Management of early rectal cancer;current surgical options and future direction
2021-07-28VijayChavdaOliverSiawSanjayChaudhriFranscoisRunau
Vijay Chavda,Oliver Siaw,Sanjay Chaudhri,Franscois Runau
Vijay Chavda,Oliver Siaw,Sanjay Chaudhri,Franscois Runau,Department of General Surgery,Leicester General Hospital,Leicester LE5 4PW,United Kingdom
Abstract Rectal cancer is the second commonest cause of cancer death within the United Kingdom.Utilization of national screening programmes have resulted in a greater proportion of patients presenting with early-stage disease.The technique of transanal endoscopic microsurgery was first described in 1984 following which further options for local excision have emerged with transanal endoscopic operation and,more recently,transanal minimally invasive surgery.Owing to the risks of local recurrence,the current role of minimally invasive techniques for local excision in the management of rectal cancer is limited to the treatment of pre-invasive disease and low risk early-stage rectal cancer(T1N0M0 disease).The roles of chemotherapy and radiotherapy for the management of early rectal cancer are yet to be fully established.However,results of high-quality research such as the GRECCAR II,TESAR and STAR-TREC randomised control trials may highlight a wider role for local excision surgery in the future,when used in combination with oncological therapies.The aim of our review is to provide an overview in the current management of early rectal cancer,the surgical options available for local excision and the future multimodal direction of early rectal cancer treatment.
Key Words:Early rectal cancer;Transanal endoscopic microsurgery;Transanal endoscopic operation;Transanal minimally invasive surgery
INTRODUCTION
Colorectal cancer is the third commonest malignancy in the United Kingdom,accounting for the second greatest number of cancer related deaths[1].Although incidence within the older population has stabilized,there has concerningly been a sharp increase in disease amongst the younger cohort(age group 20-39)within the last 20 years[2].Surgical options for the local excision of rectal lesions have developed significantly over the last four decades beginning with the technique of transanal microscopy surgery(TEM),which was first described in 1984.Following introduction of the bowel cancer screening programme within the United Kingdom in 2006 there has been a demonstrable increase in the number of patients presenting with earlystage disease as well as a 15% reduction in mortality[3,4].Furthermore,advances in the understanding of risk factors for early-stage disease has identified a cohort of patients in whom local excision techniques may lead to favorable outcomes,whilst avoiding the risks of major resectional surgery.The aim of our review is to provide an overview of the current management strategies for early rectal cancer and highlight areas of development which may dictate its direction in the future.
STAGING OF RECTAL CANCER AND DEFINITION OF EARLY DISEASE
There are variable definitions of early rectal cancer often based on endoscopic,radiological and histological characteristics;however,a consensus based on the expert opinions of the European Association of Endoscopic Surgery and the European Society of Coloproctology in 2015 defined early rectal cancer as “a rectal cancer with good prognostic features that might be safely removed preserving the rectum and that will have a very limited risk of relapse after local excision”[5].
Rectal cancers are staged according to the Union for International Cancer Control tumor-node-metastasis classification[6].Staging investigations incorporate radiological,endoscopic and histological resources where available.In general computed tomography(CT)scanning of the chest/abdomen/pelvis,together with positron emission tomography scanning where there are equivocal findings at CT,are required for the assessment of distant metastatic disease.Local staging of rectal cancer utilizes magnetic resonance imaging(MRI)scanning,endo-rectal ultrasound or a combination of the two.Endo-rectal ultrasound has been suggested to offer a higher accuracy in staging early disease compared to MRI,however offers poorer accuracy for lymph node assessment.The converse is suggested to be true for MRI scanning[7,8].Both modalities were suggested to over-stage early T1/T2 disease and showed poor differentiation between the two[9].Endoscopic evaluation allows mucosal assessment,opportunity for biopsy where indicated and exclusion of synchronous colonic lesions.
ENDOSCOPIC PREDICTORS OF DISEASE
The majority of colorectal cancers are histologically of the adenocarcinoma subtype,arising from the dysplastic progression of benign adenomateous polyps into invasive disease.The adenoma-carcinoma pathway has been extensively studied and its manifestation is widely accepted to occur due to the accumulation of genetic mutations within the dysplastic cell[10].The development of invasive disease from the dysplastic progression of adenomas is an important consideration within the context of early rectal cancer(and colorectal cancers in general),as the complete excision of a high-risk polyp would prevent its progression to invasive disease.At present the distinction between a dysplastic high-risk polyp and early invasive disease can only be conclusively determined on histological assessment of the resected specimen,owing to the inaccuracies of local staging modalities;whereby invasive disease is demonstrated by the involvement of neoplastic cells into the submucosa of the bowel wall.To allow an accurate histological assessment of resection margins and depth of invasion,a complete en-bloc excision of the specimen should be performed where possible,rather than a piecemeal retrieval in which there is specimen fragmentation.This is of particular importance when there are high risk features or suspicions of invasive disease present at endoscopy.
Not all polyps have the same malignant potential and there are several polyp characteristics assessed at endoscopy which have been shown to be predictive of invasive disease.Morphology of mucosal lesions are graded according to the Paris classification with pedunculated polyps associated with the lowest risk followed by sessile polyps(Paris classification 1p and 1s).Flat lesions(Paris classification IIa and IIb)have intermediate risk and depressed lesions(Paris classification IIc)have the highest risk[11].Excavated/ulcerated superficial lesions are graded as Paris classification III and are generally not seen within the colo-rectum[11].Increasing polyp size has also been shown to be associated with the risk of invasive disease.In general lesions < 5 mm in size are associated with a negligible risk,whereas lesions > 35 mm were found to have invasive disease in more than 75% of specimens,based on the assessment of more than 11000 polyps by Nuskoet al[12].The risk of invasive disease in depressed lesions,regardless of size,has been shown to be significant and as such they should be treated as high risk[11,12].Polyp surface pit pattern(graded according to the Kudo classification[13])and microvascular capillary architecture(grader according to the Sano classification[14])can also aid the prediction of invasive disease.
Following assessment of polyp characteristics lesions deemed to have low risk of invasive disease may be amenable to resection using endoscopic techniques.In general,smaller polyps up to 2cm in size can be exciseden-blocusing snare polypectomy[15].The technique of endoscopic mucosal resection(EMR);whereby fluid is injected to create a sub-mucosal cushion raising the overlying mucosa and increasing the vertical excision plane,can be used to facilitate snare polypectomy.Following fluid injection,non-lifting of the lesion is suggestive of sub-mucosal tethering and is indicative of invasive disease,although non-lifting can also occur due to fibrosis from inflammatory bowel disease and previous procedures at the excision site(such as biopsy,fluid injection as part of an abandoned EMR and previous attempted endoscopic resections)[16,17].
Advanced endoscopic procedures including endoscopic submucosal dissection,are currently not routinely performed in the majority of endoscopy units within the United Kingdom[18].The procedures are technically challenging and are associated with greater risk,although results from high volume centers within Japan have demonstrated positive results in the resection of larger benign lesions and low risk early cancers[19,20].The technique of endoscopic full thickness resection has more recently been described although its efficacy is yet to be validated[21].
HISTOLOGICAL PREDICTORS OF DISEASE
Histologically a number of factors have also been shown to predict invasive disease and risk of lymph node involvement.Resection margins of ≥ 1 mm are generally accepted to be adequate,whereas margins < 1 mm have been shown to have recurrence in up to 33% of patients[15-17].Depth of invasion of polyps are specifically graded according to their morphology;pedunculated polyps are graded using the Haggitt classification with level 4 Lesions associated with the highest risk[22].Depth of invasion in sessile lesions are graded according to the Kikuchi classification with sm3lesions associated with the highest risk[23].The degree of dysplasia in resected polyps can be divided into low and high grade.Similarly,in lesions that display invasive disease,the degree of differentiation of tumor cells can be histologically divided into well differentiated,moderately differentiated or poorly differentiated tumors.
Adenomateous polyps can be divided histologically into tubular,tubulovillous and villous adenomas based on their cellular architecture.Although villous adenomas are the least common of the three,they are associated with the highest risk of development of invasive disease[15].A further histological sub-type of polyp,termed serrated polyps,were historically felt to be an innocuous subset of hyperplastic polyps.However,the malignant potential of these lesions has been more recently recognized,with the adenoma-carcinoma sequence appearing to develop more rapidly with the consequence of lesions behaving more aggressively[24].The presence of lymphovascular invasion is an important prognostic feature and has been shown to be an independent risk factor for the development of lymph node metastasis[25].Finally,isolated clusters of malignant cells at the leading edge of the tumor,termed tumor budding,has also been shown to be associated with adverse outcomes[16].
LOCAL EXCISION TECHNIQUES
Prior to the advent of transanal minimally invasive procedures,surgery for local excisions of rectal adenomas and early rectal tumors were confined to the limitations of trans-anal excision(TAE)using traditional anal retractors,such as the Parks retractor,which afforded a limited degree of accessibility to the rectum.As a result,TAE procedures often lacked the visibility and working space required to perform precise,high quality oncological resections of rectal lesions.Lesions involving the proximal rectum would also be largely inaccessible to traditional TAE techniques[26,27].
The technique of TEM was first introduced in the 1980’s by Buesset al[28],in which a rigid rectoscope was used with a magnified binocular viewer,offering a 3-dimensional stereoscopic view of the rectum.This was accompanied by a dedicated rectal suction-insufflation unit in order to create the stable pneumo-rectum required for an adequate working space,as well as specialist instruments akin to those of modern laparoscopic surgery,allowing the possibility to perform precise,full thickness excisions at depth within the rectum[29].
Despite the promising reported results and premise of a technique superior to alternatives available at the time,TEM did not initially gain the popularity and widespread application which it perhaps deserved.This can likely be attributed to the steep learning curve associated with a novel minimally invasive technique,at a time where laparoscopic surgery and minimally invasive techniques in general were not common practice.There were also costs associated with procuring the specialist equipment required for the procedure and training of staff to become familiar with its use.Nevertheless,the concept of minimally invasive natural orifice surgery,together with the technology and techniques introduced with TEM,underpin modern approaches to the surgical resection of rectal lesions amenable to local excision.Compared to traditional TAE techniques,TEM has been shown to be associated with lower rates of specimen fragmentation(OR,0.096,95%CI:0.044-0.209;P< 0.001),positive resection margins(OR,5.28,95%CI:3.20-8.71;P< 0.001)and local recurrence(OR,0.248,95%CI:0.154-0.401;P< 0.001)in a systematic review conducted by Clancyet al[27]in 2015,in which outcomes of 492 TEM procedures and 435 TAE procedures were assessed.
Currently,in addition to TEM,alternative surgical options for the local excision of rectal lesions include transanal endoscopic operation(TEO)and transanal minimally invasive surgery(TAMIS).
The TEO platform has a set-up similar to that of TEM in its use of a rigid rectoscope(4 cm diameter)secured to the operating table using an articulated support arm.Rectoscopes are available in varying lengths to facilitate procedures at different depths within the rectum.The main difference between the two techniques is in the method of image acquisition,with TEO utilizing a high-definition camera to display 2-dimensional images on a dedicated monitor,similar to the set-up of laparoscopic surgery[28].Standard laparoscopic equipment,which should be available in all modern surgical units(stack system,monitors and CO2insufflator device)can be used with the TEO platform,relieving some of the costs associated with the specialist equipment unique to TEM[29].
For both TEM and TEO procedures careful consideration must be given to the location of the lesion within the rectum,which will dictate optimum patient positioning in order to provide the ideal view of the lesion to be resected within the surgical field.For posterior rectal lesions procedures are usually performed with the patient in a traditional lithotomy position.Lesions involving the anterior or lateral aspects of the rectum may require a modified prone or lateral decubitus position,lengthening procedure set up times as well as potentially increasing challenges for the anesthetist in ventilating the patient.For larger or more circumferential lesions the position of the rectoscope and that of the patient may need to be adjusted during the procedure.
Most recently the technique of TAMIS was introduced and applied the principles of single port laparoscopic surgery to transanal microsurgery[30].The single access ports used in TAMIS procedures are usually made of a flexible material,the upper lip of which anchors just proximal to the anorectal ring,creating the seal required to establish pneumo-rectum.The relatively simple design and concept of the TAMIS platform significantly reduces set-up time in comparison to TEO and TEM[31,32].In addition,the learning curve appears to be shorter for the TAMIS platform,perhaps as a result of pre-existing surgeon familiarity with laparoscopic and single access port laparoscopic techniques[33].Similar to TEM and TEO platforms,TAMIS devices have access ports which are compatible with standard laparoscopic equipment including 5mm laparoscopic cameras.Unlike TEM and TEO platforms,which have a dedicated separate optics channel in lieu of the working ports,one of the channels in TAMIS is needed for the camera.A second assistant is also required to operate the camera in TAMIS procedures unlike in TEO and TEM procedures.Given the narrow working space involved the assistant ideally needs to be suitably experienced and familiar with the procedure for efficiency and image stability.The flexibility afforded by the TAMIS platform and ability to change the camera port position enables the operator to work in all 4 quadrants of the rectum without the need to change patient position[27].
Advocates of the TAMIS platform highlight the relatively low upfront cost needed when compared to both TEM and TEO,which require specialist equipment unique to the procedures[30].However,the lower upfront costs of TAMIS may be offset by the single use nature of its equipment,unlike the reusable nature of some of the equipment in both TEM and TEO platforms[34].The relatively narrow length of the port used in TAMIS gives a wider area for triangulation of instruments within the rectum,although movements that generate high torque on the instruments may disrupt the seal of the port due to its flexible nature,potentially leading to a loss of pneumo-rectum.In comparison the rigid nature of the rectoscope used in TEO and TEM platforms may offer a more stable pneumo-rectum[35].Furthermore,the use of conventional laparoscopic insufflators,designed for abdominal laparoscopy,in TEO and TAMIS procedures may also result in a less stable pneumo-rectum in comparison to the dedicated insufflator in the TEM platform,which allows a continuous gas flow without the need to pause to measure pressure[35].
A potential limitation of the TAMIS platform is in its access to proximal lesions and operating at depth within the rectum.The rigid nature of rectoscopes used in TEO and TEM platforms may offer better access to lesions involving the upper rectum,as the scope can be advanced within the rectum to the depth required.Similarly,obstructions in view from rectal folds can also potentially be surpassed by adequate positioning of the rectoscope,stenting the rectal wall[35].Another potential limitation of the TAMIS platform is in its access to distal lesions.Due to the anchor position of the port at the anorectal ring,access to ultra-low rectal lesions can be problematic,although the use of a combined procedure incorporating TAE for the initial distal dissection with conversion to TAMIS to complete the remainder of the resection can overcome this problem[35].With the use of TEO and TEM platforms the rectoscope can be withdrawn to the desired depth to allow procedures in the distal rectum to be performed,as long as pneumo-rectum can be maintained[35].
The current body of evidence regarding the efficacy of both TEO and TAMIS platforms are not as abundant or robust in comparison to the TEM platform,which is most likely a reflection of the duration of time these procedures have been available.Owing to the similarities of TEM and TEO it may be assumed that both will be able to offer similar results.There are also a growing number of studies which demonstrate comparable outcomes to TEM in both TEO[26,29,36]and TAMIS platforms[32,37,38].Table 1 provides a brief comparison of the suggested merits of each technique.
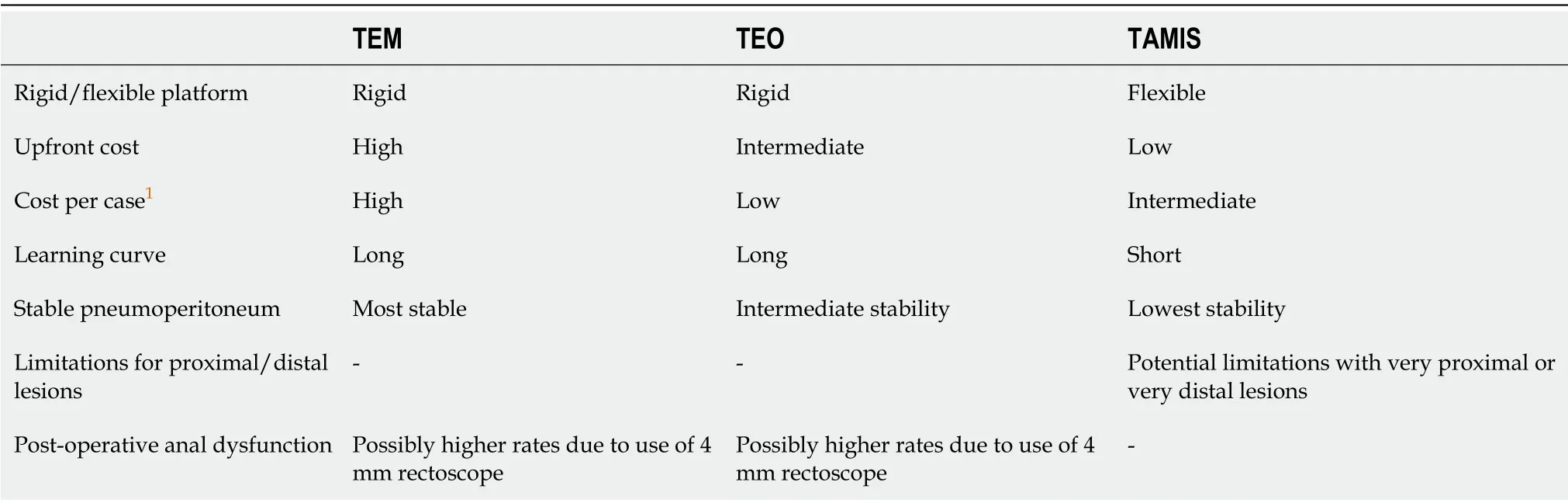
Table 1 Overview of techniques for minimally invasive transanal surgery
Regardless of the platform used,the aim of minimally invasive local excision techniques for rectal lesions would be to resect an intact specimen with adequate clearance of both the circumferential and deep margins.A resection margin of at least 1cm is regarded as satisfactory.For lesions with any suspicion of malignancy,a full thickness resection should be performed with transmural dissection up to the mesorectal fat.Following lesion excision,closure of the rectal wall defect is warranted for cases where there has been inadvertent intra-peritoneal entry or where there has been a planned dissection above the peritoneal reflection for proximal lesions[39].For resections below the peritoneal reflection there is a lack of consensus on whether the defect should be closed and it is largely dependent on surgeon preference.Closure of the rectal wall defect most commonly involves intra-corporeal suturing in a continuous fashion using a self-locking suture or suture clip forceps.Intra-corporeal suturing,particularly within the confined rectal space,is technically challenging and also increases procedure length.The advantage of routinely suturing all defects,regardless of their position,would be to allow the operator to develop the skills,experience and confidence necessary to perform in circumstances where the defect must be closed.
A meta-analysis conducted by Khanet al[39]in 2020 assessed outcomes of TEM/TAMIS in cases where the defect was sutured(n=283)in comparison to cases where it was left open(n=272).Authors found no statistically significant differences in rates of post-operative infection,length of hospital stay or duration of procedure;although there was a trend towards longer operations in the defect closure group.Authors found a statistically significant reduction in post-operative bleeding in the sutured group(2.12%vs6.99%;OR,0.26,95%CI:0.1-0.68,P=0.006).
As well as bleeding and peri-rectal infection,anorectal dysfunction and incontinence are recognized risks following TEM,TEO and TAMIS procedures,although available evidence for their incidence appears to be inconsistent and such complications are generally felt to be of short-term duration for the majority of patients.Following TEM and TEO procedures,the occurrence of anorectal dysfunction may be explained in some part by the use of the 4cm diameter rectoscope and its effect on dilatation of the anal sphincter complex.
A systematic review of functional outcomes and quality of life following TEM and TAMIS,published by Marinelloet al[40]in 2019,included 18 studies that assessed continence following TEM and 5 following TAMIS.Of the studies assessing outcomes following TEM;6 demonstrated no change,4 found improvements and 3 found worsening in incontinence scores.One study found worsening incontinence scores following repeat TEM procedures only,with no change in those patients undergoing primary TEM only.One study found worsening incontinence scores in patients having chemoradiotherapy and TEM,although contradictorily,another study found worse outcomes in patients undergoing TEM alone compared to those having both TEM and chemoradiotherapy.Of the 5 studies assessing functional outcomes following TAMIS;2 found no change in incontinence scores,2 found improvement and 1 study,which also included patients undergoing TAMIS following chemoradiotherapy,found worsening incontinence scores.Overall authors concluded that either technique did not appear to alter continence except in a minority of cases.Authors also advocated a need to standardize assessments of incontinence after local excision procedures.
OUTCOMES OF MINIMALLY INVASIVE TECHNIQUES FOR EARLY RECTAL CANCER
Total mesorectal excision(TME)of the rectum together with its lymphovascular pedicle is considered to be the definitive surgical treatment for rectal cancer[41].Traditional approaches to TME surgery are usually trans-abdominal using either open,laparoscopic or robotic techniques.
In comparison to trans-anal techniques for local excision,trans-abdominal TME surgery carries a much greater risk profile.The morbidity and mortality associated with major trans-abdominal procedures are not negligible and there are also risks of long-term disability and functional impairment,both in younger patients who will have to live with the consequences of complications for a longer duration and in the elderly population who may not have the same physiological reserve for recovery compared to a younger cohort.Bladder and sexual dysfunction are recognized complications of rectal surgery,as well as low anterior resection syndrome in those patients undergoing anastomosis following resection of distal rectal lesions.Many patients following trans-abdominal TME surgery will have a stoma,the psychological impact of which may be significant to some.A proportion of patients undergoing anterior resection may be required to have a diverting ileostomy as part of their procedure,some of which may not be amenable to future reversal.Patients undergoing abdomino-perineal excision will also have a permanent colostomy.
Although local excision techniques for early rectal cancers carry a much more favorable risk profile,the oncological results of local excision have been suggested to be inferior to the standard of traditional trans-abdominal TME surgery,particularly with regards to local recurrence.A meta-analysis conducted by van Oostendorpet al[42],published in 2020,assessed local recurrence in patients with T1 and T2 rectal cancers managed with local excision techniques.A total of 62 studies were included and consisted of 3050 patients with T1 disease and 545 patients with T2 disease who underwent no additional treatment following local excision.All included studies were required to have a median length of follow up of at least 36 mo.Local recurrence rates of 8.1%(95%CI:6.9-9.9)were found for T1 Lesions.Sub-group analysis of low risk T1 tumors;defined by the absence of lymphovascular invasion,poor differentiation,deep submucosal invasion(Kikuchi sm3,Haggitt level 4 or at least 1000 μm depth of invasion),tumor budding or positive resection margins(margin less than 1 mm or tumor in resection plane),showed recurrence rates of 6.7%(95%CI:4.8-9.3).High risk T1 Lesions were defined by the presence of one or more of the high-risk features and were found to have local recurrence rates of 13.6%(95%CI:8-22).Local recurrence rates in T2 tumors were found to be 28.9%(95%CI:22.3-36.4).It is important to note that techniques for local excision were not discriminated in results of this metaanalysis and studies incorporated used both endoscopic and surgical modalities(including traditional TAE techniques).
A meta-analysis conducted by Luet al[43],published in 2015,compared outcomes of local excision using TEM against standard TME surgery in patients with T1 rectal cancers.A total of 303 TEM and 557 TME procedures were included.Local recurrences occurred in 30/303(9.90%)of patients who underwent TEM procedures,compared to 8/557(1.44%)of patients who underwent TME surgery.This was a statistically significant finding(OR=4.62,95%CI:2.03-10.53,P=0.0003).There were no statistically significant differences found for the rate of distal metastatic recurrence,disease free survival or overall survival rates between the two groups.
A predictive model for the incidence of local recurrence following TEM,based on results of 424 procedures,was explored by Bachet al[25]in 2009.The 5-year local recurrence estimates for pT1 Lesions was 18.6% and 29.3% for pT2 Lesions.Depth of invasion(lowest risk associated with Kikuchi SM1 disease with comparably higher risks in SM2,SM3 and pT2 Lesions),increasing diameter of tumor size and lymphovascular invasion were found to be independent predictors of local recurrence.Increasing age and poorly differentiated tumors were also found to be predictive.
As evidenced within the literature,stratifying T1 lesions according to the presence of risk factors outlined above,and offering local excision procedures only for low risk T1 lesions would seem to be a viable strategy,in order to lower the risk of local recurrence.In practice this would be difficult to achieve as methods for local staging using current modalities cannot fully predict early-stage disease with a consistent certainty.Also,many of the predictive factors shown to increase the risk of local recurrence rely on the histological analysis of a completely resected specimen.An alternative strategy would be to offer local excision techniques to those patients who appear to have low-risk disease on pre-operative staging,with the intention of offering radical TME surgery to those patients subsequently found to have high-risk features,although this would subject the patients to two procedures rather than one.
Accepting the increased risks of local recurrence in favor of a less invasive procedure associated with significantly lower morbidity and mortality,as well as improved functional outcomes,may be appealing to some patients,although this approach would require careful counseling in order to allow the patient to make an informed decision.Offering a minimally invasive procedure as an oncologic compromise,but nevertheless with a considerable chance of cure,may be appropriate to a subset of patients with significant co-morbidities and poor physiological reserve who would otherwise be unsuitable for conventional TME surgery.With an ageing population,prevalence of chronic disease and increased identification of early disease secondary to screening;this scenario is likely to be encountered more commonly.
ROLE OF CHEMORADIOTHERAPY IN EARLY RECTAL CANCER
Within the United Kingdom the National Institute for Health and Care Excellence currently recommend neoadjuvant radiotherapy or chemoradiotherapy for T1/T2 M0 rectal cancers with evidence of lymph node metastasis(N1/N2)and all T3/T4 M0 cancers irrespective of lymph node status[44].Adjuvant chemotherapy is also recommended for all rectal cancers with lymph node metastasis[44].A subset of patients undergoing neoadjuvant therapies may have a complete clinical response and a “watch and wait” approach,particularly for patients with a higher risk for surgical intervention,may be appropriate[45-47].The role of adjunctive therapies for early rectal cancer,in the absence of lymph node disease,is yet to be established although there is some existing evidence for their additional benefit in patients undergoing local excision techniques.
A systematic review of local excision following neo-adjuvant chemoradiotherapy,conducted by Hallamet al[48]in 2016,included 1068 patients and found a local recurrence rate of 4%(95%CI:1.9-6.9)with a median disease free survival rate of 95%in patients who had a complete pathological response(ypT0)following their treatment.Median follow up of patients was 54 mo.Recurrence rates were 12.1%,23.6% and 59.6% for patients with ypT1,ypT2 and ypT3 disease respectively and overall local recurrence rates for patients who did not have a complete pathological response(≥ ypT1)was 21.9% with a median disease-free survival of 68%.The majority of patients included in the review had clinical stage T2 disease(46.4%)and T3 disease(30.7%)prior to commencing treatment.
A systematic review conducted by Cuttinget al[49],accepted for publication in 2018,assessed outcomes of local excision followed by radiotherapy,chemotherapy or a combination of both in 804 patients.Patients included consisted of pT1 Lesions in 35.1%,pT2 in 58.0% and pT3 in 6.9%,although one study did not explicitly specify pT stage of included patients,it assessed only lesions with pT1/pT2 staging.Where stated,indications for adjuvant therapy following local excision in pT1 Lesions included:Kikuchi depth of invasion sm2and above,lymphovascular invasion,perineural invasion,mucinous histology,poor differentiation and an R1 resection/incomplete resection margins.Chemotherapy regimens were heterogeneous for the included studies and TAE techniques for local excision were performed in the majority of patients(77.7%)with TEM accounting for 9.4%.The incidence of local recurrence was 5.8%(95%CI:3.0-9.5)in pT1 tumors,13.8%(95%CI:10.1-17.9)in pT2 tumors and 33.7%(95%CI:19.2-50.1)in pT3 tumors.The overall median disease-free survival was 88%(range 50%-100%).
The GRECCAR 2 randomized control trial,published in 2020,assessed outcomes for T2 and T3 Low rectal cancers which showed good clinical response(residual tumor < 2 cm)following neoadjuvant chemoradiotherapy[50].Participants were randomized to receive either local resection(modified intention to treat analysisn=74)or TME surgery(modified intention to treat analysisn=71)with median follow up in both groups of 60 mo.Local excision techniques in the as treated analysis consisted of 58 TAE procedures and 23 TEM procedures.Patients in the local excision group who were found to have a poor pathological response(ypT2-3)or incomplete(R1)resection following local excision(34/73 patients),underwent completion TME surgery.Due to technical difficulty,one further patient in the local excision group required completion TME surgery.Protocol deviations in the TME group,due to patient refusal or surgeon discretion,resulted in 8/71 patients undergoing local excision and 3/71 patients undergoing a watch and wait approach.At 5-year review local recurrences occurred in 6/81(7.4%)of patients in the local excision group and 2/61(3.3%)of patients in the TME group based on as-treated analysis.Local recurrence occurred in 2 further patients who had no surgery.There were local recurrences in 4 patients who underwent local excision alone within the 5 year follow up,all of whom underwent subsequent radical salvage surgery with R0 resections.Disease free survival at 5 years based on as-treated analysis was 66% in the local excision group and 80% in the TME surgery group.Overall,5-year survival was 80% in the local excision group and 87% in the TME surgery group.
The TREC randomised control trial,published in 2021,compared outcomes of patients with clinical stage T2 rectal cancer or lower,treated with neoadjuvant short course radiotherapy followed by local excision using TEM(n=27)and patients undergoing TME surgery alone(n=28)[51].The trial was primarily a feasibility study and was not formally powered to assess cancer outcomes.Following TEM 5 patients had early conversion to TME surgery(planned per-protocol conversion)due to the presence of high-risk features at histopathological specimen analysis(maximum tumor diameter greater than 30 mm,cancer within 1 mm of the circumferential or deep resection margin,predominantly poor differentiation,presence of lymphatic or venous invasion(intramural or extramural),and tumor depth of invasion of Kikuchi sm3or greater).A further 2 patients who initially refused TME surgery and underwent TEM eventually required completion TME surgery due to the presence of high-risk features.In one patient conversion to TME surgery was required as the lesion was too proximal for the TEM approach and 2 further patients were found to have lesions too large for TEM and underwent TME surgery.Overall organ preservation was achieved in 19/27(70%)of patients randomized to the TEM group.Median follow up for randomized patients was 4.28 years.In the TEM group local recurrence occurred in three patients(11%),2 of which had salvageable disease.In the remaining patient cardiovascular comorbidity precluded salvage surgery.All three patients with local recurrence in the follow up period had high risk features following initial local excision but declined completion TME surgery at this time.No local recurrences were seen in the TME cohort during the study period.There were no significant differences in overall survival or disease-free survival between randomized groups.
Two further randomized controlled trials assessing the role of adjuvant therapies for early rectal cancer are currently in progress;the TESAR trial[52]and the STAR-TREC trial[53].The TESAR trial will aim to assess outcomes of intermediate risk T1 and T2 tumors treated initially with local excision techniques,followed by randomization to either adjuvant chemoradiotherapy or completion TME surgery.The STAR-TREC trial is a three-arm phase II study aiming to assess the feasibility of recruitment for a subsequent large scale multicentre phase III study.The trial will assess outcomes for patients with clinically staged rectal cancers ≤ T3b(up to 5 mm of extramural spread)N0 M0,randomized to either TME surgery or rectum sparing management using long course chemoradiotherapy or short course radiotherapy.Patients randomised to the rectum sparing approach who have a complete clinical response will undergo active surveillance only and patients with incomplete clinical response will undergo local excision procedures.
FUTURE TECHNIQUES
The concept of natural orifice rectal surgery using minimally invasive approaches has been adopted to more advanced techniques,including robotic TAMIS(R-TAMIS)procedures and trans-anal TME excision(TaTME).The technique of TaTME is not specific to early rectal cancer as an option for local excision and is not discussed further in this review.
The technique of R-TAMIS incorporates the benefits of robotic surgery into transanal minimally invasive surgery through a single access port,similar to that used in conventional TAMIS procedures.R-TAMIS has been proposed by advocates to improve on some of the technical limitations of conventional TAMIS.Robotic surgery in general has the potential to offer better ergonomics and operator dexterity through articulated instruments,delivering 7-degrees of freedom which replicates human wrist movement.Image acquisition is also improved through magnification,threedimensional viewing and camera control by the operating surgeon,resulting in a higher quality image which has an increased stability in comparison to conventional TAMIS procedures.Motion scaling and tremor reduction also improve precision,conferring obvious benefit when operating in the narrow confines of the rectum.
To date there are relatively few studies validating the application of R-TAMIS and the majority of early evidence is based on single-center experiences.A single center retrospective review conducted by Leeet al[54]compared short term outcomes of RTAMIS(n=19)and conventional laparoscopic TAMIS(n=21)procedures.Authors found a statistically significant increased cost per procedure with R-TAMIS(added$880 per procedure).There was an increased R0 resection rate with R-TAMIS(94.74%compared to 90.48%),although this was not at a level of statistical significance.Duration of procedures were comparable in both groups.
The main limiting factors for R-TAMIS include the significant additional costs of procuring and maintaining the robotic equipment,the availability of surgeons trained in its usage and the added overall procedural time due to docking and undocking of the robot.Certainly,within the United Kingdom there are relatively fewer colorectal surgeons offering robotic procedures in comparison to those performing laparoscopic procedures.Furthermore,the availability of robotic systems are likely to be limited to large tertiary center teaching hospitals.As a consequence,the training of surgeons in robotic surgery and maintenance of skills through a high-volume caseload are only likely to be possible at a selected few centers.With the development of alternative robotic systems providing direct competition to the well-established DaVinci platform,it is likely that the costs of robotic surgery will improve in the near future,allowing its more widespread utilization.
CONCLUSION
The current multi-modal management of rectal cancer can be complex,necessitating a tailored approach for each patient.Consideration must be given to the presence of risk factors associated with aggressive disease,especially for early rectal cancers.At present the role of minimally invasive techniques for local excision are established only for the management of pre-invasive disease and early T1 rectal cancers in the absence of high-risk features and lymph node involvement.This role may be expanded in the future to more advanced,high-risk local disease when combined with additional therapies such as chemotherapy and radiotherapy.Robust evidence,however,is required before this role can be widely adopted.There are currently multiple platforms available for minimally invasive local excision of rectal lesions,each with their own merits.Careful patient selection and meticulous technique to obtain clear resection margins should be the objective,regardless of the choice of modality.
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Current status of treatments of pancreatic and peripancreatic collections of acute pancreatitis
- Efficacy and safety of early oral feeding in postoperative patients with upper gastrointestinal tumor:A systematic review and metaanalysis
- Acute mesenteric ischemia and small bowel imaging findings in COVID-19:A comprehensive review of the literature
- Novel parameter based on lipid indicators ratio improves prognostic value of plasma lipid levels in resectable colorectal cancer patients
- Gastrectomy impact on the gut microbiome in patients with gastric cancer:A comprehensive review
- Robotic donor hepatectomy:Are we there yet?
