Tangutidines A-C,Three Amphoteric Diterpene Alkaloids from Aconitum tanguticum
2021-08-10HaoYiLiBingChaoYanLiXinWeiHanDongSunPemaTenzinPuno
Hao-Yi Li·Bing-Chao Yan·Li-Xin Wei·Han-Dong Sun·Pema-Tenzin Puno
1 State Key Laboratory of Phytochemistry and Plant Resources in West China,Kunming Institute of Botany,Chinese Academy of Sciences,and Yunnan Key Laboratory of Natural Medicinal Chemistry,Kunming 650201,People’s Republic of China
2 University of Chinese Academy of Sciences,Beijing 100049,People’s Republic of China
3 Qinghai Provincial Key Laboratory of Tibetan Medicine Pharmacology and Safety Evaluation,Northwest Institute of Plateau Biology,Chinese Academy of Sciences,Xining 810008,People’s Republic of China
Abstract Three new diterpene alkaloids,tangutidines A-C(1-3),and four known alkaloids(4-7)were isolated from the whole plant of Aconitum tanguticum,from which amphoteric diterpene alkaloids(1-3)were obtained for the first time.The structures of 1-3 were elucidated by detailed interpretation of spectroscopic data,including MS and NMR data.All of them were evaluated for their cytotoxic activities.
Keywords Aconitum tanguticum·Amphoteric diterpene alkaloids·Tangutidine·Cytotoxic activity
1 Introduction
Plants in the genusAconitumof the family Ranuculaceae are abundant in C19-and C20-diterpene alkaloids with diverse structural scaffolds and important biological activities,which have long attracted scientists’attention from chemistry and pharmacology communities[1-3].Aconitum tanguticum(Maxim.)Stapf is mainly distributed in Tibet,Qinghai,Gansu,Sichuan and Yunnan Provinces in China[4].Its whole plant has long been used as a traditional Tibetan medicine for treating fever caused by various infectious diseases,influenza and poisoning for thousands of years[5].In a classic Tibetan Medical bookSman dpyad zla ba`i rgyal po,A.tanguticumis firstly recorded as one of the least toxic plants among other species in the genusAconitum[5].Previous phytochemical investigation ofA.tanguticumshowed that it contained diterpene alkaloids,flavonoids,phenolic acids,glycosides,etc.[6-16].However,most of the studies on diterpene alkaloids inA.tanguticumfocused on its fat-soluble part and few focused on the water-soluble part.In our phytochemical investigation of the whole plant ofA.tanguticum,it resulted in the discovery of three new C20-diterpene alkaloids with carboxyl groups,tangutidines A-C(1-3)from then-BuOH extract,together with four known alkaloids(4-7)(Fig.1).In this paper,we reported their isolation,structure determination,and cytotoxicity.
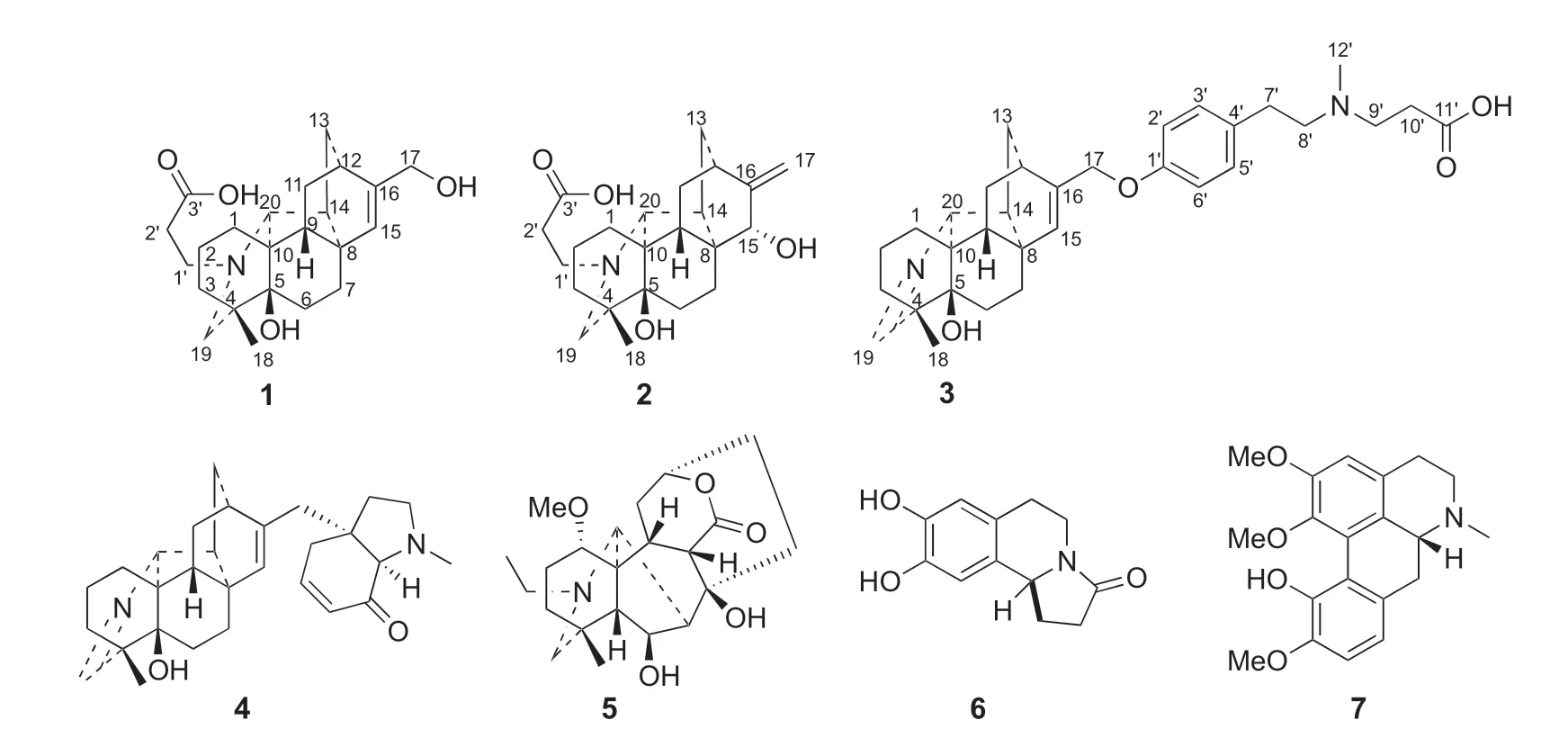
Fig.1 Structures of compounds 1-7
2 Results and Discussion
Tangutidine A(1)was isolated as colorless oil with a molecular formula of C23H33NO4,which was determined by HRESIMS([M+H]+atm/z388.2483,calcd 388.2482)with eight degrees of unsaturation.Its IR spectrum showed absorptions for hydroxyl(3428 cm−1)and carboxyl(1603 cm−1)groups.Analysis of the1H,13C NMR,DEPT and HSQC spectra of 1 revealed the existence of one olefinic bond(δH5.83,br s;δC128.6,d;150.7,s),one oxygenated methylene group(δH4.46,d,J=2.2 Hz;δC63.4,t),one tertiary methyl group(δH1.15,s;δC24.9,q),and foursp3quaternary carbon(one oxygenated)(Table 1).All the mentioned evidence suggested that 1 was a hetidine-type diterpene alkaloid containing three extra carbons.Compared with the structure of naviculine A[8]bearing a double bond between C-19 and N-atom,1 had an extra 3-N-propanoic acid moiety and two hydroxyl groups located at C-5 and C-17,respectively.The fragment C-1’/C-2’/C-3’was identified by the1H-1H COSY correlation of H2-1’/H2-2’,along with the HMBC correlation from H2-1’a(δH3.31,m)to C-3’(δC178.7,s).The connection between the fragment C-1’/C-2’/C-3’and N-atom was confirmed by the key HMBC correlation from H2-1’a(δH3.31,m)to C-20(δC79.4,d)(Fig.2)and the key ROESY correaltions from H2-1’a to H-20(δH2.70,s),H2-1’b(δH2.99,m)to H-19b(δH2.62,m)as well as H2-2’(δH2.80,m)to H-19a(δH3.11,br s)(Fig.3).The key HMBC correlations of H-18(δH1.15,s)and H-6(δH1.86 dd,J=14.2,7.0 Hz)with C-5(δC73.7,s),and H-15(δH5.83,br s)with C-17(δC63.4,t)confirmed the attachment of the hydroxyl groups to C-5 and C-17.The linkage of the hydroxyl groups to C-5 and C-17 was confirmed by the HMBC correlations from a hydroxyl group(δH4.97,br s)to C-4,C-5,and C-6,from H-18 to C-5,from H-17 to C-12 and C-16,and from H-15 to C-17.The relative configuration of 1 was established based on the ROESY spectrum,which was the same as that of naviculine A(Fig.3).Thus,the structure of 1 was elucidated with its assigned NMR spectroscopic data listed in Table 1.
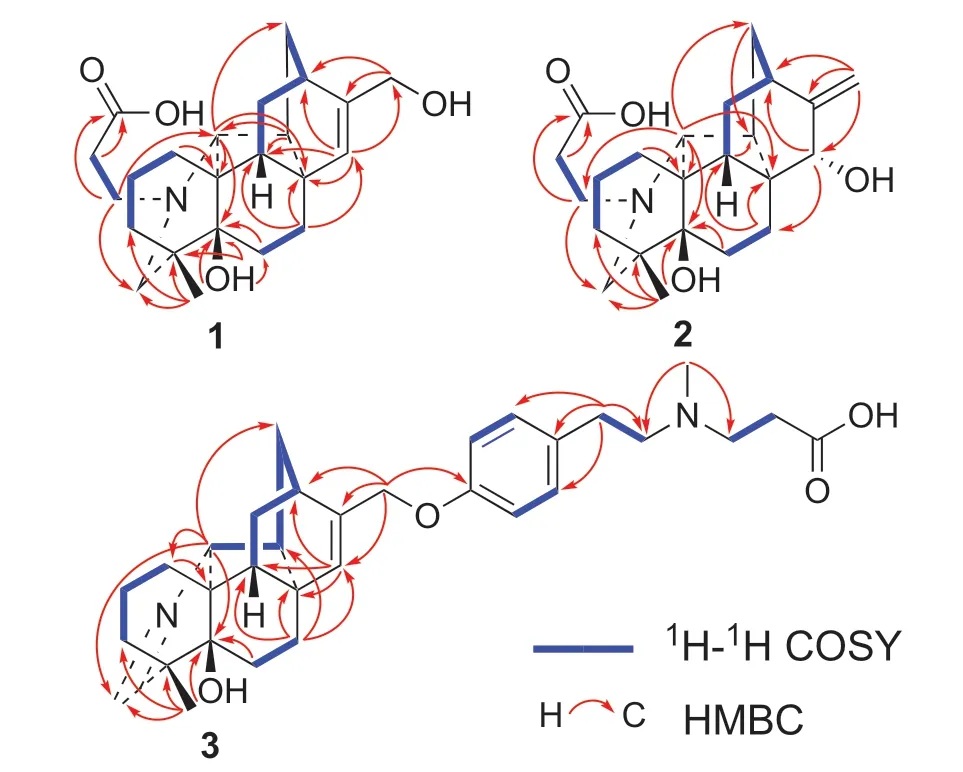
Fig.2 Key HMBC and1 H-1 H COSY correlations of compounds 1-3
Compound 2 was obtained as colorless amorphous powder.The IR spectrum showed absorptions for hydroxyl(3428 cm−1)and carboxyl(1603 cm−1)groups.Its molecular formula was assigned as C23H33NO4by the analysis of its HRESIMS(m/z388.2486[M+H]+,calcd 388.2482),indicating eight degrees of unsaturation.Comparison of the 1 H and13C NMR spectra data of 2 with that of 1(Table 1)revealed that 2 was an analogue of 1.The main diff erence between them was that an endo double bond at C-15/C-16 and a hydroxyl group at C-17 in 1 were replaced by a double bond at C-16/C-17 and a hydroxyl group at C-15 in 2.Compared with the13C NMR data of 1,some chemical shifts in that of 2 changed due to the shift of double bond:a high-field chemical shifts of C-7(δC31.6,t,Δ−1.5),C-9(δC44.3,d,Δ−6.2),C-11(δC28.4,t,Δ−0.7),C-13(δC37.9,t,Δ−4.9),C-14(δC46.8,d,Δ−2.5)and a low-field chemical shifts of C-8(δC45.7,s,Δ+1.3),C-10(δC49.0,s,Δ+1.6),and C-12(δC35.5,d,Δ+3.1).The main diff erence between 1 and 2 was further confirmed by the HMBC correlations from H-17 to C-12,C-16,and C-15,and from H-15 to C-7,C-12,and C-14.The presence of C-3’carboxyl group could be further confirmed by the HMBC correlation from both H2-1’and H2-2’to C-3’.The fragment C-1’/C-2’/C-3’connection with N-atom was confirmed by the HMBC correlation from H-20 to C-1’.The ROESY correlation of H-9β/H-15 indicated that the hydroxyl group at C-15 wasα-oriented(Fig.3).Combined with all the evidence,the structure of compound 2 was established.
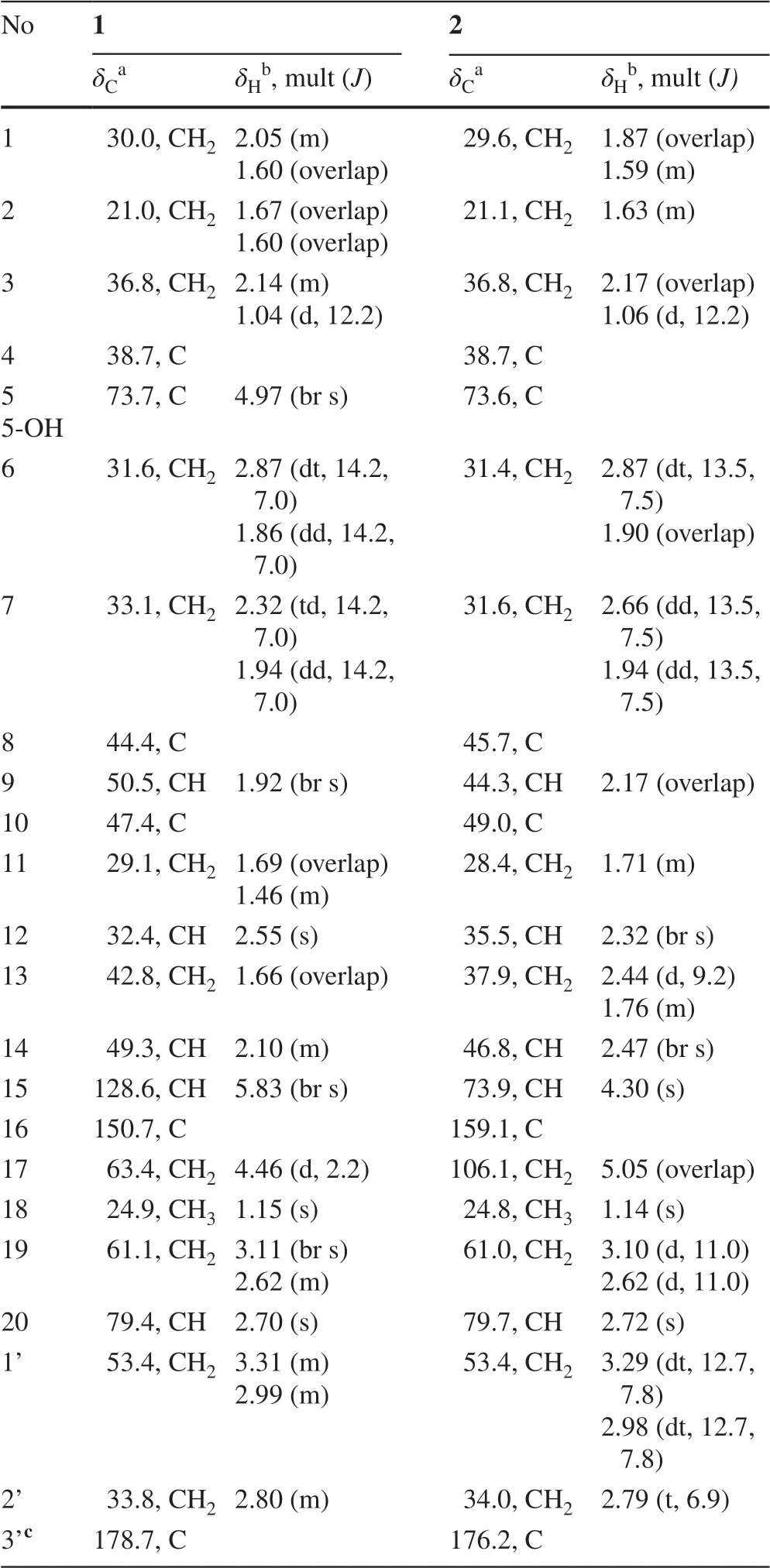
Table 1 1 H and 13 C NMR data(δ in ppm,J in Hz)of compounds 1 and 2

Fig.3 Key ROESY correlations of compounds 1-3
Compound 3 was isolated as colorless amorphous powder.Its molecular formula was deduced as C32H42N2O4by the analysis of the positive HRESIMS ion peak atm/z519.3221([M+H]+,calcd 519.3217).Its IR spectrum showed the presence of hydroxyl(3402 cm−1),phenyl(1583 cm−1,1513 cm−1,1456 cm−1),and carboxyl(1637 cm−1)groups.The1H NMR data of 3(Table 2)exhibited the signals ascribed to an imine unit(δH7.60,overlap),ap-substituted phenyl(δH7.09,d,J=8.4 Hz;7.19,overlap),a trisubstituted vinyl group(δH5.79,s),an oxygen-bearing methylene(δH4.62,s),a nitrogen-bearing methine(δH3.75,s),two methylenes connecting nitrogen(δH2.95,m;2.67,m),and two methyls(δH2.29,s;1.16,s).The13C NMR spectrum displayed the signals for two methyls,twelve methylenes(one oxygenated),ten methines(six olefinic),seven quaternary carbons(three olefinic and one oxygenated).By detailed analyses of its 2D spectra,it revealed the key1H-1H COSY correlations of H2-1/H2-2/H2-3,H2-6/H2-7,H-9/H2-11/H-12/H2-13/H-14/H-20(Fig.2),and the key HMBC correlations of H2-1/C-10,H2-6/C-5,H2-7/C-8,C-9,C-14 and C-15,H-15/C-8,C-9 and C-12,H2-17/C-12,C-15 and C-16,H3-18/C-3,C-4,C-5 and C-19,and H-20/C-1,C-5,C-13,and C-19.All the above evidence showed that 3 was also a hetidine-type diterpene alkaloid.The key1H-1H COSY correlations of H-2’/H-3’,H-5’/H-6’,H2-7’/H2-8’,and H2-9’/H2-10’,together with the key HMBC correlations from H2-7’to C-3’,C-4’,C-5’,C-8’,and correlations from H3-12’to C-8’and C-9’further confirmed the existence of this moiety.The linkage of the moiety with C-17 via a C-O bond was confirmed by the key HMBC correlation from H2-17 to C-1’.The carboxyl group was deduced to be linked with C-10’,which accounted for the residual one degree of unsaturation and an IR absorption(1637 cm−1).Thus,the structure of 3 was established,and named as tangutidine C.

Table 2 1 H and 13 C NMR data(δ in ppm,J in Hz)of compound 3
The known alkaloids(4-7)were identified to be tanaconitine[10],heteratisine[14],trolline[17]and isocorydine[18],by comparison with published physical and spectroscopic data.Alkaloids 6 and 7 were first reported fromA.tanguticum.
Additionally,compounds 1-7 were evaluated for their cytotoxicity against five human cancer cell lines(HL-60,A549,SMMC-7721,MCF-7,SW480),withcis-platin and paclitaxel as positive controls.As a result,no compounds showed activity against the tested cell lines(Table S1).
3 Experimental Section
3.1 General
Optical rotations were measured with a JASCO P-1020 polarimeter.UV spectra were obtained using a Shimadzu UV-2401 PC spectrophotometer.A Tensor 27 spectrophotometer was used for scanning IR spectroscopy with KBr pellets.HRESIMS data was acquired on an Agilent 6540 QSTAR TOF time-of-flight mass spectrometer.1D and 2D NMR spectra were recorded on Bruker DRX-600 spectrometers with TMS as internal standard.Chemical shifts(δ)are expressed in parts per million(ppm)with reference to the solvent signals.Semipreparative HPLC was performed on an Agilent 1260 liquid chromatograph with a COSMOSIL 5C18-MS-II(4.6ID×250 mm)column.Column chromatography(CC)was performed with silica gel(80-100 and 100-200 mesh;Qingdao Marine Chemical,Inc.,Qingdao,People’s Republic of China),Sephadex LH-20(Pharmacia,Uppsala,Sweden)and SEPAFlash column(Spherical C-18,20-45 μm,100Åm).Fractions were monitored by TLC(thin-layer chromatography)and spots were detected with the modified Dragendorff ’s reagent.
3.2 Plant Material
The dried whole plants ofAconitum tanguticum(Maxim.)Stapf(Ranunculaceae)were provided by Qinghai Jinke Tibetan Medicine Pharmaceutical Co.Ltd.in 2019 and identified by Prof.Yu-Bi Zhou.A voucher specimen(No.2019-WZ-01)is deposited in Qinghai Provincial Key Laboratory of Tibetan Medicine Pharmacology and Safety Evaluation,Xining,China.
3.3 Cytotoxicity Assay
Five human cancer cell lines,human myeloid leukemia HL-60,lung cancer A-549 cells,hepatocellular carcinoma SMMC-7721,breast cancer MCF-7,and colon cancer SW480,were purchased from the Shanghai Institute of Biochemistry and Cell Biology,Chinese Academy of Sciences(Shanghai,China).Cells were cultured according to the manufacturer’recommendations.All mediums were supplemented with 10% fetal bovine serum(FBS),100 units/ml penicillin G sodium and 100 μg/ml streptomycin(HyClone).All the cells were incubated at 37 °C,5% CO2in a humidified atmosphere.Cytotoxicity of compounds was determined by MTS method.Briefly,5×103cells were plated in 96-well plates 12 h before treatment and continuously exposed to test compounds for 48 h.Then MTS(Promega)was added to each well.The samples were incubated at 37 °C for 1-4 h and the optical density(OD)was measured at 492 nm using a microplate reader(MULTISKAN FC).The IC50values were calculated by Reed and Muench’s method[19].
3.4 Extraction and Isolation
Dried whole plants ofA.tanguticum.(8.8 kg)were powered and extracted with 70% EtOH(40 L each)for three times,each time for 3 days.The extract was filtered and concentrated under reduced pressure to give the crude extract.The extract was suspended in water,solution was acidified with 5% aq.HCl to pH 2.0 and the acidic solution was successively extracted with petroleum ether(PE),CHCl3andn-BuOH.Then the acidic solution was was basified with saturated NaOH solution and extracted with CHCl3.Then-BuOH part was basified with saturated NaOH solution and extracted with CHCl3,EtOAc andn-BuOH.The EtOAc part was concentrated to yield the total crude alkaloids(28 g).The part(28 g)was applied to ODS chromatography by eluting with MeOH-H2O(23:67 to 100:0)to give four fractions E1-E4.Fr.E4(12 g)was subjected to silica gel CC with CHCl3-Acetone-DEA(15:1:0.1 to 0:1)to aff ord eight fractions F1-F8.Then Fr.F3(800 mg)was chromatographed on flash column by eluting with MeOH-H2O(3:7 to 10:0)to yield five fractions F3A-F3E.Fr.F3B(100 mg)was subjected on Sephadex LH-20(CHCl3-MeOH,1:1)to give subfractions B1-B6.Fr.B2(50 mg)was purified by semi-preparative HPLC(3 ml/min,MeCN/H2O 15.9:84.1)to yield 1(2.8 mg,tR=7.2 min),2(4.0 mg,tR=11.5 min)and 3(1.0 mg,tR=21.1 min).Fr.F2(1 g)was chromatographed on flash column with a MeOH-H2O(3:7 to 10:0)gradient system to yield five fractions F2A-F2E.Fr.F2D(18.7 mg)was purified by semi-preparative HPLC(3 ml/min,MeCN/H2O 20:80)to yield 6(1.5 mg,tR=25.2 min).Fr.F2A(30 mg)was purified by semi-preparative HPLC(3 ml/min,MeCN/H2O 16:84)to yield 7(20.3 mg,tR=4 min).The CHCl3extract(1 g)was subjected to silica gel CC with a Acetone-PE-DEA(diethylamine)gredient system(10:1:0.1,8:1:0.1,5:1:0.1,3:1:0.1,1:1:0.1 and 1:0:0.1)to yield six frictions A-F.Fr.C(120 mg)was subjected on Sephadex LH-20(CHCl3-MeOH,1:1)to give subfrictions C1-C5.Fr.C3(25 mg)was purified by semi-preparative HPLC(3 ml/min,MeCN/H2O/1‰ triethylamine 63:37)to yield 4(19.5 mg,tR=15.6 min).Fr.F(56.7 mg)was purified by semi-preparative HPLC(3 ml/min,MeCN/H2O/1 ‰ triethylamine 55:45)to yield 5(15.3 mg,tR=45 min).
3.5 Physical Constants and Spectroscopic Data of Compounds 1-3
3.5.1 Tangutidine A(1)
3.5.2 Tangutidine B(2)
Colorless amorphous powder;[α]21D−50.8(c0.09,MeOH);UV(MeOH)λmax(log ε)196(5.02)nm;For1H NMR and 13C NMR spectroscopic data,see Table 1;HRESIMSm/z388.2486[M+H]+(calcd for C23H34NO4,388.2482).
3.5.3 Tangutidine C(3)
Supplementary InformationThe online version contains supplementary material available at https://doi.org/10.1007/s13659-021-00310-3.
AcknowledgementsThis project was supported financially by the Second Tibetan Plateau Scientific Expedition and Research(STEP)program(2019QZKK0502)and the National Natural Science Foundation of China(No.81673329).Authors are grateful for Dr.Ming Zhang from northwest institute of plateau biology,Chinese Academy of Sciences,for the assistance for obtaining plant materials.
Declarations
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Natural Products and Bioprospecting的其它文章
- Hinokiflavone and Related C-O-C-Type Biflavonoids as Anti-cancer Compounds:Properties and Mechanism of Action
- α-C(sp3)-H Arylation of Cyclic Carbonyl Compounds
- Naturally Occurring Terpenes:A Promising Class of Organic Molecules to Address Influenza Pandemics
- Diterpenoid Alkaloids from the Aerial Parts of Aconitum flavum Hand.-Mazz
- Screening and Purification of Natural Products from Actinomycetes that Induce a“Rounded”Morphological Phenotype in Fission Yeast
- Psathyrellins A-E,Antibacterial Guanacastane Diterpenoids from Mushroom Psathyrella candolleana
