a progressive loss of neurons and synaptic plasticity in brain areas involved in cognitive performance.[50−52]These alterations are often accompanied by a significant surge in reactive oxygen species (ROS).[53]
Interestingly, BDNF shows substantial protective effects against oxidative stress during neurodegenerative disorders affecting the old population.[54,55]Likewise, aging is associated with significant cerebral brain impairment due to the progressive downregulation of the expression of neurotrophic factors,such as BDNF and NGF, which, in turn, alters neurogenesis, affecting many brain regions (especially the dentate gyrus and hippocampus). This process starts during adulthood and worsens with time.[56]BDNF is a pleiotropic and almost ubiquitous protein that is secreted/expressed in several body compartments, including vascular and smooth muscle cells, skeletal muscles, platelets, and, not least, the heart.[57,58]Here, we show that aged rats have significantly reduced levels of circulating BDNF.This, in turn, can lead to a marked reduction in the stimulation of cardiac tropomyosin-like receptor kinase B (TrkB). We and others have shown that BDNF/TrkB stimulation is essential to optimize basal cardiac contraction and relaxation.[59]Thus,analogous to HF patients who harbor significantly lower circulating BDNF levels, older adults can exhibit signs of subclinical left ventricular impairment[60,61]due to depleted levels of circulating BDNF. BDNF is a primary promoter of angiogenesis in the same vein,[62]and a substantial loss in capillaries parallels maladaptive hypertrophy in the aged myocardium.[63]This deficiency impairs myocardial contractile function and relaxation. Our study adds to this scenario that loss in circulating and cardiac BDNF levels, and thus its trophic effects on myocytes and cardiac vessels, is likely central to the agerelated myocyte size/vascularization mismatch. In aggregate, this evidence suggests that loss of viable BDNF, and thus deficient cardiac TrkB activation,accounts for the significant impairment in cardiac performance observed even during normal senescence while predisposing aging people to cardiovascular disease of different natures and etiologies. That BDNF loss has a cardinal, unique contributory role in the senescence of cardiovascular function is indirectly supported by the current findings with NGF.
This neurotrophin is the most highly expressed and studied neurotrophic factor,[18,64,65]yet our evidence portends that aging eminently jeopardizes only BDNF expression. Accordingly, in total protein lysates taken from the myocardium and serum of old rats, we found that only BDNF expression was markedly downregulated. Moreover, in aged hearts, the BDNF reduction at the cardiac and systemic levels was associated with a sizable decline in the neuronal regeneration marker GAP-43, with severe denervation of ventricular tissue that affected both branches of the ANS. Of relevance, ourin vitrodata corroborated the findings obtainedin vivo. Indeed, we noticed an impairment in SH-SY5Y functionality, seen as reduced neuronal sprouting,in response to stimulation with serum withdrawn from old rats compared with that seen in the cells treated with the serum drawn from young rats. Intriguingly, the serum obtained from old rats harbored markedly reduced levels of both BDNF and GAP-43 neuronal cell expression, thus confirming that a lack of bioavailable circulating BDNF (regardless of its initial source) is pivotally involved in the deterioration of cardiovascular function during senescence.
The present contribution comes with some limitations. First, we did not monitor LV function in our rat model. However, the main goal of the present study was to determine whether aging affects myocardial autonomic innervation. Second, serum withdrawn from senescent rats can lack or miss other factors in addition to neurotrophins compared to serum circulating in young animals. However, we set out to investigate the role of neurotrophins such as BDNF and NGF because they are among the best-characterized trophic factors liable for developing and maintaining autonomic nerve fibers. However, we cannot exclude that abnormalities in the expression/release of other neurotrophic factors, such as NT3 and NT4, may also be at play in the aged myocardium.Third, previous studies showed that endothelial cells produce BDNF and that a mutual influence intervenes between BDNF and vascular endothelial growth factor (VEGF),[66]where these factors control the expression of each other. Notably, VEGF is downregulated in the aging heart,[67]and we have shown that a decline in VEGF expression/activity accounts for the transition from compensatory hypertrophy to cardiac failure.[68]Thus, BDNF loss can also be liable for VEGF reduction in the aged heart.Finally, the causes of BDNF depletion deserve further in-depth investigation.
In conclusion, these data suggest that the aging process alters the well-being of autonomic nerve fibers, thus modulating cardiac function, and this deterioration is, at least in part, ascribable to a reduction in circulating BDNF levels. In turn, as concluded before, this autonomic change increases the risk of developing cardiovascular disease. Thus,analogous to degenerative or other etiologic neuronal disorders, exogenous (recombinant) BDNF or TrkB agonists could be considered novel, exciting therapeutic tools to be used in an attempt to correct or delay some of the effects caused by age in the cardiovascular system.
FUNDING
The present work was supported by R01 HL136918 (to N.P.); STAR 2016 program (to GR).The present study was also partly supported by the Italian Ministry of Education, Universities, and Research- Rita Levi Montalcini 2016 (to AC).
AUTHOR CONTRIBUTION
A.E. designed the study, performed the experiments, and analyzed the data; A.C. wrote and revised the manuscript; G.G. and M.C. performed the experiments; N.F. R.K. and N.P. revised the manuscript; G.R. wrote and revised the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Leadless cardiac pacemaker implantations after infected pacemaker system removals in octogenarians
- Association between coronary artery calcification and cognitive function in a Chinese community-based population
- Prevalence and modifiable risk factors of degenerative valvular heart disease among elderly population in southern China
- Cardiovascular injuries and SARS-COV-2 infection: focus on elderly people
- Acute heart failure in elderly patients: a review of invasive and non-invasive management
- Vulnerable atherosclerotic plaque features: findings from coronary imaging
