Ocular prodrugs: Attributes and challenges
2021-07-21JagpreetkourNehaKumariBhartiSapra
Jagpreet kour,Neha Kumari,Bharti Sapra
Department of Pharmaceutical Sciences and Drug Research,Punjabi University,Patiala 147001,India
Keywords:Bioavailability Corneal permeability Esterase Stability Lipophilicity Ocular prodrugs
ABSTRACT Ocular drug delivery is one of the most attention-grabbing and challenging endeavors among the numerous existing drug delivery systems.From a drug delivery point of view,eye is an intricate organ to investigate and explore.In spite of many limitations,advancements have been made with the intention of improving the residence time or permeation of the drug in the ocular region.Poor bioavailability of topically administered drugs is the major issue pertaining to ocular drug delivery.Several efforts have been made towards improving precorneal residence time and corneal penetration,e.g.iontophoresis,prodrugs and ionpairing,etc.Prodrug approach (chemical approach) has been explored by the formulation scientists to optimize the physicochemical and biochemical properties of drug molecules for improving ocular bioavailability.Formulation of ocular prodrugs is a challenging task as they should exhibit optimum chemical stability as well as enzymatic liability so that they are converted into parent drug after administration at the desired pace.This review will encompass the concept of derivatization and recent academic and industrial advancements in the field of ocular prodrugs.The progression in prodrug designing holds a potential future for ophthalmic drug delivery.
1.Introduction
The foremost problem in the ocular drug delivery is the attainment of an optimal drug concentration at the site of action.The main route for the topically instillation of drugs into eyes is the cornea,whereas conjunctival and scleral route can also be utilized [ 1,2 ].There have been number of positive attributes of topical administration due to which they are more preferred like expeditious onset of action,small dose,safety,patient compliance,non-invasive etc.On the other hand,negative attributes are also reported such as precorneal drop,restricted concentration of drug for lipophilic agents and physical barriers of cornea [ 3,4 ].Despite of being easily accessible to eyes,topical ocular application inflicts a lot of constraints that are responsible for drainage of the drug that leads to rapid elimination which simultaneously affect bioavailability.The constraints include precorneal factors that rapidly remove drugs from the conjunctiva sac (where it is applied) and structure of the cornea that restricts the passage of drug molecules [5—7].It becomes quite exigent to deliver drugs to the required site due to the presence of various constraints e.g.physiological limitations (blinking,nasolacrimal drainage,wash out by tears) and anatomical limitations (blood aqueous and retinal barrier,physical membrane barrier,conjunctival blood and lymph flow) [8—10].When dose instilled in the eye,some part of medication is expelled out by tear mechanism and remaining leftover dose resides for a small period of time to penetrate from cornea into the eye but poor permeability of cornea allows only small concentrations of active ingredient to pass through it [ 11,12 ].1% or even less of applied drug is absorbed and eliminated by metabolic action [13].Blood ocular barrier comprises of both blood aqueous and retinal barrier that restricts the movement of medicament into ocular compartments.Blood aqueous barrier limits the molecular motion to reach the aqueous humor via iris,ciliary capillaries from blood [ 14,15 ].Exterior surface of epithelial cells form tight junctions which act as corneal epithelium barrier.The presence of these tight junctions along with lipophilic property of epithelium,forms a highly structured barrier [16—18].Ultimately small concentration of dose reaches at the site of action.Hence for effective delivery,attempts have been made to improve the bioavailability either by increasing the residence time in conjunctiva sac,or by improving the penetration across cornea.Regardless of ease of topical applied ocular drugs,the required amount of medication does not reach to the target site which in turn builds certain strategies to overcome the issue of bioavailability.Numerous strategies have been used to improve the bioavailability of topically instilled drugs.These strategies include the solubility enhancers improve solubility of drug in the formulation,penetration enhancers alter the drug permeation through corneal epithelium,retention strategies which consist of viscosity enhancing polymers,nanoparticles,in situ gelling system,mucoadhesives etc.,ocular inserts and ocular implants etc.[19—26].Numerous dosage forms e.g.solutions,ointments,gels,microparticles,nanoparticles,and micelles have been developed to tackle the issue of poor ocular bioavailability upon topical instillation of drugs [27].There are several approaches to improve the bioavailability and corneal penetration but the most challenging and novel approach is prodrugs.The concept of prodrugs was introduced in 1958 by Albert for improving the corneal penetration [28].The first commercial prodrug was methanamine available in 1899 by Schering [29].Prodrug design is a chemical approach to transport parent drug molecule to the target site in order to achieve improved drug absorption [30—32].It remains inactive until unless reach to target site and become active to exert its therapeutic action by altering the structure of drug.
Ocular prodrugs are designed in order to achieve one or more objectives,e.g.improving the efficacy of drugs,reducing side effects,alteration of duration of action or drug targeting,to ameliorate active drug solubility which may improve bioavailability,permeability as well as absorption and thus may alter the distribution profile of the drug that reaches to the target site.It mainly optimizes ADME (absorption,distribution,metabolism,excretion) profile [33].The present review will be a revisit of utilization of prodrug strategy in ocular drug delivery.There are few imperative factors which are to be considered while formulating ophthalmic prodrugs e.g.the parent drug is required to possess a functional group amenable to chemical derivatization,the chemical modification at the functional group of parent drug must be reversible,prodrug or pro-moiety attached to parent compound must be safe and nontoxic,biological enzymes such as esterase and peptidase should be involved in bioreversion,prodrug should be safe and stable,log P value of prodrug is a significant parameter and lastly prodrug should exhibit high affinity and targeted delivery of parent drug.
2.Prodrug strategy –a novel developing approach
Prodrugs are the medicinal agents that remain inactive until they reach the target site.But when it reaches target site,becomes active to exert its required action [ 32,34 ].Prodrugs increase corneal drug permeability which is a major issue in ocular drug delivery by modification of hydrophilicity or lipophilicity of the drug.It involves different methods like alteration of chemical structure of drug moiety.Prodrug approach is used for the production of safe ocular drug delivery system that implicit action on the target site.Several factors such as instability,poor solubility,irritation,pain,low absorption,shorter duration of action,poor penetration and permeability are vanquished by the use of prodrugs[35].The main objectives behind prodrugs in any delivery system are to ameliorate stability,intensify solubility,to improve patient compliance,decrease side effects,increase bioavailability,extended duration of action,and enhanced penetration and permeability [35—37].The commonly used conventional ophthalmic drug delivery include solutions [ 38,39 ],suspensions [40],emulsions [41]and ointments [ 38,40 ].However,various new strategies that have been incorporated in ocular delivery are nanomicelles,liposomes,dendrimers,contact lens,nanoparticles,nanosuspension,implants etc.Ophthalmic formulation based on nanotechnology is a novel approach which is used for anterior as well as for posterior segment drug delivery.Several nano carriers are designed to ensure adequate bioavailability,low irritation and ocular tissue compatibility.These have been summarized in Table 1 .
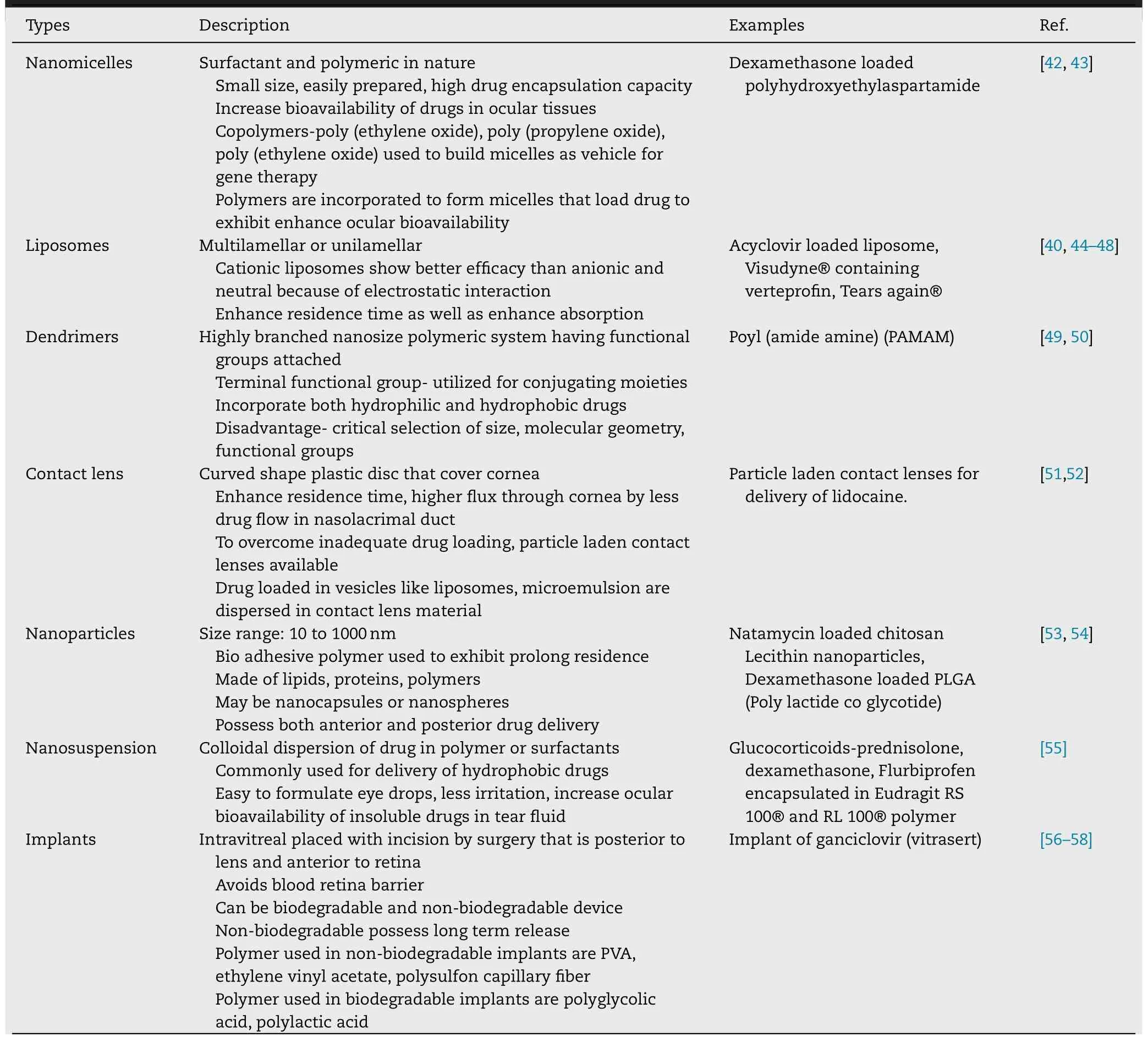
Table 1–New strategies of ocular drug delivery system.
2.1.Consideration in prodrug designing
While considering prodrug derivatization,the important factors which are required to be considered are transport or mechanism of penetration of drugs,the presence of vulnerable or receptive functional group essential for derivatization,and enumeration of the characteristics or description of enzymes which will be responsible for bioconversion [59—61].The derived prodrug should be stable in the finally designed dosage form,should possess optimum log P value in order to attain appropriate permeation across dynamic as well as static lipophilic ocular barriers.Parent drug must have the presence of functional group that is susceptible to chemical modification and derivatization at the site of parent drug attached with functional groups should be reversible.In addition,it is important that the parent drug and pro moiety linked with parent compound must be nontoxic and safe.The prodrug should manifest appropriate propinquity and should have targeted or site specific delivery of the parent drug.The significant factors which should be considered for ocular drug delivery are explained through Fig.1 [62—66].
2.2.Consideration in ocular delivery
Nearly in all cases,prodrugs are the chemical derivatives which are one or more chemical or enzymatic steps away from the parent drugs.Many ophthalmic drugs have hydroxyl or carboxyl groups that can be esterified to ester-prodrugs which can be more lipophilic.The esterase activity in corneal epithelium is ~2.5 times higher than that in the stromal endothelium [67].In addition it is worth noting that acetylcholinesterase (AChE) and butyrylcholinesterase(BuChE) are absent in rabbit’s tears [68],this further ensures the absorption of intact ester-prodrug into the corneal epithelium.Hence,along with presence of enzymes,chemical structure of drugs play a significant role in designing and derivatization of ocular prodrugs.
2.2.1.Adrenergic agonist prodrugs
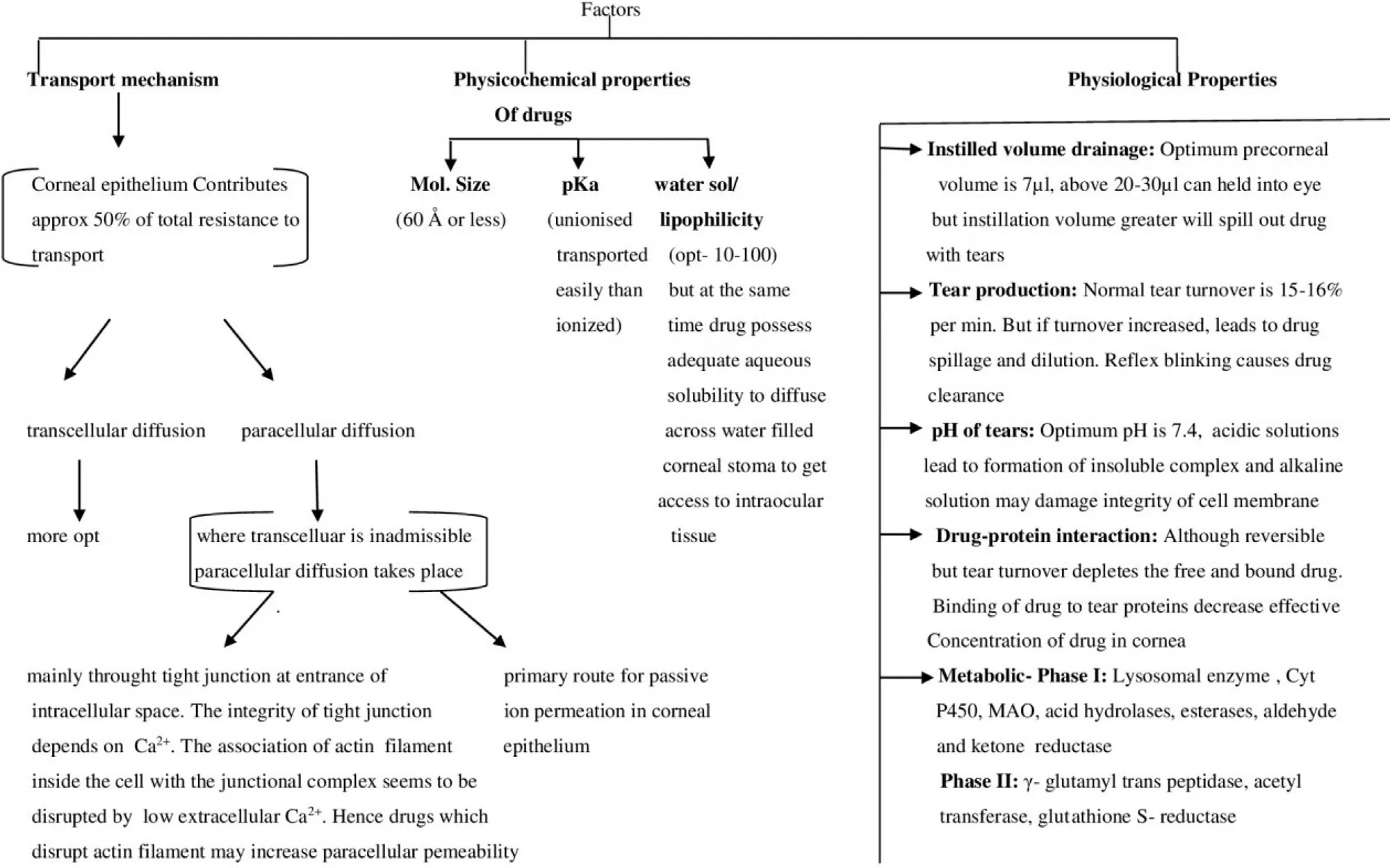
Fig.1–Schematic representation of consideration of factors in designing ocular delivery.
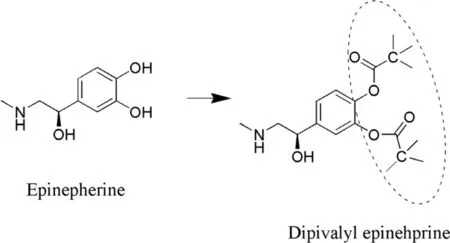
Fig.2–Conversion of EPI (parent drug) to prodrug (Dipivalyl EPI).
Epinephrine prodrugs: Epinephrine (EPI) is a choice used in the treatment of glaucoma by exerting its action by blocking beta adrenergic receptors.However,the major drawback associated is cardiovascular unenviable effects when the drug passes into systemic circulation through the tear duct [69].Enhanced polarity manifests decreased transport across the corneal epithelium which is considered as lipoidal barrier.In consequence,resistance to the permeation occurs due to top two cell layers.Hence,to overcome this problem the prodrug had been designed.A prodrug of EPI is supposed to have an optimum water solubility and lipophilicity in order to deliver drugs to the target site in the eye.Dipivefrin (Dipivalyl epinephrine) (Fig.2 ) is a prodrug that diffuses quickly across ocular tissue and is bio transformed into epinephrine with the help of enzyme called of corneal esterase.It exhibits better therapeutic index than EPI [ 70,71 ].In addition the study elucidated that absorption and subsequent hydrolysis of dipivefrin takes place in the conjunctiva,which is accounted for ~60% −75% of instilled prodrug recovered in the eye.Furthermore,the investigation revealed the age and pigmentation related variation of esterase activity in case of rabbits [71].Lipophilicity of Dipivefrin is approximately~600 fold because of which it exhibits improved corneal penetration [71].Table 2 summarizes the log P values of EPI and dipivefrin in different solvents.In another investigation,dipivefrin and related compounds have been reported to inhibit passive anaphylactic reactions in rat conjunctiva.The order of activity has been reported as isoproterenol>dipivefrin >EPI >Nor EPI.Dipivefrin was suggested to exert anaphylactic action due to activation of beta adrenergic receptors [72].
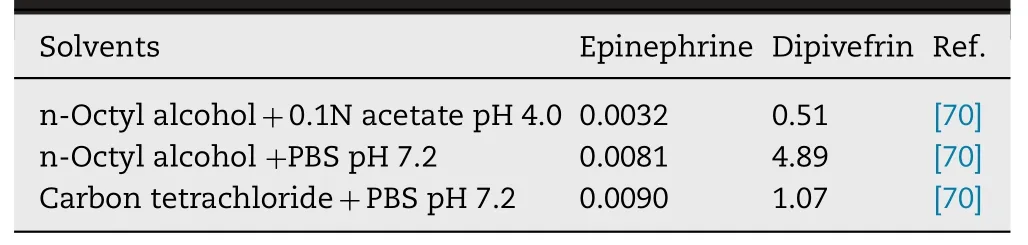
Table 2– Partition coefficient of epinephrine in different solvents.
Phenylephrine prodrugs: Phenylephrine (PE) is a class of drug that is used for the dilation of pupil mainly during surgery and examination [73].Its main drawback is lower ocular bioavailability because of high hydrophilicity (log P is −1.89)[74].It does not permeate well across the cornea because of low lipophilicity.Hence,to overcome these limitations oxazolidines phenylephrine (OPE) was synthesized as a prodrug (Fig.3 ) which enhanced lipophilic character and ultimately bioavailability.Oxazolidines are weaker bases(pKa= 6—7) and more lipophilic at physiological pH.Due to enhanced lipophilicity (log P= 1.38) it easily permeates across cornea [75].OPE lead to ~2 fold more mydriatic effect as compared to PE.10% suspension of OPE lead to ~8 fold enhanced bioavailability in the aqueous humor,which may be the reason of faster transport of OPE across the cornea,which further may lead to decrease in the dose of PE [76].In another study improved lipophilicity of OPE lead to ~3 fold more distribution coefficient and further exerted significant improved mydriatic effect (9 mm) with low dose (1% prodrug)as compared to PE [77].
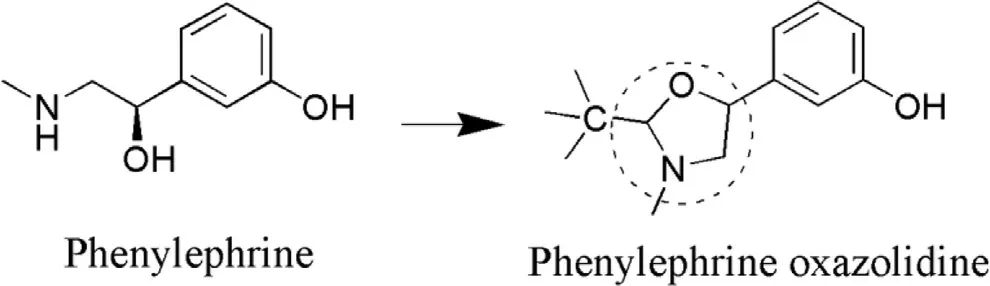
Fig.3–Synthesis of OPE from PE.
2.2.2.Antiviral prodrugs
Acyclovir: Acyclovir (ACV) is mainly given in patients having herpetic keratitis and corneal disease.ACV has low penetration across the cornea due to its hydrophilicity (log P= −1.22) [78]which may be the reason of decreased ocular absorption and in turn lesser ocular bioavailability.In order to overcome decreased permeability,ACV was converted into prodrug.Biotinylated lipid ACV prodrug was developed to improve ocular bioavailability and absorption of ACV [79].The most common mean of increasing water solubility of drugs containing OH group is by the formation of water soluble ester prodrugs.The ideal characteristics of such prodrugs include high water solubility at pH of optimum stability leading to improved shelf life (more than 2 years) of ready to use solutions.However,practically this is demanding and ungovernable as generally they are hydrolyzed by enzymes very quickly leading to decreased stability.
Anand and Mitra investigated the permeation and enzymatic hydrolysis of valacyclovir (VACV) [80].The corneal permeation of VACV (l-valyl ester of acyclovir) was ~3 fold more than ACV.β-lactam antibiotics and angiotensin converting enzyme inhibitors inhibited the transport of VACV which elucidated that the corneal permeation of VACV was dependent on carrier mediated transport system specific for peptides.The flux was pH dependent and was found to be 1.087 ±0.05 nmole/cm/min [80].Numerous esters (valyl,benzoyl,acyl,amino,methyl,glycine,benzoate) of ACV have been synthesized to improve its corneal transport.It is well established that enzymatic process plays a more vital role than chemical processes in hydrolysis.Stability of different ester prodrugs of ACV was studied and stability was found to be in the order of EACV (γ glutamate) >> SACV (L-serine)> IACV (L-isoleucine) > VACV (L-valine) > AACV (L-alanine).Different ester prodrugs of ACV have been mentioned along with their structures that represent the moiety attached responsible for the prodrug activity (Fig.4 ).In addition,halflives of these prodrugs was significantly less and the only exception in this case was EACV whose half-life 82 ±6 min,MRT (mean residence time) was 149 ±11 min and T max (time to reach maximum concentration) was 160 ±11 min.The reason of this was the lack of the amino terminus near the hydraulic site,i.e.the ester linkage [81].Physiological data of various esters linked with ACV has been mentioned in Table 3 .

Table 3–Physicochemical data of various esters of ACV.
Ganciclovir: It is also an antiviral drug that exhibits action against human cytomegalovirus that leads to infection to AIDS patient,and if remain untreated leads to blindness.Its bioavailability is less due to which it does not get rapidly penetrated into ocular tissues.Its partition coefficient is low (1.55) [78]which contribute towards low corneal bioavailability.Hence,to overcome this drawback,it has been modified into prodrug (Fig.5 ).An investigation on ganciclovir (GCV) was carried out by Macha and Mitra in order to determine its intravitreal pharmacokinetics[85].Acyl monoester prodrugs of GCV were being used to achieve improved and sustained concentration of GCV in the vitreous fluid.The metabolic enzymes responsible for the bioconversion are primarily acetylcholine esterase and butyrylcholine esterase.Hydrolysis of prodrug were found to increase with increase in ester chain length and it followed the order as butyrate > valerate > propionate > acetate.Also the MRT increased ~3 to 4 folds with the administration of GCV.Their elimination rate was found to increase with the increase in their lipophilicity.This investigation had concluded that the prodrug approach is better than the use of sustained release implants in case of eyes [85].An another investigation carried out by the same research group involved replacement of monoester prodrugs of GCV by diesters and then the hydrolytic rate followed the order as dibutyrate > dipropionate > diacetate and MRT was enhanced by ~2 folds following prodrug administration as compared to GCV [86].
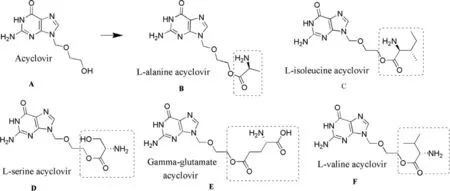
Fig.4–Structure of Acyclovir (A) and its ester prodrugs (B–F).
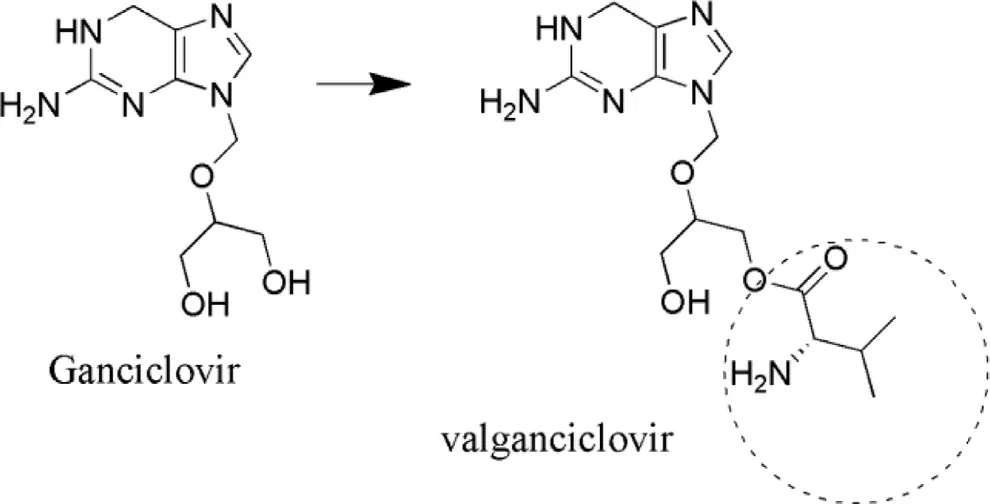
Fig.5–Structure of ValGCV (prodrug) converted from GCV(parent drug).
2.2.3.Prostaglandins
The most common complication is glaucoma that leads to loss of vision,the destruction of the optic nerve and the use of prostaglandins can overcome this.Prostaglandins are the polar compounds that do not have enough penetration and possess several unwanted side effects.The major focus is given to PGF 2 α and its analogues.The initial PGF 2 α prodrugs were PGFZ 2-L -methyl and PGF 2-L -isopropyl esters that inhibited increased corneal permeability [87].Latanoprost(LTP) and travoprost (TVP) are isopropyl esters of PGFα analogue on which modification was done on one side of omega chain.LTP was approved by FDA in 1996 (marketed as Xalatan®) followed by bimatoprost (BMP) (Lumigan®),TVP(travatan) and unprostone isopropyl (rescula®) by early 2000s[88].The analogs possess lower side effects.LTP and TVP gets hydrolyzed to free acid by the enzymatic action of corneal esterases.TVP was found to decrease intra ocular pressure (IOP) up to the levels of 6.5—9.0 mmHg [89],however,with a combination of timolol-dorzolamide and latanoprosttimolol IOP decreased up to 4.5 to 4.8 mmHg [90]and 6.4—6.8,respectively [91].This data clearly shows the efficacy of TVP in maintaining the IOP in comparison to combinational therapy.In addition,TVP was found to maintain the decreased IOP up to 63 h after the last dose.However,it was Suzuki,who suggested once in a day dosing of TVP as first line therapy and its fixed dose combination with Timolol to enhance IOP in case of failure of monotherapy [90].Yucel and Ariturk compared efficacy of LTP (0.005%),TVP (0.004%)and bimatoprost (0.03%) in open angle glaucoma and ocular hypertension.The baseline IOP in case of LTP,TVP and BMP was found to be 26.50 ±3.14,25.58 ±3.62 and 24.66 ±3.62 mm Hg,respectively.However,the mean IOP was found to be similar in all the three cases.All the three drugs were found to be equipotent although LTP was considered to be the most preferred because of lesser side effects [92].Structure of different prostaglandins and their conversion to prodrugs has been represented in Fig.6 .
2.2.4.Beta adrenergic antagonist prodrugs
These are the hypotensive agents for ocular use that have the potential to diminish IOP.Timolol (TM) (Fig.7 ),tilisolol(Fig.8 ),oxeprenolol (Fig.9 ) are the commonly used beta adrenergic antagonist whose prodrugs have been synthesized.FDA approval to TM was granted in 1979 for ocular delivery,which was used as a gold standard for IOP reduction.Prodrugs of TM were synthesized with the aim to decrease the extent of systemic absorption of the drug following ocular administration,thereby decreasing side effects and improving the ratio of corneal versus conjunctival penetration.TM has low lipophilicity (log P= −0.04) at physiological pH,which is hostile for corneal absorption [93].Esterification of the hydroxyl group of TM with aromatic/aliphatic acids increased the lipophilicity and thereby also corneal absorption.Halflives and lipophilicity of various esters of TM has been mentioned in Table 4 .
The penetration of TM esters across cornea was found to be more than the parent molecule due to increased lipophilicity,however,the magnitude of corneal penetration was not in parallel to that through conjunctiva membrane and ultimately to systemic absorption.The major challenge which is associated with delivery of TM esters is its stability in ophthalmic formulations for sufficient time which is required for enzymatic activity to ensure its complete bioconversion in the eye.Now investigations are being conducted by various researchers to overcome the limitation of the prodrug i.e.stability and also to further improve its action,e.g.for sustained effect/depot effect in order to improve the efficacy and patient compliance.Such novel drug delivery systems have been summarized in Table 5 .
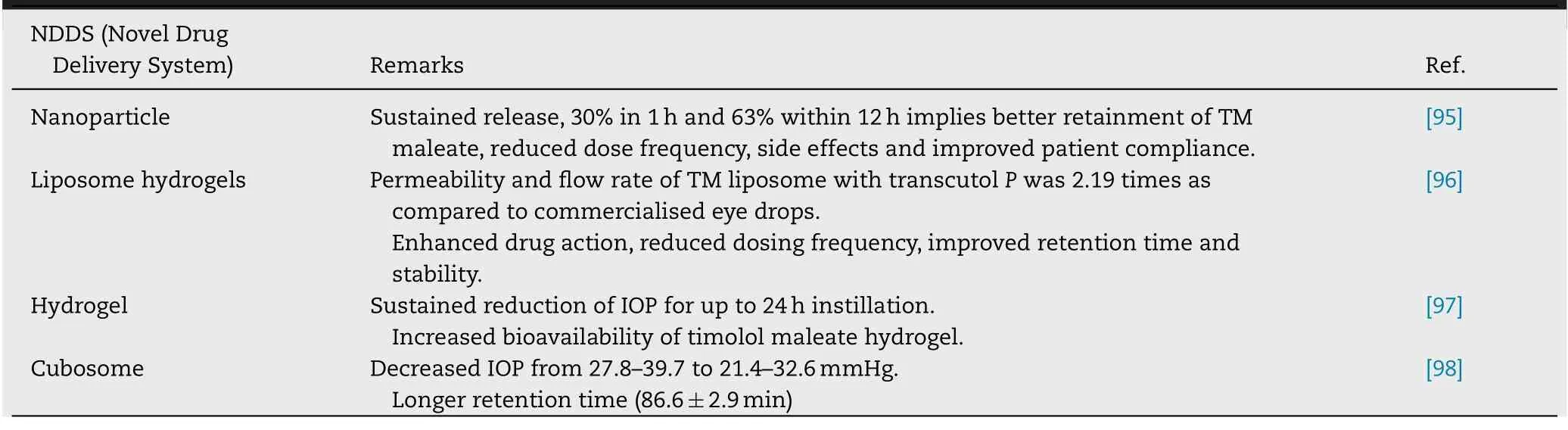
Table 5–Novel formulations of prodrugs of timolol.
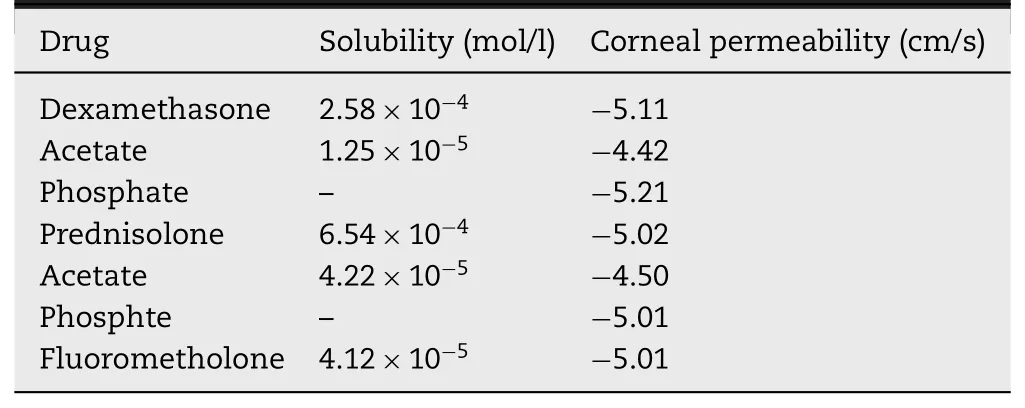
Table 6–Data of some steroids and their prodrugs [105].
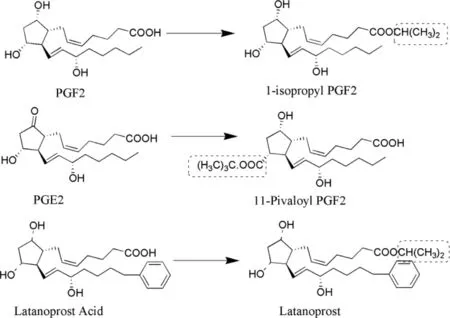
Fig.6–Structures of different prostaglandins (parent drug) and their prodrugs.
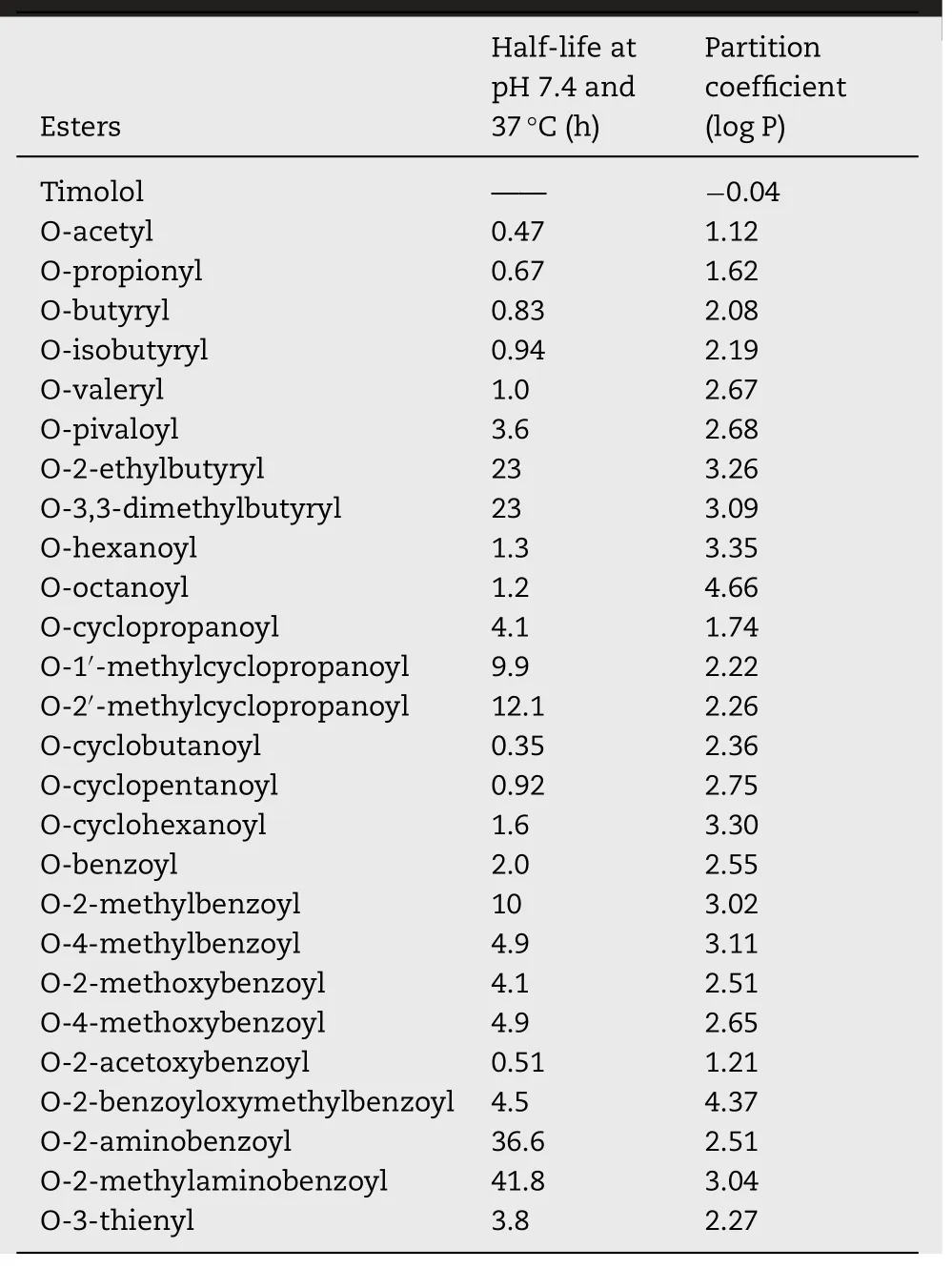
Table 4– Half-lives and partition coefficients of various esters of timolol [94].
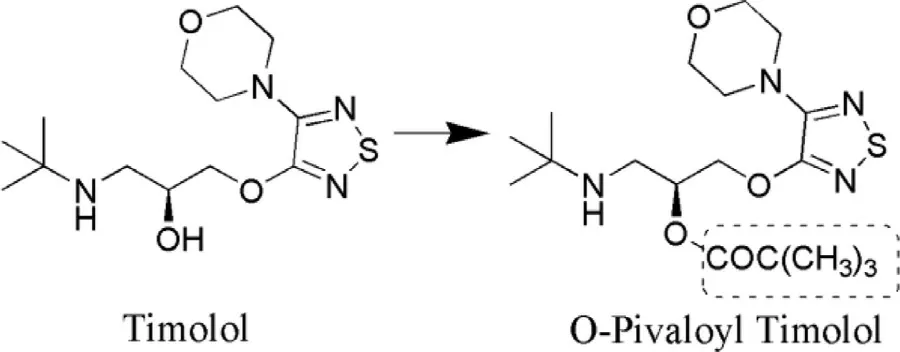
Fig.7–Synthesis of O-pivaloyl (prodrug) from timolol(parent drug).
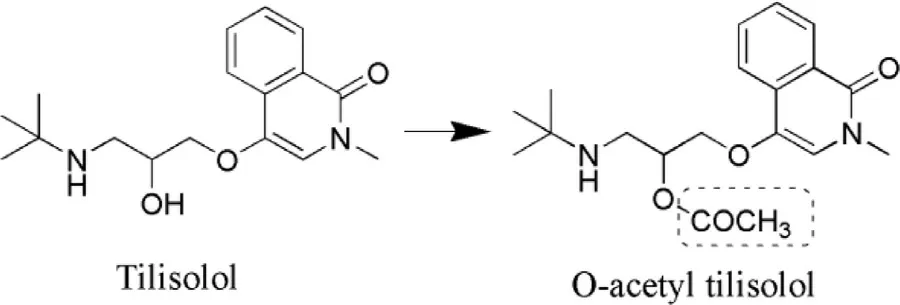
Fig.8–Synthesis of O-acetyl tilisolol (prodrug) from tilisolol(parent drug).
O-Palmitoyl tilisolol (PaTL) were synthesized as its prodrug by Kawakami et al.[99].They have reported enhanced transit time as well as increased retention of PaTL in precorneal areas as compared to the parent drug in ocular tissues.Hence improved effects with prodrugs have been demonstrated[99].The same group has reported O-butyryl (BuTL) and Opalmitoyl (PaTL) derivatives of tilisolol as prodrugs.Ocular inserts of tilisolol prodrug were formulated in order to achieve sustained release/controlled release of drug through the cornea.Lipophilic index of tilisolol,BuTL and PaTL have been reported as 1.56,2.18 and 3.75,respectively [100].Number of prodrugs of oxeprenolol (OPL) were also investigated for determining the stability profile.O-acetyl,O-propionyl,O-butyryl,O-valeryl and O-pivaloyl were synthesized as prodrugs and their half-lives in buffer at physiological pH were determined and found to be 9.1,10.4,19.1,21.1 and 2035.5 h,respectively and half lives were found to be 1.4,1.5,2.9,3.1 and 308.4 h,respectively.It was concluded that Opivaloyl exhibited the maximum stability,which is reflected from its shelf life that determines quality and efficacy over a specific period of time.O-acetyl was considered to be unstable amongst the rest of the prodrugs of OPL [101].
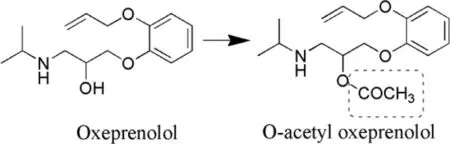
Fig.9–Synthesis of O-acetyl oxeprenolol (prodrug) from oxeprenolol (parent drug).
2.2.5.Cholinergics
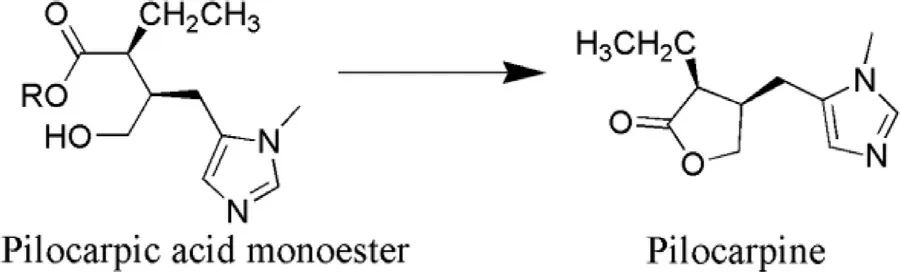
Fig.10–Conversion of pilocarpic acid monoester (prodrug)into pilocarpine (parent drug).
Pilocarpine prodrugs: Pilocarpine is well known cholinergic agent used in the treatment or management of glaucoma.It exhibits low level of absorption in the corneal site due to which it cannot penetrate into ocular tissues to exhibit its effect.Its log P value is −0.15 [102].So the answer to the above problem was in the synthesis of pilocarpine prodrug (Fig.10 ) that would improve the corneal absorption of parent drug and prove beneficial in ocular drug delivery.Bispilocarpine diesters (BPD) prodrug is one of the best examples to elaborate the effect of pilocarpine prodrug over parent drug.BPD prodrug exhibits unimpeachable amount of water solubility and lipophilic character due to esterification of lactone which is required for the better corneal penetration which further aids in releasing Pilocarpine at predetermined rate [103].During investigations permeability coefficients of bispilocarpic acid diesters was found to be (6.5—20.2) ×10cm/s more than the pilocarpine.Lipophilicity of the ester was improved from −0.77 to 2.49 at pH 5.0 which leads to augmented corneal permeation [104].Double prodrug is an auspicious means to overcome the stability issues with the help of derivatization of prodrug.In such a way that enzymatic release approach became necessary antecedent to release prodrug spontaneously e.g.pilocarpic acid diesters (double prodrug) by the action of ocular esterase,which further hydrolyzed into pilocarpic monoesters (prodrug) and ultimately leads to the cyclization of pilocarpine (parent drug) without enzymatic action (Fig.11 )[105].
2.2.6.Carbonic anhydrase inhibitors (CAIs)
Ethoxzolamide,methazolamide and acetazolamide are CAIs that have been exploited for derivatization in order to improve aqueous solubility and permeability,and to reduce unwanted side effects of the parent molecule [106].As mentioned earlier,reduced lipophilicity is the main culprit in the reduced permeation of this category of drugs.During the early periods,to overcome the limitation of these parent drugs different formulations were prepared like suspension,soft contact lenses,etc.Afterwards to surmount the same,chemical modification of existing CAIs was carried out to synthesize prodrugs with appropriate and desired physicochemical properties.However,it was further reported that the moiety which was considered to be amenable for derivatization was not appropriately amenable for prodrug synthesis,however,on the other side,it mainly emphasized on molecular manipulation of CAIs in order to improve physicochemical properties with the help of tail and ring approach [106].In continuation to this synthesis based problem,it was found in vivo experiments that substitution on sulphonamide nitrogen either diminishes or completely nullifies CAI activity.Because of these limitations,the progress of work on synthesis of a prodrug of CAIs was not appreciated by the researchers.
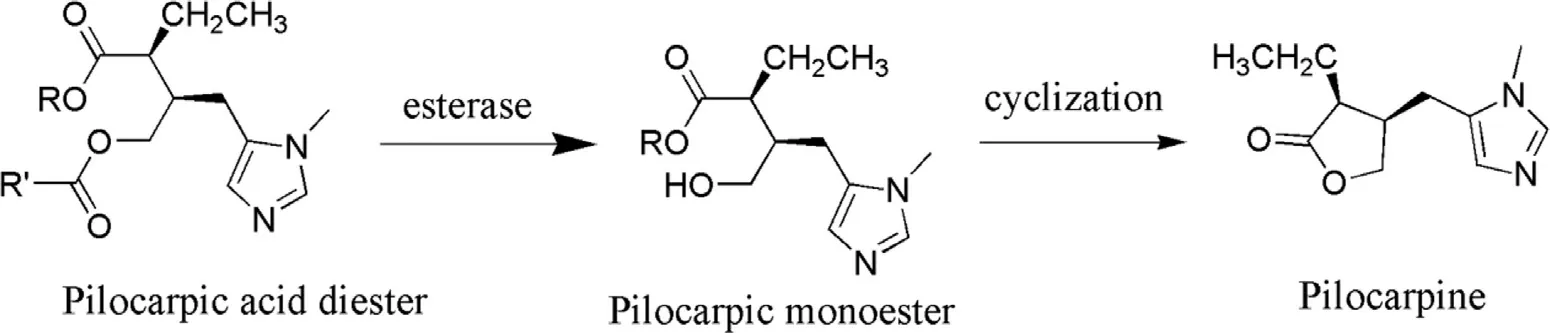
Fig.11–Concept of double prodrug.
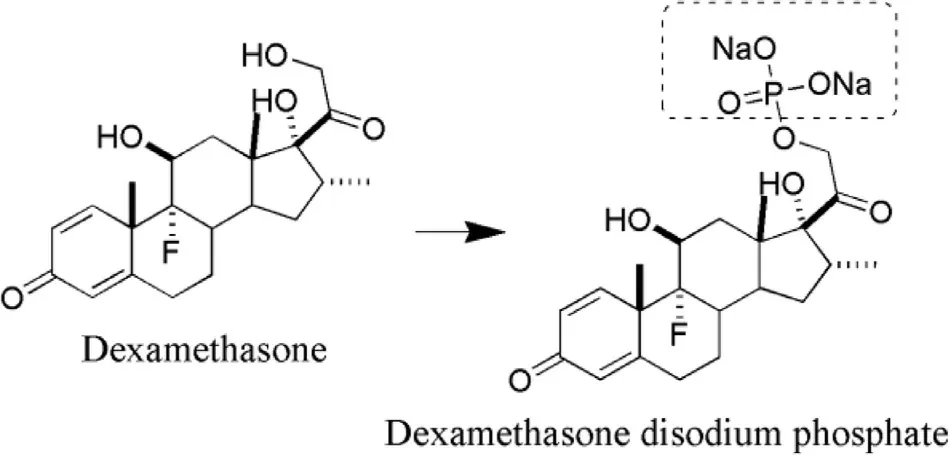
Fig.12–Synthesis of dexamethasone disodium phosphate(prodrug) from dexamethasone (parent drug).
2.2.7.Steroids
The steroid is the first class of drugs on which concept of prodrug was practically applied by researchers [107].The posterior segment of eye has always faced a challenge for efficient drug delivery.Dexamethasone has been used for treating anterior as well as posterior segment ocular problems [107].The ester prodrugs of steroidal class have been synthesized to improve their absorption through cornea and also to improve the hydrophilicity of parent molecules which build a possible way for the preparation of aqueous eye drops solution,leading to improved patient compliance as well as the instillation of an accurate dose[108].However,the data of few steroids suggest that there is no correlation of permeability through the cornea with a partition coefficient against the normal principle of permeation.Dexamethasone acetate possesses log P value of 2.4 (highly lipophilic) however,its corneal permeation is low [105].In a study,concentrations of dexamethasone following sub conjunctival administration of dexamethasone disodium phosphate were lower at initial time points (15 min),however,higher concentrations were achieved at 2 h time period,which revealed its lower residence time or depot effect in ocular tissues [109].Structures of dexamethasone disodium phosphate (prodrug) and dexamethasone (parent drug) are represented in Fig.12 .Barot and co-researchers developed a transporter targeted prodrug strategy which could be recognized by peptide transporter in retina.They synthesized amino acid and peptide prodrugs of dexamethasone and studied their physicochemical properties.The water solubility of Valine—Valine dexamethasone,a peptide prodrug has been reported as 7.07 ±1.86 mg/ml,which is significantly greater than that of dexamethasone (0.14 ±0.09 mg/ml).In addition,stability studies revealed the fast bioconversion of parent drug from the prodrug [110].Data on some of steroids has been mentioned below in Table 6 .
3.Patents
Ocular drug therapy has been considered to be a major challenge in the turf of drug delivery.The presence of blood ocular barriers and efflux pumps has always been of a great concern.Pharmaceutical industries and research institutes have embraced a collaborative way to meet the contemporary challenges which is demonstrated by the progression in the published and filed patents.Various approaches have been employed to achieve sustained and targeted delivery in the segments of the ocular diseases.Prodrug strategy has been implied to overcome ophthalmic challenge and obstacles which is evident from an increasing number of patents filed in this field.Patents are essential for promoting new directions in the research as well as for illuminating possibilities for future prospective in the field of ophthalmic.Few ocular drugs along with their prodrugs have been summarized in Table 7 and few patents on ocular prodrugs have been enlisted with their respective data in Table 8 .Quinidine is a known substrate of efflux transporters due to which it leads to a significant decrease in the permeation.Valine conjugated prodrugs of the quinidine (Val-quinidine and Val—Val-quinidine) depicted higher affinity for the peptide transporters and lesser affinity for the efflux transporters.A carrier-mediated transport of the quinidine prodrug resulted in improved permeation (1.5 and 3 times higher permeability of val-quinidine and Val—Val-quinidine in comparison to quinidine) [143].This approach was further exploited by scientists of Allergan Inc.These researchers anticipated glycyl and tryptophyl ester prodrug of bimatoprost(targeting amino acid transporters); glycylsarcosine ester of bimatoprost (targeting peptide transporters),succinate ester of bimatoprost (targeting monocarboxylic acid transporter),uridine di-ester of bimatoprost (targeting nucleoside transporters),and D-glucopyranosyl ester of dexamethasone(targeting glucose transporter) [134].These types of researches build the gap between academic and industry based research and proves to be beneficial for the society.
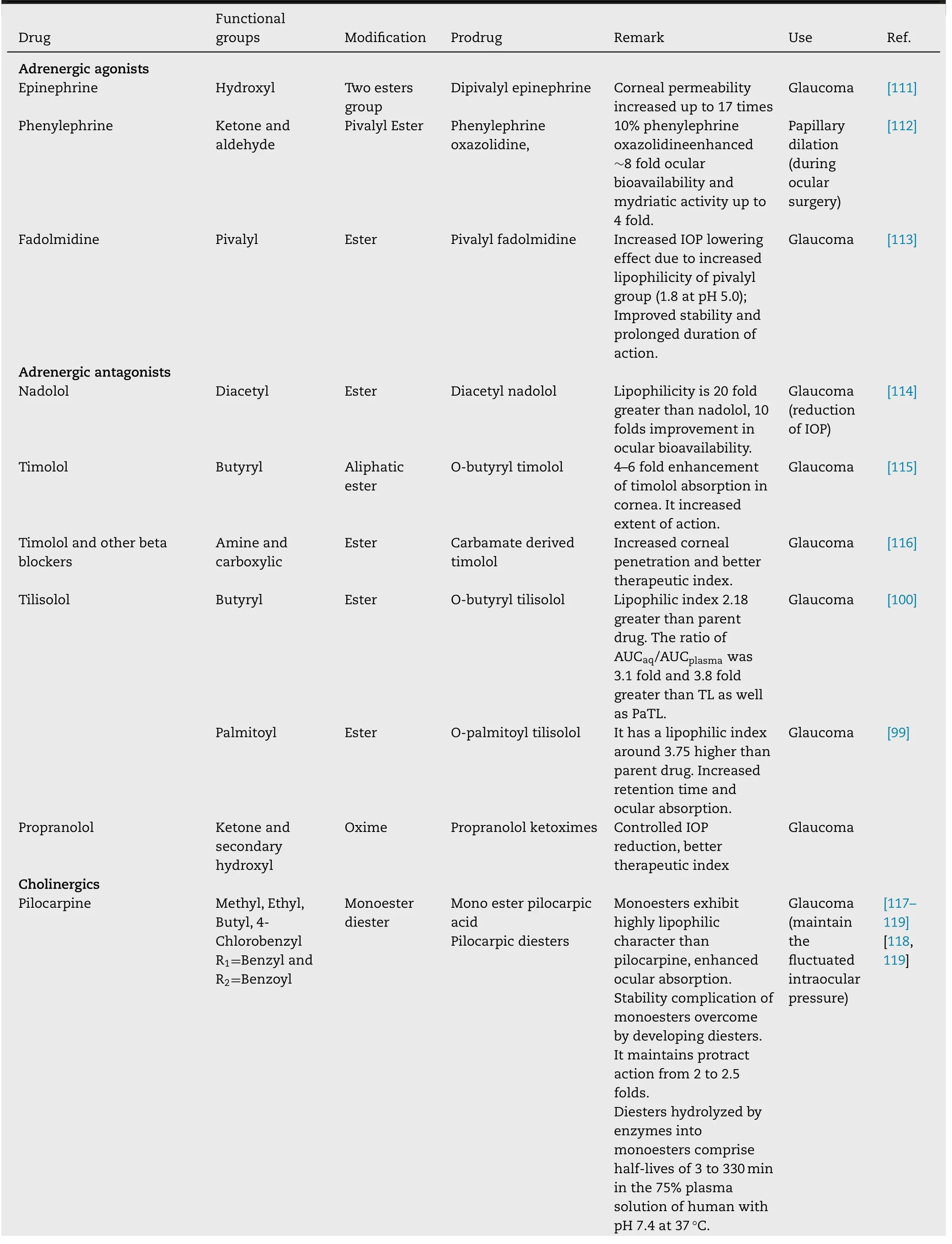
Table 7–Summary of ocular drugs with their prodrugs.
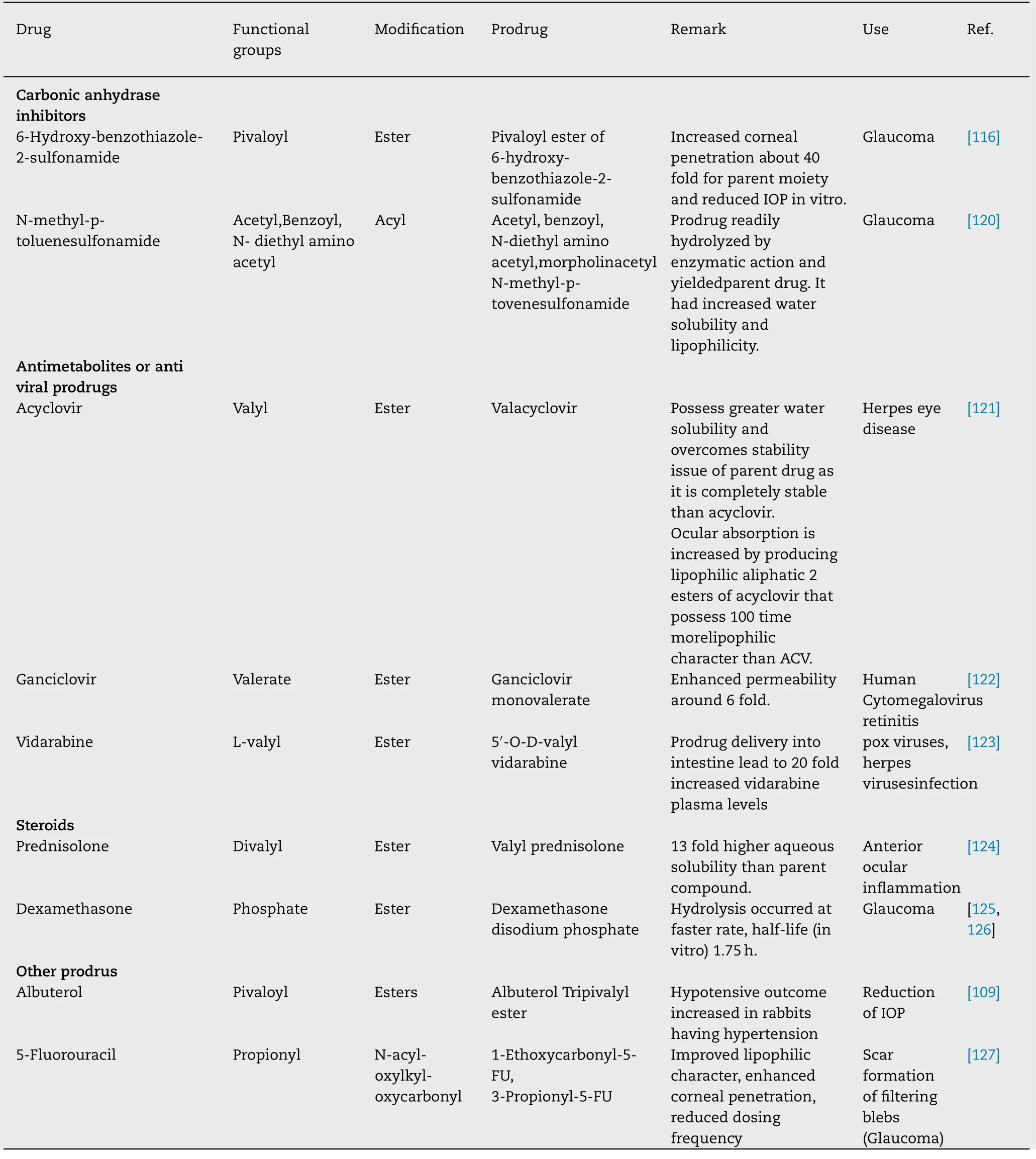
Table 7 (continued )
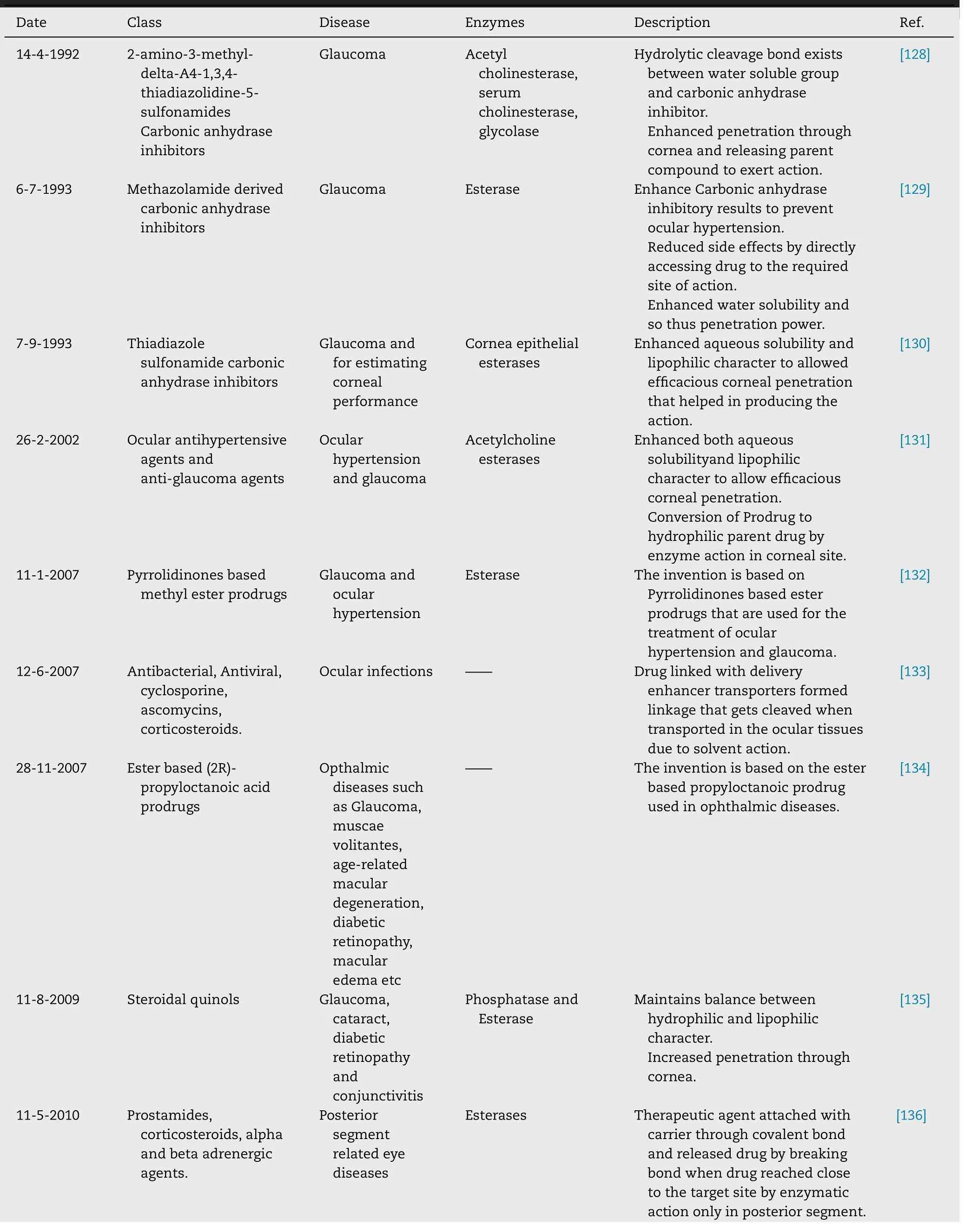
Table 8–Patented ocular prodrugs.
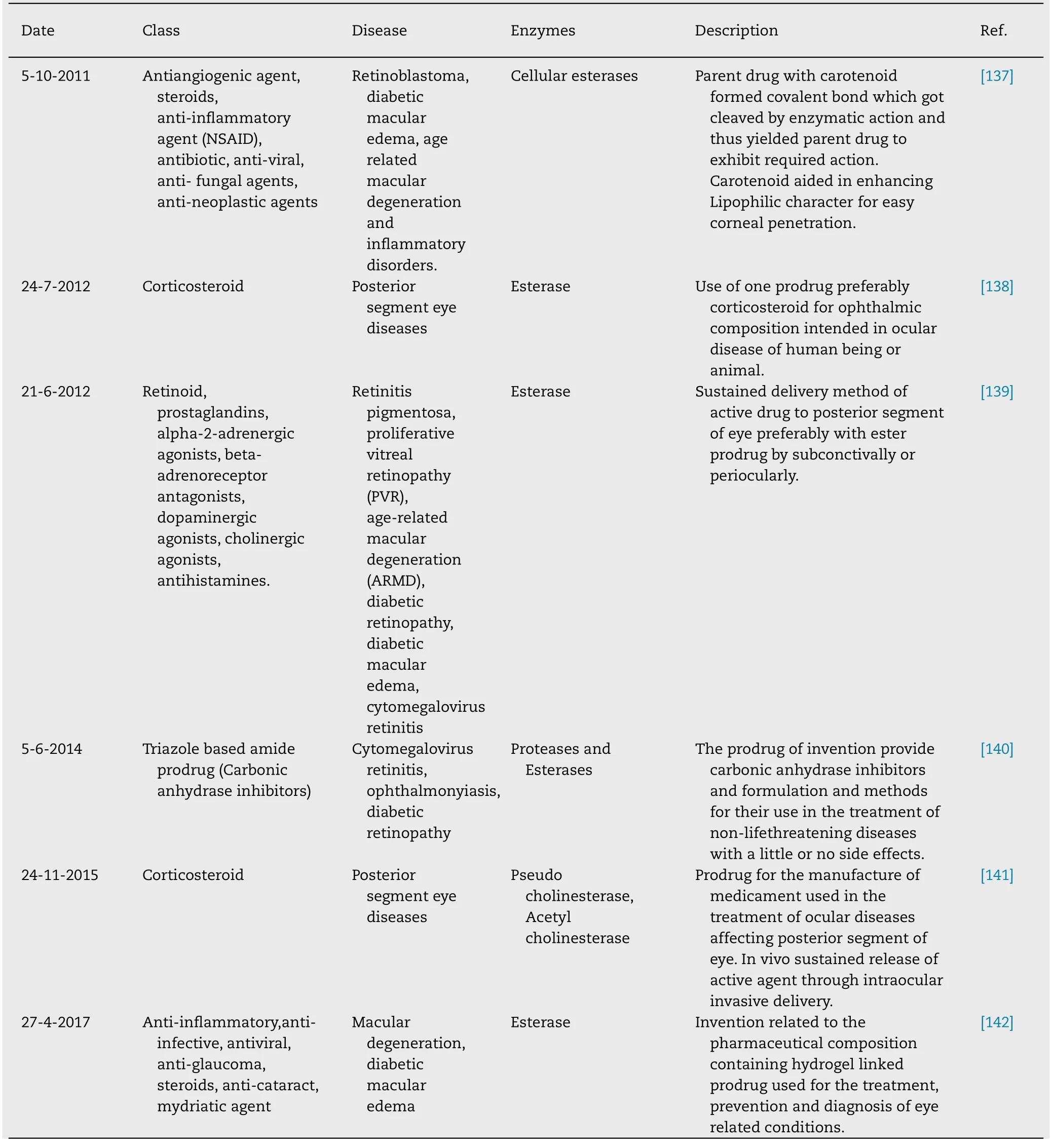
Table 8 (continued )
4.Conclusion
Drug development for ocular prodrug is extremely exigent approach for the researchers,as prodrugs have to pass defined basic standards like adequate solubility,stability,rational bioconversion into parent drug,optimum lipophilicity,safety etc.for the fortunate target drug delivery.Unfortunately,only a few of them are optimized for ocular absorption considering pharmacokinetic properties,and in addition poor water solubility that present a censorious pace in preclinical phases.Constraints of ocular drug delivery include short residence time,narrow pH range that can be tolerated,highly impermeable cornea,small surface area for corneal absorption,and large surface area for systemic drug loss.Attempts to improve ocular bioavailability have focused primarily on improving precorneal drug retention.Overcoming of poor permeability of the cornea and providing a possible alternate for the refinement of undesirable ADME properties and done with the help of prodrug approach.Due to flexibility in prodrug approach,many drug molecules are approved by FDA in the market for clinical use and are classified under prodrug.The rational prodrug derivatization has become an integral part in drug designing and development as illustrated by increasing number of approved prodrugs and patents.
5.Current and future developments
Effective topical administration of drugs using ocular drug delivery systems has always been a challenging task for scientists,researchers and industries.The paramount challenge is because of membrane barriers (reduce drug transport/permeation) and contact time.The extensive research in ocular drug delivery system has been going on since last many years and numerous new approaches like prodrug solution,implants and nana carriers have been developed.Gene therapy has also emerged as an interesting area of research in the field of ocular delivery recently.The reason behind this is that the eye is easily accessible,highly compartmentalized and immune privileged organ.However,this technique also faces extracellular and intracellular challenges.Hence it is expected that still there is a need of designing and synthesizing new molecules with expected physicochemical and pharmacokinetic properties,which can be achieved by in silico screening.Furthermore,if one approach is not proving to have desirable effects,then the combination of approaches might solve the issue.
Conflict of interest
The authors declare no conflict of interest.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi: 10.1016/j.ajps.2020.08.002 .
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- A cleavable self-delivery nanoparticle for tumor photo-immunotherapy
- SARS-CoV-2 vaccine research and development:Conventional vaccines and biomimetic nanotechnology strategies
- Prospective of extracellular matrix and drug correlations in disease management
- Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor
- Gas-blasting nanocapsules to accelerate carboplatin lysosome release and nucleus delivery for prostate cancer treatment
- A computer-aided chem-photodynamic drugs self-delivery system for synergistically enhanced cancer therapy
