Prediction of Clostridium difficile infection based on gut microbial traits in patients with Clostridium difficile colonization
2021-07-24DongYnYnDiHungYunBoChenToLvChunXiZhuJinRongHungLnJunLi
Dong Yn ,Yn-Di Hung ,Yun-Bo Chen ,To Lv ,Chun-Xi Zhu ,Jin-Rong Hung ,Ln-Jun Li ,∗
a State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b Department of Laboratory Medicine, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
TotheEditor:
Cirrhotic patients usually require multiple hospitalizations due to upper gastrointestinal hemorrhage,abdominal infection,and hepatic encephalopathy.These patients need long-term hospital stay,and long-term application of proton pump inhibitors and antibiotics,which may result inClostridiumdifficileinfection(CDI) [1].To our best knowledge,Clostridiumdifficilecolonization(CDC) is the major risk factor for the pathogenesis of CDI.Our previous study demonstrated that 19.8% patients with hepatic cirrhosis have CDC,and about 26% of the CDC patients may develop CDI during hospitalization [2].In cirrhotic patients,gut microbial structure and diversity may lead to overgrowth of intestinal bacteria,which may trigger the overgrowth of conditioned pathogens and opportunistic infection [3].The antibiotics could further destroy the gut microbiota which makes the intestinal tract vulnerable to the growth of pathogenic bacteria [4].A previous study showed that decline of gut microbial diversity and structural changes were the main etiology of CDI [5].In this study,we aimed to analyze the roles of gut microbial traits in the pathogenesis of CDI among patients carryingClostridium(C.)difficile.
We enrolled cirrhotic patients who receivedC.difficilescreening in the First Affiliated Hospital,Zhejiang University School of Medicine between May 2015 and December 2015.C.difficilepositive subjects were followed up until discharge.For each patient,the stool sample was collected within 48 h upon hospitalization,followed by subsequentC.difficileculture and with identification of toxins.Only the subjects positive forC.difficileculture and identification of toxins were included.Illumina MiSeq sequencing was performed for the qualitative analysis of 16S rRNA V3 region obtained from the stool samples.Then the gut microbial diversity for each subject was analyzed,followed by investigation of CDI pathogenesis and prevention.Among the 526 cirrhotic patients,52(9.9%) met the inclusion criteria.We dynamically observed the diarrhea and the medication during the hospitalization.According to the presence ofC.difficile,the patients were divided into the CDI group (n= 22) and non-CDI group (n= 30).Twenty-two cirrhotic patients with no CDC who were hospitalized in our hospital were served as control.There were no differences in the clinical traits except ALB among the three groups ( Table 1 ).
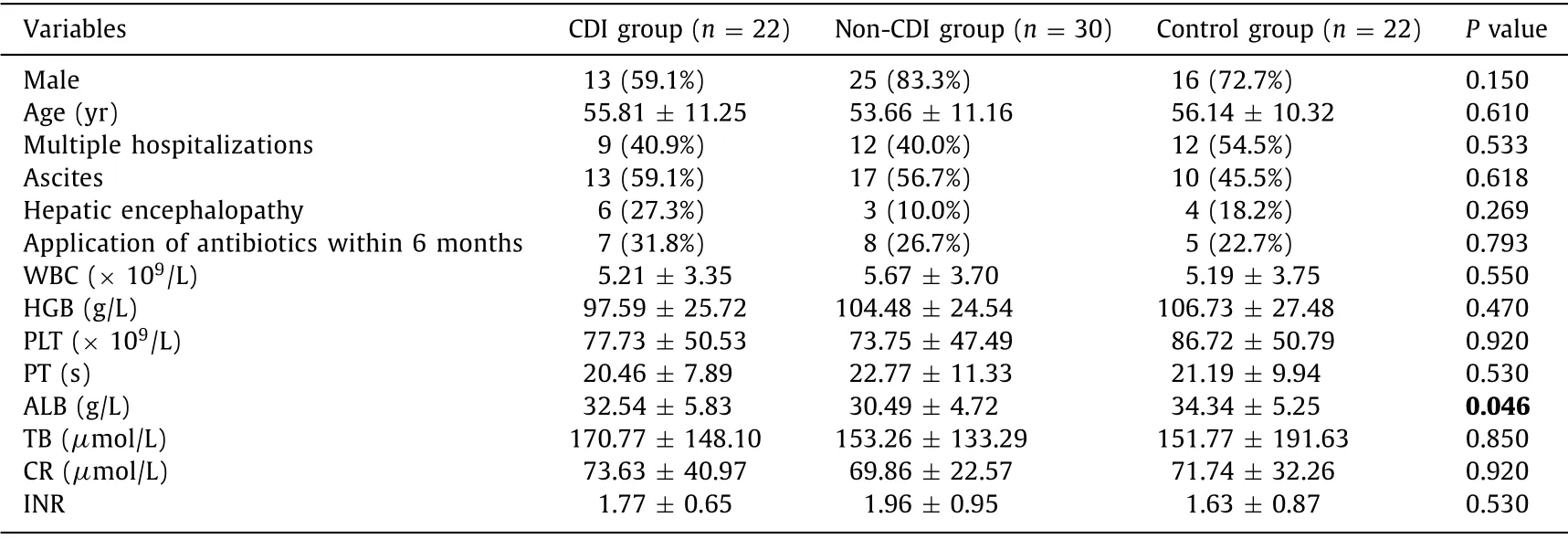
Table 1 Comparison of clinical traits upon hospitalization.
Compared with the non-CDI group,the diversity of gut microbiota was significantly declined in the CDI group which included OTU (86.95 ± 41.97 vs.121.67 ± 45.41,P<0.05),ACE index(132.41 ± 53.41 vs.168.28 ± 58.38,P<0.05),and Chaol index(113.94 ± 51.21 vs.157.71 ± 55.64,P<0.05).Bacterial community barplot analysis showed that the predominant bacteria in the non-CDI group wereBacteroides(64.6%) andRuminococcus(7.1%).In the CDI group,the predominant bacteria wereEnterococcus(66.3%) andEscherichia(10.1%).Sequencing category comparison indicated that the abundance ofAcinetobacterin the CDI group was significantly higher than that of the non-CDI group (0.5% vs.0.1%,P<0.05).The abundance of unclassified_Lachnospiraceaein the CDI group was significantly lower than that of the non-CDI group (0.05% vs.0.25%,P<0.05).Linear discriminant analysis (LDA) effect size (LEfSe)analysis indicated the featured microbiota in the CDI group wereMoraxellaceaeandParabacteroides,while those in the control group wereClostridia,ClostridialesandBacteroidaceae( Fig.1 ).
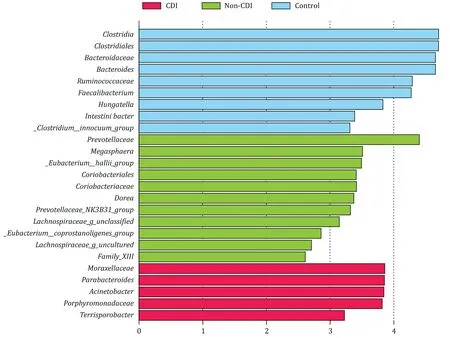
Fig.1.Comparison of LEfSe analysis among the CDI,non-CDI and control groups.LEfSe analysis indicated that the featured microbiota in the CDI group were Moraxellaceae and Parabacteroides,while those in the control group were Clostridia, Clostridiales and Bacteroidaceae.LEfSe: LDA effect size.
Rare studies have been conducted to identify the predictive factors of CDI in theC.difficilecarriers,as well as its potential mechanisms.Pakpour et al.[6]showed that the decline of fecal microbiome diversity could predict the occurrence ofC.difficile-associated diarrhea,especially theFirmicutesandProteobacteria.Besides,Veillonellacould serve as a promising candidate for predicting CDI recurrence.In this study,we dynamically followed up 52 cirrhotic patients carryingC.difficile,including 22 who progressed into CDI and 30 in a state of non-CDI.Our data demonstrated that the gut microbial diversity in the CDI group was significantly declined compared to that of the control group and non-CDI group.The decreased microbial diversity may promote the pathogenesis of CDI [7].Therefore,declined microbial diversity may be a predictor of CDI.
Gut microbial structure is a crucial index for evaluating the outcome of fecal microbiota transplantation (FMT).CDI patients usu-ally present declined intestinal microbial diversity,which is featured by predominance ofBacilliandGammaproteobacteriaand declinedBacteroidiaandClostridia[8].The predominant bacteria in the CDI patients wereLactobacillus,followed byStreptococcusandEnterobactergenera.Meanwhile,theClostridiawere predominant type in the donor community,and the diversity of microbiota showed significant increase after FMT [8].The predominant type in the CDI patients who received FMT wasCoprococcusandClostridiumleptum,which was similar to that of the microbial structure of the donor [8].According to the previous description,the elevation ofRuminococcaceae,Rikenellaceae,Clostridiaceae,Bacteroides,FaecalibacteriumandRothiawas an important factor for the CDI recurrence [9].There were significant differences in the bacterial structure among the CDI,non-CDI and control groups.For patients carryingC.difficile,several factors could serve as candidates for the prediction of CDI including elevation ofEnterococcus,and decline ofBacteroides,Faecalibacterium,VeillonellaceaeandPrevotellaceae.On this basis,theFaecalibacteriumcontributed to the gut microbial stability,which then may prevent CDI in theC.difficilecarriers.Upon FMT therapy,theBacteroidetesandFirmicutesshowed significant elevation in the CDI group.The proportion ofProteobacteriaandActinobacteriashowed significant decline [10].For patients who received FMT,the number ofBacteroidetesshowed significant increase after gut microbial analysis
such asBacteroides,Ruminococcus,Lachnospiraceae,VerrucomicrobiaceaeandRikenellaceae.This was in line with our LEfSe analysis in which theClostridium,Clostridiales,Bacteroidaceae,Bacteroides,VerrucomicrobiaceaeandClostridiuminnocuumshowed significant elevation in the control group.These bacteria may prevent patients from developing CDI.
In summary,our data showed that the gut microbial diversity was declined before CDI.The elevation ofEnterococcusandAcinetobacter,and decline ofE.faecalismay be important for monitoring CDI among cirrhotic patients.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
DongYan:Data curation,Formal analysis,Writing - original draft.Yan-DiHuang:Data curation,Writing - original draft.Yun-BoChen:Supervision.TaoLv:D ata curation.Chun-XiaZhu:Data curation.Jian-RongHuang:Data curation.Lan-JuanLi:Conceptualization,Funding acquisition,Supervision,Writing - review and editing.
Funding
This study was supported by a grant from the National S&T Major Project (2018ZX10101-001).
Ethicalapproval
This study was approved by the Ethics Committee of the First Affiliated Hospital,Zhejiang University School of Medicine (2017-674).Written informed consent was obtained from all participants.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Reply to: Contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma: A cost-effective alternative for low-resource settings
- Contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma: A cost-effective alternative for low-resource settings
- Transarterial chemoembolization as adjuvant treatment after surgery:The cure of huge hepatocellular carcinoma?
- RELEVANT CONTENT
- Variations of the hilar biliary confluence from postoperative cholangiography in Chinese population
