SARS-CoV-2 vaccine research and development:Conventional vaccines and biomimetic nanotechnology strategies
2021-07-21LnxingHungYunRongQinPnKezhenYiXunTngQinZhngWeiWngJinyunWuFuingWng
Lnxing Hung,1,Yun Rong,1,Qin Pn,Kezhen Yi,Xun Tng,Qin Zhng,Wei Wng,Jinyun Wu,Fuing Wng,∗
a Department of Laboratory Medicine,Zhongnan Hospital of Wuhan University,Wuhan 430071,China
b State Key Laboratory of Virology and Medical Research Institute,Hubei Province Key Laboratory of Allergy and Immunology and Department of Immunology,Wuhan University School of Basic Medical Sciences,Wuhan 430071,China
c Clinical Trial Center,Zhongnan Hospital of Wuhan University,Wuhan 430071,China
Keywords:SARS-CoV-2 COVID-19 Vaccine Biomimetic nanotechnology Virus-like nanoparticles
ABSTRACT The development of a massively producible vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),a novel coronavirus,is essential for stopping the current coronavirus disease (COVID-19) pandemic.A vaccine must stimulate effective antibody and T cell responses in vivo to induce long-term protection.Scientific researchers have been developing vaccine candidates for the severe acute respiratory syndrome (SARS)and Middle East respiratory syndrome (MERS) since the outbreaks of these diseases.The prevalence of new biotechnologies such as genetic engineering has shed light on the generation of vaccines against novel viruses.In this review,we present the status of the development of coronavirus vaccines,focusing particularly on the biomimetic nanoparticle technology platform,which is likely to have a major role in future developments of personalized medicine.
1.Introduction
The coronavirus disease (COVID-19) outbreak started in China at the end of 2019.As of April 30,2020,there were 84,369 confirmed cases in China and 3,077,229 confirmed cases outside of China [1].The outbreak and current pandemic have attracted worldwide attention following the WHO’s declaration that the outbreak was a global health emergency.The widespread and severe clinical impact of COVID-19 compelled the Chinese government to take strict measures to prevent the spread of the virus [2].In addition to the development of therapeutic agents against SARS-CoV-2,there is an urgent need to development an efficacious vaccine tocombat the rapid spread of SARS-CoV-2.Since the birth of the first vaccine in 1796,vaccines have profoundly reduced the prevalence and fatality rates of various infectious diseases,including measles and smallpox [3].Owing to rapid advances in technology,novel materials have been employed for vaccine preparation,such as virus-like particles (VLPs).For example,a VLP-based HPV vaccine,which is now commercially available,has improved HPV prevention.
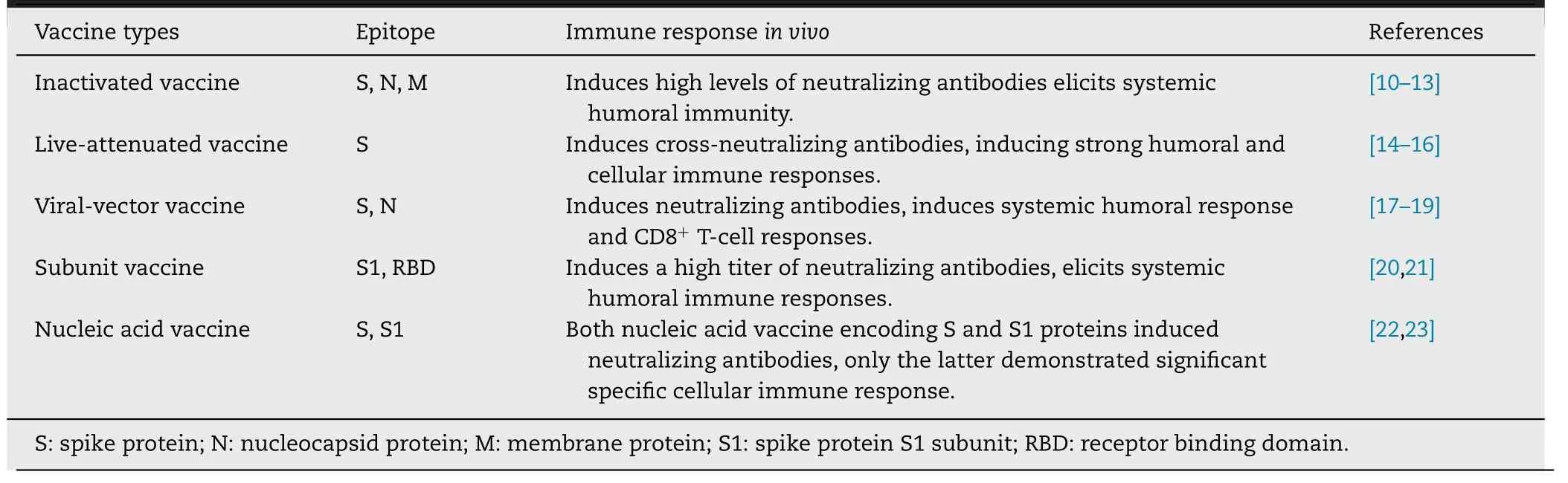
Table 1–Types of traditional vaccines and possible immune responses.
2.Conventional coronavirus vaccines
Conventional coronavirus vaccines include inactivated vaccines,live-attenuated vaccines,viral vector vaccines,subunit vaccines,and nucleic acid vaccines.
2.1.Inactivated vaccine
Inactivated vaccines are viral particles killed by irradiation or chemicals.Their production is relatively easy,and they induce a robust immune response owing to the multiple epitopes on the viral surface [9].However,it is reported that SARS-CoV inactivated vaccine increase eosinophilic pro-inflammatory pulmonary response upon challenge [24,25].Studies have shown that SARS-CoV N protein vaccination enhances the immunopathological changes in the lungs.SARS-CoV N protein-specific T cells and Th2-skewed cytokine profile may be the cause of adverse reactions [26,27].Therefore,it is necessary to find methods to enhance the protective Sspecific immune response while minimizing the potentially pathological anti-N response.We should keep in mind that the side effects of inactivated vaccines must be intensively evaluated before clinical application.Furthermore,most research on coronavirus must be performed cautiously in biosafety level-3 (BSL-3) laboratories,which has hindered the development of coronavirus vaccines.
Inactivated SARS-CoV vaccines induce the production of high levels of specific neutralizing antibodies in animal models [10,28,29],including antibodies against the viral S,N and M proteins.The results from in vivo experiments showed that the levels of antibodies against the N and S proteins were higher than the levels of antibodies against M proteins in antisera from vaccinated mice,suggesting that epitope polypeptides from the N or S proteins may be potential targets for the generation of recombinant SARS vaccines [10].On April 12,2020,the inactivated SARS-CoV-2 vaccine developed in Wuhan,China was approved for clinical trials,it is the world’s first inactivated SARS-CoV-2 vaccine to enter clinical trials[30].The inactivated SARS-CoV-2 vaccine is currently in Phase II clinical trials.
2.2.Live-attenuated vaccine
Live-attenuated vaccines are created by weakening infectious organisms that can still replicate and induce protective immune responses,but do not cause disease in the host.The live-attenuated vaccine against coronavirus may be generated by depleting or mutating virulent genes in the viral genome.These live-attenuated vaccines diminish virulence while maintaining immunogenicity.Live-attenuated vaccines are good at driving both innate and adaptive immunity with longterm immunological memory.Furthermore,these vaccines can be produced quickly and at a low cost,thus,they are ideal for responding to coronavirus outbreaks [31].Researchers have generated a series of live-attenuated SARS or MERS vaccine candidates that have successfully induced immune responses in vivo [24,32—35].However,it is vital to avoid the restoration of virulence in these vaccines owing to the backmutation of attenuating mutations,compensatory mutations elsewhere in the genome,recombination or reassortment.
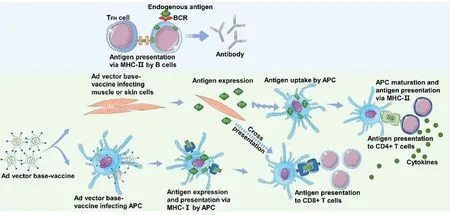
Fig.1–Immune response to an Ad vector vaccine.When the Ad vector vaccine infects non-APCs,the infected cells express and secrete antigenic proteins,which are taken by antigen-presenting cells (APCs) and B cells.The antigenic proteins are degraded into antigenic peptides.APCs present exogenous antigenic peptides to CD4+ T cells through MHC-II.Cytokines produced by activated CD4+ T cells contribute to the activation of CD8+ T cells.B cells present antigenic peptides to follicular T helper cells through MHC-II,thereby promoting further B cell differentiation into long-lived antibody-producing plasma cells.When the Ad vector vaccine infects APCs,the vaccine expresses the antigen protein which is subsequently digested into antigenic peptides.The antigenic peptides are then presented to CD8+ T cells through MHC-I.In this way,both T cell subsets are activated.
2.3.Viral vector vaccine
Viral vector vaccines use live recombinant viruses to deliver DNA into human cells.The DNA sequences loaded into the viral vector vaccines encode one or more antigens.After these vector vaccines infect cells,the antigen protein is expressed in the cells,and the antigen is processed to activate the subsequent immune response (Fig.1 ).Several viral vectors have been used to construct coronavirus vaccines,including measles virus,vaccinia virus Ankara (MVA),and recombinant adenovirus vectors (ADVs).The genes encoding the N protein and the S protein have been loaded into the viral vectors to trigger anti-coronavirus immunity in the host [17,18,36—40].The effectiveness of SARS and MERS viral vector vaccines have been validated in animal models.These viral vector vaccines are capable of triggering specific CD8T cell response and generating high levels of virus-neutralizing antibodies.
Adenovirus vectors are the most commonly used vectors in clinical trials [41].They are widely used in gene therapy,vaccination and other fields.It is one of the best candidates for vaccine development,mainly owing to its low pathogenicity,genetic safety,lack of host genome integration steps in its replication cycle,and induction of strong humoral and cellular immune responses.Adenovirus type-5 (Ad5) vector is the most commonly used vector.Ad5 vector vaccines against human immunodeficiency virus type 1 (HIV-1) and Ebola virus have been investigated in Phase II clinical trials to verify their safety and effectiveness [42,43].For other pathogens,recombinant adenovirus type 5 (rAd5) vector vaccines have also elicited antigen-specific cellular immune responses [44].Professor Wei Chen and colleagues reported the safety,tolerability and immunogenicity of a rAd5 vectored COVID-19 vaccine in The Lancet [45].Using genetic engineering technology,replication-deficient Ad5 was used as a vector to prepare a vaccine that expresses the SARS-CoV-2 S protein.
However,adenovirus infection induces a strong innate and adaptive immune response and establishes long-term immune memory.Ad5 is a common human serotype and has a high infection rate,as such,anti-Ad5 antibodies may already be present in the human body,especially in individuals in developing countries [46—48].Thus,the immunogenicity and clinical utility of the Ad5 vector vaccine is limited.Research has shown that a homologous priming-boosting regimen with an Ad-5-type vaccine at 6-month intervals was able to elicit strong antibody responses with a longer duration [49].The results supported the use of an immunization strategy to implement a booster injection to effectively overcome the effect of pre-existing immunization on vaccine efficacy.This indicated that the clinical trial of Ad5-nCoV may also require a strategy that uses a booster injection.
Existing immunity to Ad5 in humans is highly prevalent,consequently,this has led to the development of vectors derived from rare Ad serotypes.Ad26 vectors have been proven to have the highest immunogenicity of the rare serotype Ad vectors studied,which has shown the potential of the Ad26 as a vaccine vector in developing countries [50].Another company has developed a vaccine based on Ad26 vector,the company expects to initiate human clinical studies of its lead vaccine candidate by September 2020 at the latest and anticipates the first batches of a SARS-CoV-2 vaccine could be available for emergency use authorization in early 2021 [51].The company’s research strategy is promising.
So if you ever have a chance to talk with Marianne and ask her if there is any truth to fairy tales like Cinderella and Snow White, she ll probably say she s learned a lot about such stories in her lifetime.She s likely to say: There sure are a lot of Prince Clods out there. But there sure are some Prince Charmings, and there are really a lot of Cinderellas, too.
2.4.Subunit vaccine
A subunit vaccine is a vaccine produced from selected viral antigenic protein subunits.Thus,this type of vaccine has a lower risk of adverse reactions compared with a whole virus vaccine.Subunit vaccines for the S protein fragment of SARS-CoV and MERS-CoV have been reported previously [52,53].However,the MERS-CoV S protein likely contains non-neutralizing epitopes,which may hinder the production of neutralizing antibodies and the anti-MERSCoV immune responses.Therefore,it is necessary to identify the critical neutralizing epitopes in the MERS-CoV S protein and exclude non-neutralizing epitopes without the expense of domain integrity and stability in the design of the subunit vaccine for inducing the host immune response[54].The spike glycoprotein of MERS-CoV is a type I trimeric membrane protein expressed on the viral surface.It binds to dipeptidyl peptidase 4 (DPP4) on target cells[55].Currently,the neutralizing epitopes and functional mechanisms of monoclonal antibodies including 4C2,2E6,7D10,D12,m336,MCA1,MERS-27,JC57-14,CDC-C2,MERS-4,and MERS-GD27 were elucidated at the atomic level by structural and functional studies [56—58].These antibodies target the receptor-binding subdomain of MERS-CoV and overlap with the DPP4 binding surface.These studies are the basis for the development of subunit vaccines.
2.5.Nucleic acid vaccine
Nucleic acid vaccines include DNA vaccines and mRNA vaccines.After nucleic acid vaccines are inoculated into the cells,the antigenic proteins encoded by the vaccines are expressed on the cells,and the immune system in the host stimulates specific immune reactions [59—61].DNA vaccines are based on bacterial plasmid vectors that encode vaccine antigens driven by efficient eukaryotic promoters [62].Application of a DNA vaccine encoding the MERS virus S protein is successful in Phase I clinical trial.Vaccine-induced humoral and cellular responses have been detected in the trial [63,64].Nucleic acid vaccines can be produced on a massive scale and could be easily purified from genetically modified bacteria.Consequently,nucleic acid vaccines may be ideal for the fast,inexpensive and large-scale preparation of SARS-CoV-2 vaccines.The DNA vaccine platform was one of the first technologies to receive support from the Coalition for Epidemic Preparedness Innovations (CEPI) to accelerate SARS-CoV-2 vaccine development.The SARS-CoV-2 vaccine candidate (INO-4800) is in Phase I/II clinical trials in South Korea and the United States [65].
Over the past decade,significant technological innovations and research investments have made mRNA a promising therapeutic tool in the field of vaccine development and protein replacement therapy [66].mRNA is the intermediate molecule between DNA and proteins.The mRNA vaccine aims to deliver antigen-encoding mRNA into the ribosome to produce viral antigens [67].The mRNA vaccine is taken by different types of cells and activates the immune system through the MHC-I pathway and the MHC-II pathway after vaccination (Fig.2 ).When the mRNA vaccine is taken by APCs,APCs processes and express the target protein as an endogenous antigen,and then activates CD8T cells through the MHC-I pathway.When the mRNA vaccine is taken by non-APCs,the cells translate and secrete the target protein which is then taken by APC.The APCs subsequently activate CD4T cells through the MHC-II pathway.Exogenous antigens can also be processed and loaded onto MHC-I molecules through cross-presentation [68].mRNA-based vaccines are a promising new platform that is highly versatile,safe,effective,simplified,scalable and cheap,has the potential to be free of cold chains [67,69,70].Importantly,mRNA-based vaccines can meet the requirement for rapid and large amounts of effective vaccines against emerging pandemic infectious diseases [67].This multi-functional RNA vaccine platform can increase the speed and cost of vaccine development and quickly produce effective vaccines for new epidemics [71].By optimizing the mRNA manufacturing platform and design of intracellular delivery methods,clinical transformation of mRNA therapy has been improved [72].
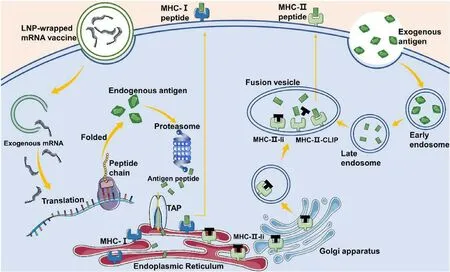
Fig.2–mRNA vaccine-mediated antigen presentation via MHC-I and MHC-II pathways.When an mRNA vaccine is taken by APCs,the antigenic proteins are translated in these cells.These proteins become endogenous proteins and are degraded by the proteasome into small peptides.The peptides are transported to the endoplasmic reticulum and loaded onto MHC-I molecules,and then activate CD8+T cells through the MHC-I pathway.In the endoplasmic reticulum,MHC-II molecules are protected by an invariant chain (Ii) to prevent them from binding to endogenous peptides.The MHC-II-Ii complex is exported to the fusion vesicle through the Golgi apparatus,and then the invariant chain is replaced by exogenous antigenic peptides.When the vaccine is taken by non-APC cells,it will express and secreted antigenic proteins.These exogenous proteins enter APCs through endocytosis and activate CD4+ T cells through the MHC-II pathway.
On January 13,2020,the U.S.National Institutes of Health(NIH) finalized the sequence for a potential mRNA vaccine,mRNA-1273 [73].mRNA-1273 is a vaccine that encodes for a prefusion stabilized form of the S protein of SARS-CoV-2.On March 16,2020,the NIH announced that the first participant in its Phase I study for mRNA-1273 was dosed.The mRNA platform offers significant advantages in terms of speed and efficiency from the perspectives of basic science,manufacturing and clinical development.
3.Biomimetic nanotechnology in the development of vaccines
Although conventional viral vaccines have delivered considerable success,their ability to generate strong antiviral responses is limited and great challenges remain in achieving effective responses to sudden epidemics.Further,traditional vaccine preparation methods cannot produce vaccines against viruses with high antigen diversity and mutation rates,thereby limiting the development of vaccines.To address these obstacles with conventional vaccines,many researchers have turned to nanotechnology to design and produce nanoparticle vaccines with good effectiveness,specificity and long-lasting antiviral response.Taking the VLP vaccine as an example,highly conserved antigens shared by different virus subtypes can be inserted into VLPs as potent antigenic proteins [74].Structural informatics can be used to reconstruct and modify conserved epitopes to expose highly immunogenic regions or insert multiple immunodominant epitopes into the same VLP to create a broadly crossprotective vaccine [75].This is of great significance for viruses that exhibit highly variable antigenicity and enormous mutations.
Biomimetic nanoparticle technology,a novel type of nanotechnology,is a rapidly developing field that has undergone tremendous advances in the last decade.Taking advantage of the specific delivery and translocation mechanisms adopted by pathogens,bioinspired nanoparticles have been produced with diverse functions,good biocompatibility,extended circulation time and enhanced accumulation at the infection sites [76].Biomimetic nanoparticles have unique antigenic characteristics and immunostimulatory properties and have been used to design more effective vaccine formulations.This strategy directly uses the principles of natural design to manufacture multifunctional and multi-antigen nanosystems that may play an important role in the future [77,78].Compared with traditional vaccines,the careful manipulation of nanoparticle parameters and conscious design can yield significant improvements in efficacy.
3.1.Advantages of biomimetic nanoparticles for vaccine development
MERS-CoV-specific IgA protects patients with the infection,but cannot persist for a long period [79].MERS-CoV-specific memory CD8T cells can be detected in the body for up to 6 years and exert a strong antiviral effect on various subtypes of viruses [80,81].Live-attenuated vaccines are capable of inducing the formation of memory T cells.However,the potential risk of restoration of virulence hampers the application of conventional coronavirus vaccines in clinical trials.Therefore,non-live virus vaccines that robustly induce memory T cells are in great demand.
As next-generation medical agents,biomimetic nanoparticles simulate the physiochemical nature of the pathogens while retaining the surface features of nanoparticles [78].Biomimetic nanoparticle vaccines usually consist of three elements: viral antigen molecules,adjuvants and a protein scaffold.The viral antigens induce the adaptive immune response,and the adjuvants enhance innate immune response [82—85].This vaccine type is safer and more effective than conventional vaccines.Biomimetic nanoparticle delivery not only protects the adjuvant from degradation but also protects the body from systemic toxicity caused by free adjuvants,which may cause side effects such as fever,drowsiness,diarrhea,and nausea[86].Current biomimetic nanoparticles available for vaccine preparation include virosomes,VLPs,self-assembling protein nanoparticles and synthetic nanoparticles,with sizes ranging from 10 to 200 nm [76,87].VLPs are the most frequently used biomimetic nanoparticles in vaccine preparation.VLPs carry both antigens and adjuvants [88],and prime immune cells to induce immune responses after vaccination.
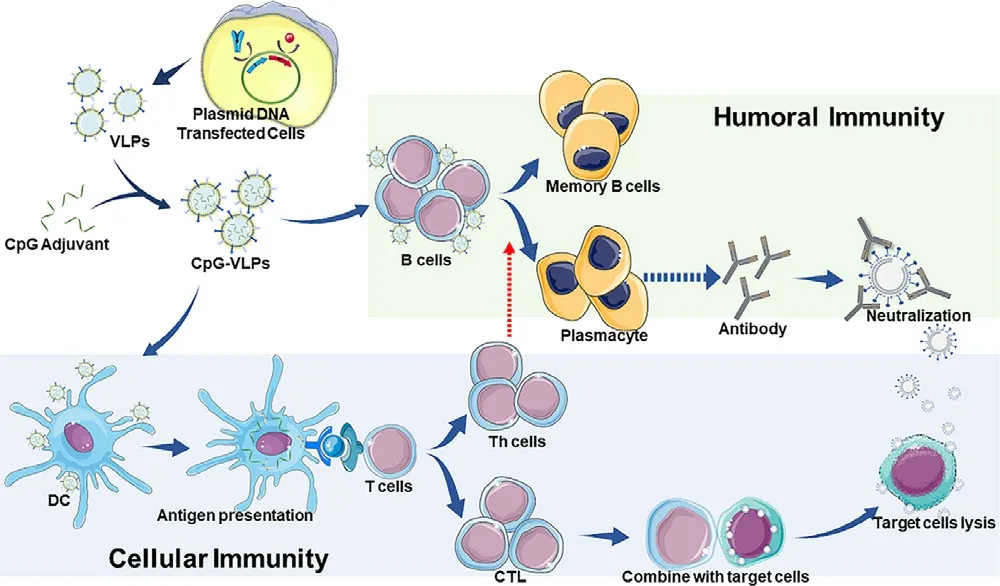
Fig.3–Generation of SARS-CoV-2 CpG-VLPs vaccine and the Cor111responding immune response.VLPs induce humoral immunity through the B cells and cross-presentation.VLPs induce cellular immunity through antigen-presenting cells.
3.2.Immune responses induced by biomimetic nanoparticle vaccines
VLP vaccines are capable of conferring humoral immune responses and triggering the production of neutralizing antibodies (Fig.3 ).B cells specifically bind to the antigens carried by VLPs through B cell receptors (BCRs) and internalize VLPs.The recognition and internalization of VLP vaccines provide signals for B cell activation.Consequently,activated B cells initially differentiate into plasmablasts and ultimately into plasma cells to produce antibodies [89]including IgG and IgA [90].Under the induction of T follicular helper cells (TFH),memory B cells and plasmacytes are produced and sustained in the germinal centers [91].
VLP vaccines also activate the cell-mediated immune response (Fig.3 ).The maturation of dendritic cells (DCs) is critical to successfully trigger cellular the immune response induced by a vaccine.DC maturation requires both antigen presentation and co-stimulatory molecules.Antigens are engulfed and processed by DCs,subsequently,the antigenic peptide binds to MHC molecules and is presented on the surface of DCs.T cells will be activated by recognizing the antigenic peptides presented by MHC molecules,as well as by binding to c molecules on DCs.The adjuvants in biomimetic nanoparticle vaccines act as costimulatory molecules,whereas viral proteins provide antigens.These interactions between VLP vaccines and DCs induce maturation of DCs and the subsequent T cell activation [92,93],including the activation of both helper T (Th) cells and cytotoxic T lymphocytes (CTLs) [94,95].Activated Th cells induce cytokines to promote antiviral immunity and to upregulate the expression of costimulatory molecules for B cell activation and differentiation into plasma cells.CTLs inhibit virus replication by lysing infected cells to release the virus and neutralizing antibodies to destroy the virus.
Therefore,biomimetic nanoparticle vaccines provide sufficient stimulatory signals to launch both cellular and humoral immunity.
3.3.VLPs for vaccine development
VLPs are the noninfectious protein shells or capsids conjugated with virus-derived structural proteins that are modified to be of use in nanotechnology.The structure of VLPs is similar to natural viruses but has no viral genome.VLPs are strongly immunogenic and biologically active [96],capable of inducing both cellular and humoral immune reactions [97,98].VLPs’ sizes range from 20 to 100 nm [99].In comparison with conventional virus vaccines such as inactive or live-attenuated vaccines,VLPs are much safer and can be more easily produced at a lower cost.VLPs have more diverse immunogenicity than subunit vaccines and nucleic acid vaccines [100,101].
Evidence has accumulated to show that VLPs promote protective immunity against infectious diseases including flu,hand,foot,and mouth disease,hepatitis B,and HPV infection [98,102—106].The immunogenicity of HBV VLP was significantly higher than the recombinant protein vaccine.HBV VLP elicits neutralizing antibodies and is capable of stimulating specific CD8and CD4T cell responses in vivo[107].VLPs may also be used as a potential therapeutic vaccine owing to their powerful effect on the induction of antiviral immunity [108].Some VLP vaccines for HPV are already commercially available,including Cervarix,Gardasil,and Gardasil9○,and Sci-B-Vacis an HBV vaccine [102,105,109].VLPs are generated in insect or yeast expression systems,which can express complex viral protein antigens on a large scale for commercial purposes [110].
VLPs vaccines are considered to be a powerful candidate for the prevention of the SARS-CoV-2 pandemic.VLP-based coronavirus vaccines have previously been developed using a variety of antigen combinations or expression systems.These vaccines are reported to inhibit coronavirus infections and are thus promising or future clinical applications [111—119].However,to date,few studies have investigated whether the combination of VLPs with adjuvants enhanced the protective effects of VLP-based vaccines.A research team uses its proprietary recombinant protein nanoparticle technology platform to develop a SARS-CoV-2 vaccine candidate containing the coronavirus S protein.They recommend utilizing its proprietary Matrix-Madjuvant to enhance immune responses during the vaccination with the vaccine.It is expected to advance the SARS-CoV-2 vaccine candidate to Phase I clinical testing in May or June [120].
3.4.Modification of VLPs
Researchers are attempting to modify the surface of VLPs with small molecules or chemical groups to protect the “cargo”from the negative effects of enzymes or serum proteins and increase the targeting accuracy [121].This approach has been evaluated in tumor therapy.However,the necessity of surface modification to VLP-based vaccines remains controversial.VLPs have a unique repetitive surface structure that is easily recognized by the innate immune system.This structure is the basis of VLPs’ strong immunogenicity because it induces the opsonization of antigen-presenting cells [121].Moreover,this structure promotes B-cell activation,resulting in a stronger humoral immune response,as well as enhanced T-cell stimulation and cellular mediated immunity [122].Therefore,it may be unnecessary to increase the uptake of VLPs by modifying the surface of VLPs.
VLPs are able to spontaneously assemble nucleic acids,enabling them to effectively deliver CpG [123].Cytidine monophosphate guanosine oligodeoxynucleotides (CpG or CpG ODN) are a class of DNA analog composed of unmethylated cytosine.As a robust adjuvant,CpG-ODN binds to TLR9 to trigger the maturation of antigen presenting cells,resulting in the promotion of CD4and CD8T cell responses,as well as B cell activation and proliferation.Consequently,both T cell response and antibody production are conferred to provide comprehensive protection of immunized individuals from virus infection [124—129].The CpG adjuvanted VLP vaccines were shown to enhance cellular immunity in preclinical models of infections for the foot-and-mouthdisease virus and influenza virus [130,131].The combination of a CpG adjuvant is an important strategy to enhance the efficacy,potency,and safety of VLP vaccines.
4.Perspectives
We have proposed a promising strategy for the development of a novel SARS-CoV-2 vaccine: biosynthetic SARS-CoV-2 VLPs combined with CpG (termed CpG-VLP SARS-CoV-2 vaccine).Most vaccines consist of antigens and immune adjuvants,it is the spatial colocalization of these two components that ensures a prompt immune response against the antigen of interest [132].VLP-based SARS-CoV-2 vaccines may show advantages in the induction of persistent immune reactions for the prevention of SARS-CoV-2 infection.In the face of a rapidly emerging epidemic,reduction of vaccine development time and costs is important.Existing vaccine technology cannot quickly respond to new virus outbreaks; thus,further developments are required to address practicality at the industrial scale.VLP platform technology has the potential to address the rapid spread of emerging viruses.
VLP technology can overcome numerous drawbacks associated with traditional methods of vaccine production,such as infectiousness and lengthy production time.The yield and cost of the vaccine depend on the choice of expression systems and the purification process.VLP technology is rapidly developing especially in the optimization of the expression system and VLP purity.For example,for the expression system,a study reported that when produced in E.coli,the murine polyomavirus (MuPyV) capsomere platform was capable of producing 320 million vaccine doses in a few days,at a low cost.This shows its suitability as a rapid response and low-cost vaccine platform [133].The recently optimized Insect Cell-Baculovirus Expression Vector System(IC-BEVS) has a similar potential [134].IC-BEVS is used to manufacture approved VLP vaccines such as Cervarix○and Porcilis PCV○.The purification of VLPs is usually performed via polyethylene glycol precipitation,diafiltration,chromatography,density gradient ultracentrifugation,ultracentrifugation or continuous flow ultracentrifugation[135,136].Although these technologies are widely used,they have the following disadvantages: the inability to remove host cell-derived contaminants,the rupture of particles that affect immunogenicity,the expensive equipment required for continuous flow ultracentrifugation,and the potentially hazardous materials generated by viral ultracentrifugation[137].Chromatography is still used in industry to purify VLP vaccines,but this method is time-consuming and costly.There is still a need to develop more efficient and cost-effective purification methods.The purification method based on sulfated cellulose membrane adsorbers is significantly better than the traditional method [138].As it is easy to popularize and simplifies the purification steps,it can be used as a general purification platform for VLP vaccines,and this technology is expected to be used to purify VLPs on a large scale.
The clinical transformation of vaccines depends on the mature vaccine production process to ensure a stable and reliable vaccine supply and to expand vaccine production in a safe,effective,and cost-effective manner.As a noninfectious universal vaccine platform,VLPs are easy to standardize in the production process of vaccines and to minimize the unstable factors in the production process to achieve the rapid production of safe and cheap vaccines.Regulatory agencies manage each stage of vaccine production to ensure high-quality and consistent products.In the future,mechanistic studies should be conducted to examine the biological mechanism and the in vivo pharmacokinetic profiles of biomimetic nanoparticles.There is still a need to explore better-optimized expression systems and purification methods to actualize high-throughput production.Standardized work processes also need to be consistent with good manufacturing practices to meet the quality requirements approved by regulatory authorities.Simultaneously,VLP characterization is a key step to ensure the stability and effectiveness of VLP vaccines.The development of rapid analysis methods that can support the analysis of a large number of VLPs will help more effective vaccine development.
Conflicts of interest
The authors have declared that no competing interest exists.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No.2042020kf1015 ),and National Natural Science Foundation of China (No.81672114,81702627 ).This work was also funded by the Medical talented youth development project in the Health Commission of Hubei Province (No.WJ2019Q049 ).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- A cleavable self-delivery nanoparticle for tumor photo-immunotherapy
- Prospective of extracellular matrix and drug correlations in disease management
- Ocular prodrugs: Attributes and challenges
- Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor
- Gas-blasting nanocapsules to accelerate carboplatin lysosome release and nucleus delivery for prostate cancer treatment
- A computer-aided chem-photodynamic drugs self-delivery system for synergistically enhanced cancer therapy
