Psathyrellins A-E,Antibacterial Guanacastane Diterpenoids from Mushroom Psathyrella candolleana
2021-08-10HanWuHuiXiangYangZhengHuiLiTaoFengJiKaiLiu
Han Wu·Hui-Xiang Yang·Zheng-Hui Li·Tao Feng,·Ji-Kai Liu,
1 Anhui Key Laboratory of Modern Chinese Materia Medica,School of Pharmacy,Anhui University of Chinese Medicine,Hefei 230012,People’s Republic of China
2 School of Pharmaceutical Sciences,South-Central University for Nationalities,Wuhan 430074,People’s Republic of China
Abstract Five previously undescribed guanacastane diterpenoids,namely psathyrellins A-E(1-5),were obtained from cultures of the mushroom Psathyrella candolleana.Their structures with absolute configurations were elucidated by extensive spectroscopic methods.Compounds 1-3 showed antibacterial activity against four strains with MIC values in a range of 16-128 μg/mL.
Keywords Psathyrella candolleana·Guanacastane diterpenoids·Antibacterial activity
1 Introduction
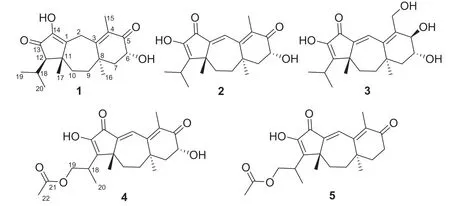
Fig.1 Structures of compounds 1-5
2 Results and Discussion
Psathyrellin A(1)was isolated as colorless crystals.Its molecular formula was determined as C20H28O4on the basis of HRESIMS atm/z333.20584(calcd for C20H28O4[M+H]+,333.30604),implying seven degrees of unsaturation.The IR absorption bands at 3421,1710,1640,1462 cm−1revealed the existence of hydroxy,carbonyl,and double bonds.In the1H NMR spectrum(Table 1),three singlets atδH1.06,1.09,1.85,two doublets atδH0.79(3H,d,J=6.9 Hz),1.12(3H,d,J=6.9 Hz)were readily assigned for five methyl groups.In addition,one doublet atδH4.46(1H,dd,J=14.0,5.2 Hz)indicated an oxidized methine carbon.In the13C NMR spectrum,a total of 20 carbon resonances were detected.They were classified into five CH3,four CH2,three CH,and eight non-protonated carbons bythe DEPT and HSQC spectra(Table 2).Of them,two carbonyl signals atδC200.8(s,C-5)and 205.4(s,C-13),and four olefinic signals four two double bonds atδC150.2(s,C-1),162.5(s,C-3),130.5(s,C-4),and 149.3(s,C-14)occupied four degrees of unsaturation,suggesting a tricyclic backbone of 1.All these data,as well as the literature survey[5,6,14],suggested that 1 should be a guanacastane diterpenoid characterized with a 5/7/6-fused ring system.Preliminary analysis of 2D NMR data(especially the HMBC data)revealed twoα,β-unsaturated keto moieties in rings A and B,respectively(Fig.2).A HMBC correlation fromδH4.46(1H,dd,J=14.0,5.2 Hz,H-6)to C-5,as well as a 1 H-1H COSY correlation between H-6 and H-7,suggested a hydroxy group placed at C-6.After many attempts,a single crystal of 1 was obtained from methanol,while the single crystal X-ray diff raction revealed the absolute configuration of 1 as shown in Fig.3(Flack parameter=0.05(3);CCDC:2068966).
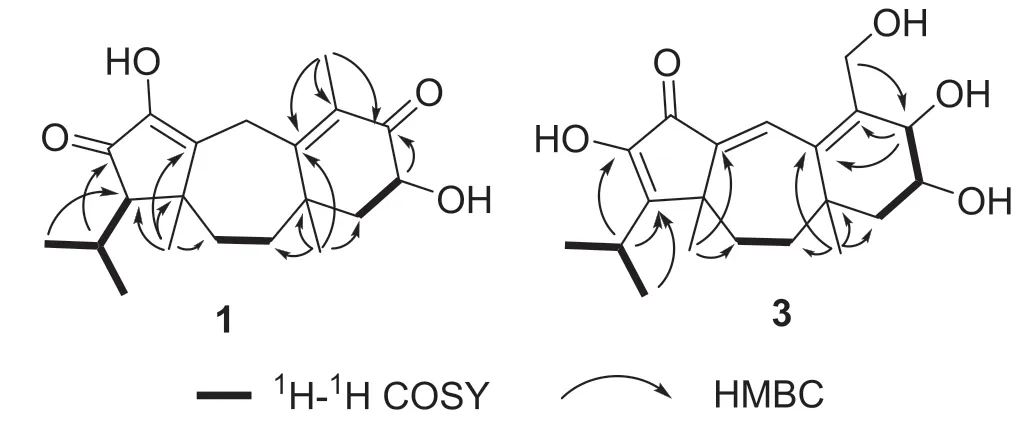
Fig.2 Key1 H-1 H COSY and HMBC correlations of 1 and 3
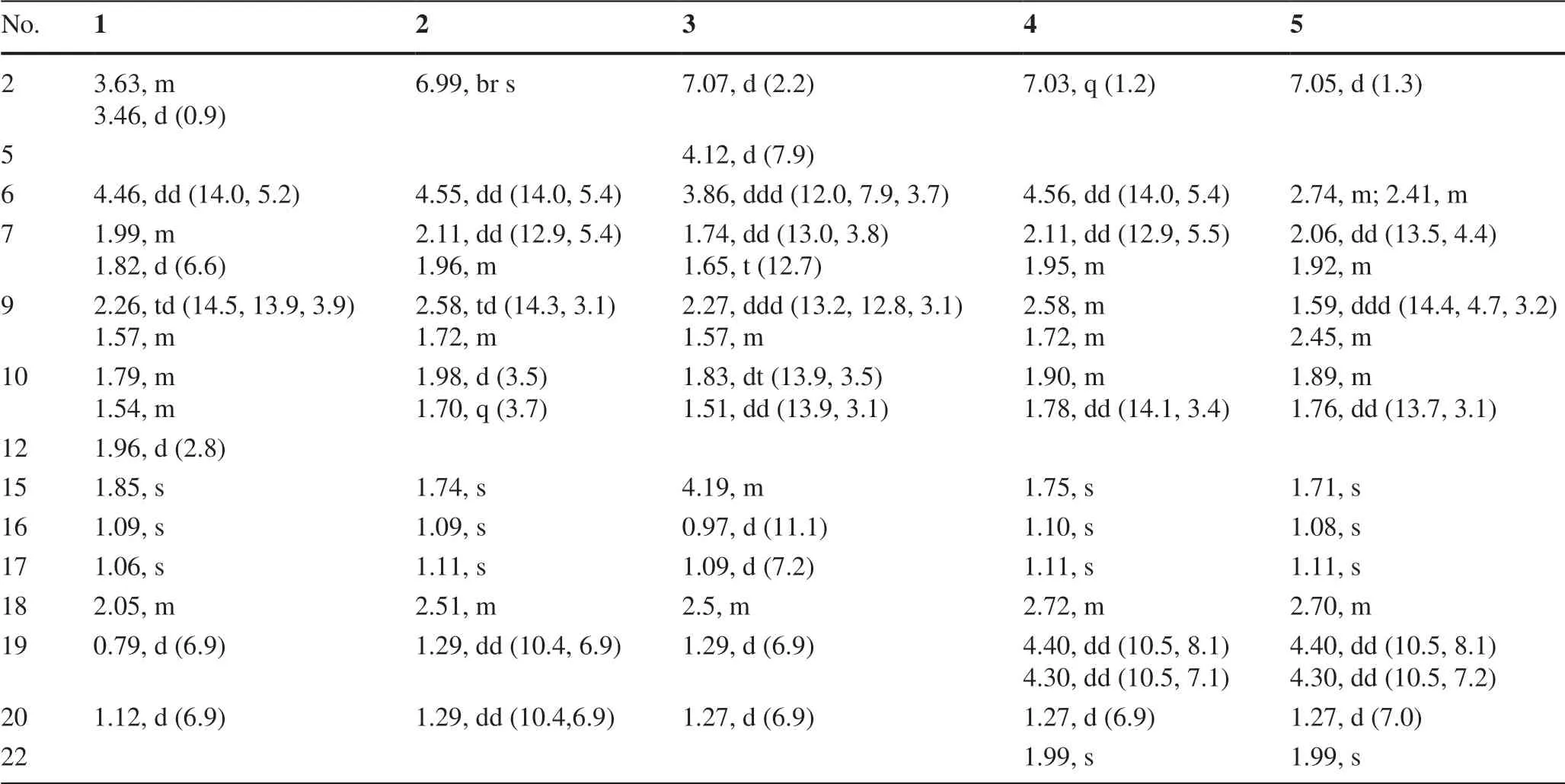
Table 1 1 H NMR Data for 1-5 in Methanosl-d4(δ in ppm,J in Hz)
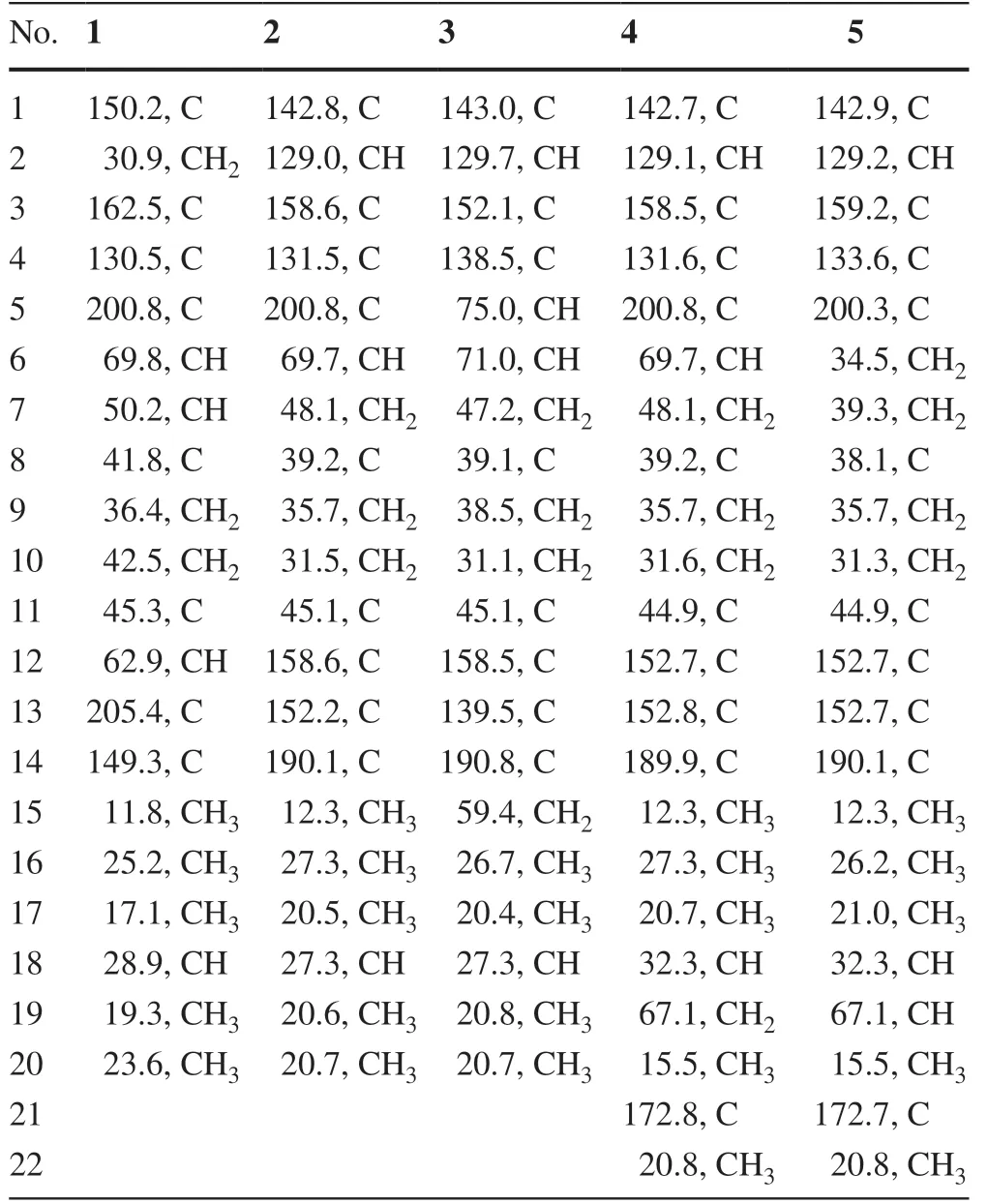
Table 2 13 C NMR Data for 1-5 in Methanol-d4(150 MHz,δ in ppm)
Psathyrellin B(2)was isolated as colorless crystals.Its molecular formula was determined as C20H26O4by HRESIMS data atm/z331.19031[M+H]+(calcd for,331.19039).The UV data at 282 nm suggested a conjugated system in 2.The 1D and 2D NMR spectra revealed similar patterns to those of 1 except that one more double bond in 2.In the HMBC spectrum,correlations fromδH1.11(3H,s,H3-17)toδC142.8(s,C-1)and 158.6(s,C-12),fromδH6.99(1H,br s,H-2)to C-1 andδC158.6(s,C-3),and fromδH1.74(3H,s,H3-15)to C-3 andδC131.5(s,C-4)suggested that three double bonds were distributed at C-12/C-13,C-1/C-2,and C-3/C-4,respectively.Detailed analysis of 2D NMR data suggested that the other parts of 2 were the same to those of 1.A single crystal X-ray diff raction not only proved the planar structure,but also determined the absolute configuration of 1 as shown in Fig.3(Flack parameter=−0.12(14);CCDC:2068967).
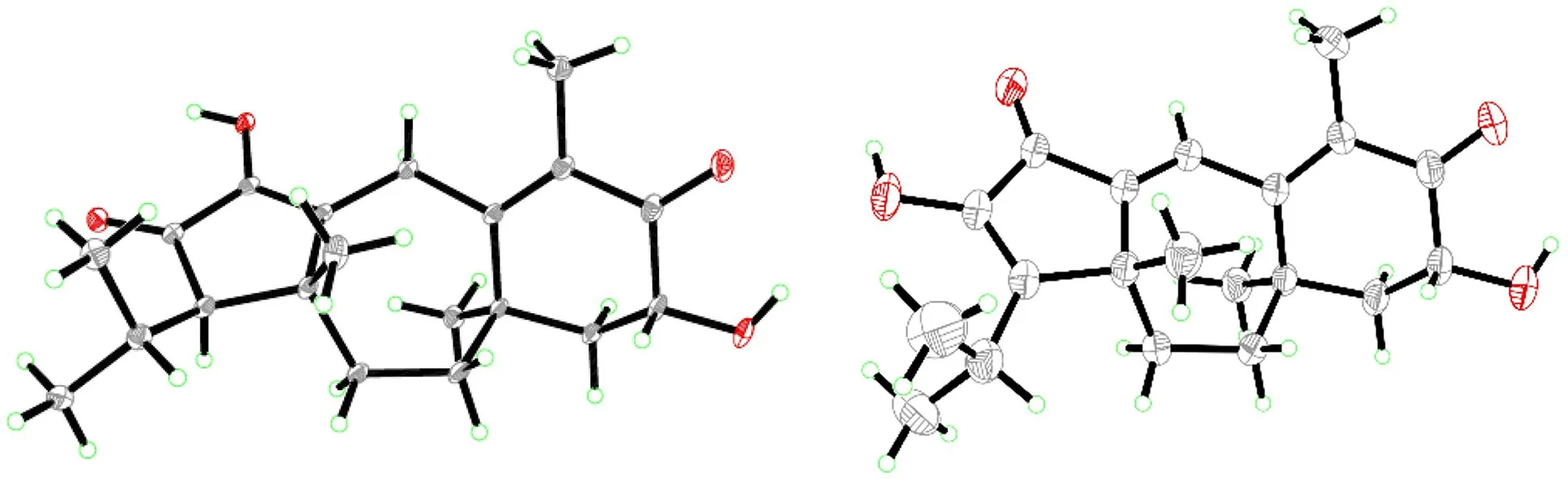
Fig.3 ORTEP diagrams of 1(left)and 2(right)
Psathyrellin C(3)was isolated as a yellow oil.The molecular formula was determined as C20H28O5on the basis of HRESIMS data atm/z349.20093[M+H]+(calcd for,349.20095).All NMR data suggested that 3 was structurally similar to that of 2(Fig.2).In compound 3,C-15 was oxidized into a hydroxymethylene atδC59.4.In addition,C-5 was reduced into a hydroxymethine atδC75.0.These information were supported by the HMBC and 1 H-1H COSY data.In the ROESY spectrum,correlation of H-6 with H-9 suggested that OH at C-6 should beαoriented.Based on this information,the coupling constant ofJ5,6=7.9 Hz indicated that the OH at C-5 should beβoriented.The CD spectrum of 3 revealed similar Cotton eff ects with those of 2(Fig.4),indicating the absolute configuration of 3 to be the same to that of 2.
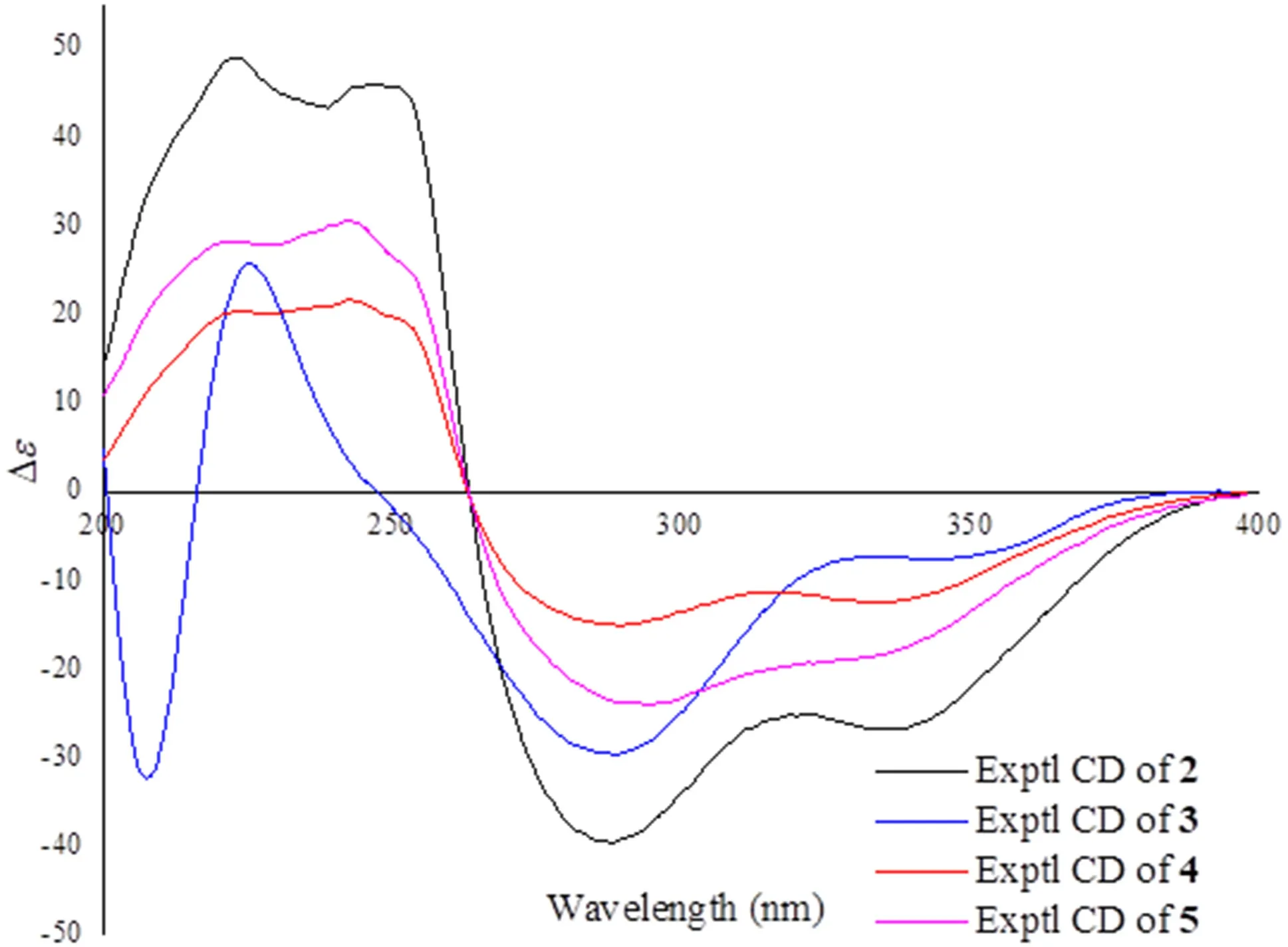
Fig.4 CD curves of compounds 2-5
Psathyrellin D(4)was isolated as a yellow oil.The molecular formula was determined as C22H28O6on the basis of HRESIMS data atm/z411.17749[M+Na]+(calcd for C22H28O6Na+,411.17781).All spectroscopic data of 4 were similar to those of 2 excepted two additional carbons atδC20.8(q)and 172.8(s)in 4 that were easily assigned as anO-acetyl moiety.The HMBC correlations fromδH4.40 and 4.30 toδC172.8(s)and fromδH1.27(3H,d,J=6.9 Hz,H3-20)toδC32.3(d,C-18),67.1(t,C-19)suggested that theO-acetyl moiety should be placed at C-19.Detailed analysis of 2D NMR data suggested that the other parts of 4 were the same to those of 2.The CD spectrum almost showed the same curve to that of 2,indicating the absolute configuration of the main backbone in 4 to be the same to that of 2(Fig.4).However,the stereochemistry of C-18 could not be established currently.
Psathyrellin E(5)was isolated as a yellow oil.The molecular formula was determined as C22H28O5on the basis of HRESIMS data atm/z373.20084[M+H]+(calcd for,373.20095).According to analysis of 1D and 2D NMR data,compound 5 was easily identified as a 19-O-acetyl derivative of 2,which was also similar to 4.The only diff erence was that the hydroxymethine of C-6 in 4 was reduced into a methylene atδC34.5(t,C-6)in 5,as supported by the HMBC correlation from H-6 to C-5 and the 1 H-1H COSY data between H-6 and H-7.The CD spectrum almost indicated the same curve to that of 2,indicating the absolute configuration of the backbone in 5 to be the same to that of 2(Fig.4).However,the stereochemistry of C-18 could not be established currently.
Compounds 1-5 were evaluated for their antibacterial activities againstEscherichia coli,Staphylococcus aureus,Salmonella enterica,andPseudomonas aeruginosa.As a result,compounds 1-3 showed antibacterial activity with MIC values in a range of 16-128 μg/mL(Table 3).
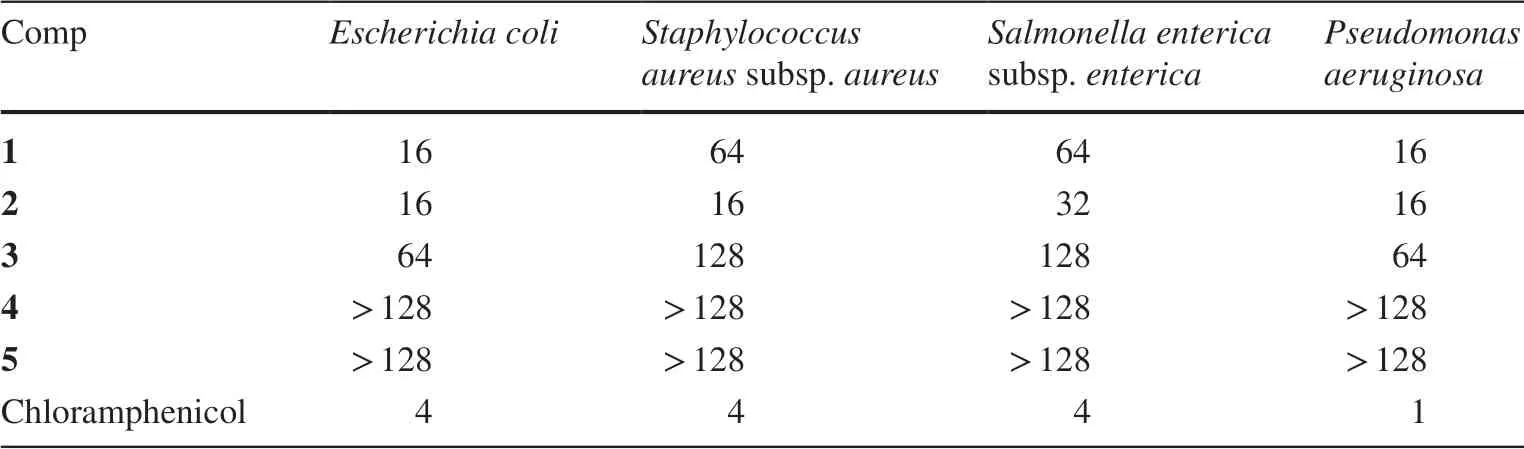
Table 3 Antibacterial activity of 1-5(MIC,μg/mL)
3 Experimental Section
3.1 General Experimental Procedures
Optical rotations were measured on a Rudolph Autopol IV polarimeter.UV spectra were obtained on a UH5300 UV-VIS Double Beam Spectrophotometer.IR spectra were obtained by using a Shimadzu Fourier Transform Infrared spectrometer with KBr pellets.NMR spectra were acquired with a Bruker Avance III 600 instrument.CD spectra were recorded with an Applied Photophysics Chirascan-Plus spectrometer.High resolution electrospray ionization mass spectra(HRESIMS)were recorded on a LC-MS system consisting of a Q Exactive™ Orbitrap mass spectrometer with an ESI ion source used in ultra-high resolution mode(140,000,atm/z200)and a Dionex UltiMate 3000 RSLC UPLC system.Silica gel(200-300 mesh and 500-800 mesh),RP-18 gel(40-75 μm)and Sephadex LH-20 were used for column chromatography(CC).Preparative HPLC was performed on an Agilent 1260 liquid chromatography system with a Zorbax SB-C18(5 μm,9.4×150 mm)column,a Daicel chiral column(AS-H,5 μm,4.6×250 mm)and a DAD detector.
The blanket slid to the floor as the old man took the fiddle and stood up. He tuned4 up for a minute, and then said, This is one you ll like to remember.
3.2 Fungal Material and Cultivation Conditions
Fruiting bodies ofP.candolleanawere collected at Jingdong of Yunnan Province,China in 2003.They were identified by Prof.Zhu-Liang Yang of Kunming Institute of Botany,Chinese Academy of Sciences.The voucher specimen(NO.CGBWSHF00118.2)was deposited at School of Pharmaceutical Sciences,South-Central University for Nationalities.The strains were cultured in PDA and stored at−4 °C.Culture medium was composed of glucose(5%),pork pepton(0.15%),yeast(0.5%),KH2PO4(0.05%)and MgSO4(0.05%).Initial pH was adjusted to 6.0,the fermentation was first carried out on an erlenmeyer flask for 6 days till the mycelium biomass reached to the maximum.Then it was transferred to rice medium at 24 °C in dark culture for 40 days.The rice medium in each 250 mL-Erlenmeyer flask was composed of rice(50 g)and water(50 mL).A total of 180 bottles were used in this study.
3.3 Extraction and Isolation
The rice fermentation(9 kg)was extracted four times with EtOAc.The organic layer was evaporated to give a crude extract(90 g).The extract was subjected to silica gel CC(200-300 mesh)eluted with a gradient solvent system of petroleum ether(PE)/Me2CO(from 20:1 to 1:1)to aff ord eight fractions A-H.Fraction C(3.8 g)was isolated by CC over silica gel using PE/Me2CO(6/1)to give subfractions C1-C6.Compound 1 was deposited from fraction C4 ascolorless crystals(6 mg;purity >95%).Fraction E(4 g)was first isolated by silica gel CC(200-300 mesh)eluted with PE/Me2CO(5/1)to give five subfractions E1-E5.Fraction E2(800 mg)was further isolated by CC using RP-C18 silica gel(MeOH/H2O from 6/4 to 9/1)to give subfractions E2a-E2e.HPLC preparation(MeCN/H2O from 7/3 to 8/2 in 20 min)on fraction E2d(82 mg)aff orded compounds 3(1.8 mg,retention time(tR)=12.1 min;purity 90%),4(2.8 mg,tR=12.8 min;purity 90%),and 2(4.3 mg,tR=14.6 min;purity >95%).Fraction E2e(70 mg)was separated by CC over Sephadex LH-20(MeOH)to give a mixture.The mixture was subjected to HPLC(MeCN/H2O from 7/3 to 8/2 in 20 min)to give compound 5(2.6 mg,tR=15.1 min;purity 90%).
3.4 Spectroscopic Data of Compounds
3.4.1 Psathyrellin A(1)
3.4.2 Psathyrellin B(2)
3.4.3 Psathyrellin C(3)
3.4.4 Psathyrellin D(4)
3.4.5 Psathyrellin E(5)
3.4.6 X-Ray Crystallographic Data for Psathyrellin A(1)
C20H28O4,M=332.42,a=14.0862(3)Å,b=7.26370(10)Å,c=17.3636(3)Å,α=90°,β=99.5460(10)°,γ=90°,V=1752.01(5)Å3,T=100.(2)K,space groupP1211,Z=4,μ(Cu Kα)=0.692 mm−1,33652 reflections measured,6741 independent reflections(Rint=0.0259).The finalR1values were 0.0306[I>2σ(I)].The finalwR(F2)values were 0.0799[I>2σ(I)].The finalR1values were 0.0307(all data).The finalwR(F2)values were 0.0800(all data).The goodness of fit onF2was 1.056.Flack parameter=0.05(3).CCDC:2068966(www.ccdc.cam.ac.uk).
3.4.7 X-Ray Crystallographic Data for Psathyrellin B(2)
C20H26O4,M=330.41,a=8.0026(8)Å,b=8.3729(9)Å,c=14.4078(15)Å,α=90.00°,β=105.783(3)°,γ=90.00°,V=929.00(17)Å3,T=150.(2)K,space groupP1211,Z=2,μ(CuKα)=1.54178 mm−1,12059 reflections measured,3552 independent reflections(Rint=0.0456).The finalR1values were 0.0559[I>2σ(I)].The finalR1values were 0.0415(all data).The finalwR(F2)values were 0.1156(all data).The goodness of fit onF2was 1.092.Flack parameter=−0.12(14).CCDC:2068967(www.ccdc.cam.ac.uk).
3.5 Antibacterial Assay
The tested bacteria strainsEscherichia coliATCC25922,Staphylococcus aureussubsp.aureusATCC29213,Salmonella entericasubsp.entericaATCC14028,Pseudomonas aeruginosaATCC27853 were purchased from China General Microbiological Culture Collection Center,(CGMCC).All these strains were cultured in Mueller Hinton broth(MHB)(Guangdong Huankai Microbial Sci.&Tech.Co.,Ltd.)at 37 °C overnight with shaking(200 rpm).A sample of each culture was then diluted 40-fold in fresh MHB broth and incubated with shaking(200 rpm)at 37 °C for 2.5 h.The resultant mid-log phase cultures were diluted to a concentration of 5×105CFU/mL,then 50 mL was added to each well of the compound-containing plates.The minimum inhibition concentration(MIC)was determined by measuring bacterial growth after 24 h on performing 1:2 serial dilutions of each compound ranging from 1 to 128 μg/mL.Chloramphenicol was used as a positive control.
Supplementary InformationThe online version contains supplementary material available at https://doi.org/10.1007/s13659-021-00316-x.
AcknowledgementsThe work is financially supported by the National Key Research and Development Program of China(Grant No.2017YFC1704007).The authors thank Analytical &Measuring Centre,South-Central University for Nationalities for the spectra measurements.
Declarations
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Natural Products and Bioprospecting的其它文章
- Hinokiflavone and Related C-O-C-Type Biflavonoids as Anti-cancer Compounds:Properties and Mechanism of Action
- α-C(sp3)-H Arylation of Cyclic Carbonyl Compounds
- Naturally Occurring Terpenes:A Promising Class of Organic Molecules to Address Influenza Pandemics
- Diterpenoid Alkaloids from the Aerial Parts of Aconitum flavum Hand.-Mazz
- Screening and Purification of Natural Products from Actinomycetes that Induce a“Rounded”Morphological Phenotype in Fission Yeast
- New Secodaphnane-Type Alkaloids with Cytotoxic Activities from Daphniphyllum angustifolium Hutch
