Alginate and alginate composites for biomedical applications
2021-07-21RhAhmdRusWnMohdFzliWnNwwiRiccRhmnNsruddin
Rh Ahmd Rus ,Wn Mohd Fzli Wn Nwwi ,b,∗,Ricc Rhmn Nsruddin ,b
a Department of Biotechnology Engineering,International Islamic University Malaysia,Kuala Lumpur 50728,Malaysia
b Nanoscience and Nanotechnology Research Group (NanoRG),International Islamic University Malaysia,Kuala Lumpur 50728,Malaysia
Keywords:Alginate Alginate composite Tissue engineering Wound dressing Drug delivery
ABSTRACT Alginate is an edible heteropolysaccharide that abundantly available in the brown seaweed and the capsule of bacteria such as Azotobacter sp.and Pseudomonas sp.Owing to alginate gel forming capability,it is widely used in food,textile and paper industries;and to a lesser extent in biomedical applications as biomaterial to promote wound healing and tissue regeneration.This is evident from the rising use of alginate-based dressing for heavily exuding wound and their mass availability in the market nowadays.However,alginate also has limitation.When in contact with physiological environment,alginate could gelate into softer structure,consequently limits its potential in the soft tissue regeneration and becomes inappropriate for the usage related to load bearing body parts.To cater this problem,wide range of materials have been added to alginate structure,producing sturdy composite materials.For instance,the incorporation of adhesive peptide and natural polymer or synthetic polymer to alginate moieties creates an improved composite material,which not only possesses better mechanical properties compared to native alginate,but also grants additional healing capability and promote better tissue regeneration.In addition,drug release kinetic and cell viability can be further improved when alginate composite is used as encapsulating agent.In this review,preparation of alginate and alginate composite in various forms (fibre,bead,hydrogel,and 3D-printed matrices) used for biomedical application is described first,followed by the discussion of latest trend related to alginate composite utilization in wound dressing,drug delivery,and tissue engineering applications.
1.Introduction
Biomaterials either natural or synthetic are widely used in biomedical applications to support,enhance,or replace damaged tissues or biological functions.In clinical setting,biomaterials are used as medical implants and devices to promote healing and serve as a carrier for cells during human tissues regeneration process.It can also be utilised as a part of biosensors architecture,drug delivery carrier,and medical imaging material [1] .
Utilisation of biomaterials dates far back into ancient civilisations in which Egyptians used animal sinew as suture while Chinese and Indians used waxes,glues,and tissues to reconstruct affected and injured body parts [2] .Over the centuries,as surgical techniques and production of synthetic material advances,detailed knowledge on biomaterial was steadily gained.Although natural material such as glue,rubber,wood and tissues are preferred choice during early days,continuous effort by biomaterial researchers around the globe have made synthetic materials like metals,ceramics,plastics and glass to be equally accepted for biomaterial construction.Nowadays,knowledge on which biomaterial will be tolerated or rejected by the body are largely known.In addition,synergistic performance while having compatible interaction between composite biomaterial and bodily tissues compared to non-composite biomaterial are also almost well understood [3] .With regards to healing and regenerating human tissues,natural materials still often utilised as they are biocompatible and most importantly biodegradable within the body.Although all biomaterials are biocompatible to prevent post-acute or -chronic adverse effects,biomaterials that able to degrade will avoid a second surgical event for removal of the material after the tissues are healed and regenerated [4] .Ability to control the rate of degradation of the biomaterials is another important criterion to ensure the tissues heal and regenerate properly [5] .Additionally,the degraded biomaterials should not be toxic and can be easily removed from the body [6] .
Alginate is one of the biological substances that is continually used for healing and regenerating human tissue particularly for wound dressing [7] .As alginate is used in food preparation for human consumption,it is considered as safe for biomedical applications.Alginate is biodegradable as well since it dissolved slowly in the body when crosslinking agents in the alginate release and exchange reaction with the monovalent cations found in the body fluids.The rate of dissolution of alginate can be controlled by oxidation[8] and reduction of molecular weight [9] of the alginate.Ultimately,alginate capacity to form gel is the major reason for its uses mostly in soft tissue engineering and wound healing.Nowadays,alginate is mixed with other distinct materials to form alginate composites that suit the need of other types of biomedical applications [10-13] and also to enhance its capacity in soft tissue engineering and healing[11,14,15] .In this review paper,we describe the preparation of alginate and alginate composites and discuss its biomedical applications,specifically wound dressing,drug delivery,and tissue engineering.Future outlooks on the preparation and biomedical applications of alginate and alginate composite are also included.
2.Alginate and alginate composites
Alginate is a polysaccharide that occurs naturally in the cell wall of algae and bacterial capsule of Azotobacter sp.and Pseudomonas sp.In brown algae (also called brown seaweeds),the presence of alginate in their cell walls provide flexibility and strong structure to the algae and buffer them from possible injury when the algae are exposed to strong sea water waves [16] .In bacteria,it forms protective capsule,aids in biofilm formation [17] and assists bacterial adherence and colonisation [18] .Because of alginate capability of forming hydrogel,alginate is widely known as stabiliser,thickener,gelling agent and emulsifier [19] since its discovery from kelp in 1881 by Stanford.After the patent of alginate extraction from algae was granted in 1930s,alginate had been extracted in large scale from brown algae (Phaeophyceae sp.) such as Laminaria hyperborea,Laminaria digitata,Laminaria japonica,Ascophyllum nodosum,and Macrocystis pyrifera [19] .Chemical companies around the world such as China Seaweed Industrial Association,Danisno Cultor (Denmark),Degussa Texturant Systems (Germany),FMC BioPolymer (USA),ISP Alginates Ltd (UK),and Kimitsu Chemical Industries Co.Ltd(Japan) have commercially extracted alginate [20] with total production is estimated 30 000 tons,primarily from the genera Laminaria and Macrocystis [17] .
Extraction of alginate from brown algae involves multiple processes [21],including initial treatment with mineral acid to change salts of alginic acid (alginates) in the algae into free alginic acid.This is followed by neutralisation with either sodium carbonate or sodium hydroxide to form water soluble sodium alginate.To recover the soluble alginate,precipitation with either calcium chloride or mineral acid can be applied and insoluble calcium alginate fibre or alginic acid gel is obtained,respectively.Both calcium alginate and alginic acid are later mixed with sodium carbonate to produce sodium alginate.Sodium alginate is the common and largest alginate-base salt produced in the industry [22] .Other types of alginate salt produced are calcium alginate,potassium alginate and ammonium alginate [23] .Calcium alginate is produced in the process of alginate extraction as described earlier while potassium alginate and ammonium alginate are produced by adding appropriate alkali,usually potassium carbonate or ammonium hydroxide,respectively to the alginic acid gel.
Alginates composed of 1,4-linked β-D -mannuronic acid (M)and 1,4 α-L -guluronic acid (G) residues (Fig.1) that form blocks of repeated G residues,repeated M residues and alternating G and M residues [24] .The composition and the sequence of G and M residues depends on type of natural resources used to extract alginate.Alginate extracted from algae,L.hyperborean has 60% of G content while alginates from other commercial algae species have 14% −31% G content.It was suggested that only carboxylate groups of the G residues crosslink with divalent cations such as Ca,Mgto form hydrogels.Thus,alginates with high G content form stiffer hydrogel while alginates with high M content generate softer elastic hydrogel[25] .The molecular weight of alginate ranges between 32 000 to 400 000 g/mol.The higher the molecular weight of the alginate,the more viscous it gets in the gel preparation.Although alginate is considered as biocompatible,non-toxic materials and non-immunogenic [26],there are claims that alginate with high M content tends be more immunogenic than alginate with high G content [27] .
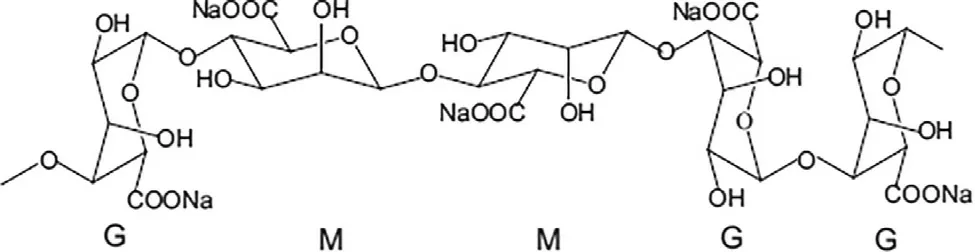
Fig.1–Chemical structure of alginate composed of 1,4-linked β-D -mannuronic acid M and 1,4 α-L -guluronic acid G residues.Adapted with permission from [24] .Copyright (2011),Elsevier.
To enhance alginate physical characteristics,other substances had been mixed with alginate to form alginate composite.Alginate composites are formed by adding natural polymer such as collagen,chitosan and gelatin,synthetic polymer such as polylactide and polypyrrole,and inorganic compounds such as tetraethylorthosilicate(TEOS) and hydroxyapatite (HA) [28,29] .Blending other types of material,for example ceramic,bioglass,inorganic nanoparticle,and inorganic carbon-based material had been explored as well [29-31] .Several alginate composites had shown successful results and available commercially particularly for wound dressing application [19] while others are still under investigation.
For both alginate and alginate composite to be used for various biomedical applications,they had to be made into different forms such as fibre,bead,hydrogel,or 3D printed material depending on the specific demand of each biomedical applications.In the following subsections,preparation of each form of both alginate and alginate composite are discussed.
2.1.Fibre form
The use of alginate in fibre form as biomedical materials has attracted extensive interest because of their high surface area,ease of handling,and its ability to retain mechanical integrity while in wet state.The later properties being crucial given the absorbent nature of alginate,especially when used as dressing for heavily exuding wound [32] .For example,3MTegadermAlginate High Integrity and High Gelling Alginate Dressing,made from hydro-entangled nonwoven web of calcium alginate fibres [33],readily form a gelatinous mass which maintain a moist healing environment while maintaining its form factor integrity upon contact with exudates.Nevertheless,at low weight basis,alginate fibrous webs can collapse under its own weight when swollen due to insufficient entanglement,leaving fibrous residue at the wound site when the dressing is removed.
Blending the alginate fibres with fibres that do not swell in saline — a common solution used when cleaning wound site — not only increase the strength of overall fabric,but also reduce the cost of overall dressing.Improvement can be sought by incorporating reinforcing or bioactive material within the alginate fibre itself,creating a composite fibre which are not only stronger,but able to confer additional healing properties.Below are preparations of alginate fibre and alginate composite fibre using traditional wet-spinning method,electrospinning and microfluidic system.
2.1.1. Fibre formation via wet spinning
During traditional wet spinning process of pure alginate fibre,extruded spinning dope (i.e. water soluble sodium alginate)precipitate upon contact with calcium chloride solution in coagulation bath to produce water insoluble calcium alginate fibre [34] .The as-made calcium alginate fibre can then be stretched,washed and dried.Because physical stretching or drawing increases the degree of molecular alignment within the fibre which in turn improve overall fibre strength [35],care must be taken so that breakage does not occur during drawing.This care usually accomplished by adjusting the drawing ratio between output and input filament velocity in the drawing zone or ratio between length of drawn and undrawn fibre [36] .
To prepare composite wet spun fibre,sodium alginate usually blended with substance of interest at certain ratio and extruded via spinneret with multiple holes or syringe.The extruded fibre then passed into coagulation bath that typically comprise of CaCl 2 solution to crosslink the alginate.These fibres often stretched further to improve its final mechanical properties.To enhance wet-spun alginate fibre mechanical and liquid absorption capability,wet spun alginate fibre has been incorporated with reinforcing materials like (1)natural-based polymer such as chitin [37,38],chitosan[39-41],cellulose [42] carrageenan [43],gelatin [44],starch[45],(2) synthetic-based polymer such as polypyrrole [46],(3)carbon based material such as graphene oxide [47],carbon nanotube [30],or (4) inorganic nanoparticle such as silver[31] to produce composite fibre.Table 1 shows few additive materials that have been used lately to prepare alginate fibre composite using wet spinning method.
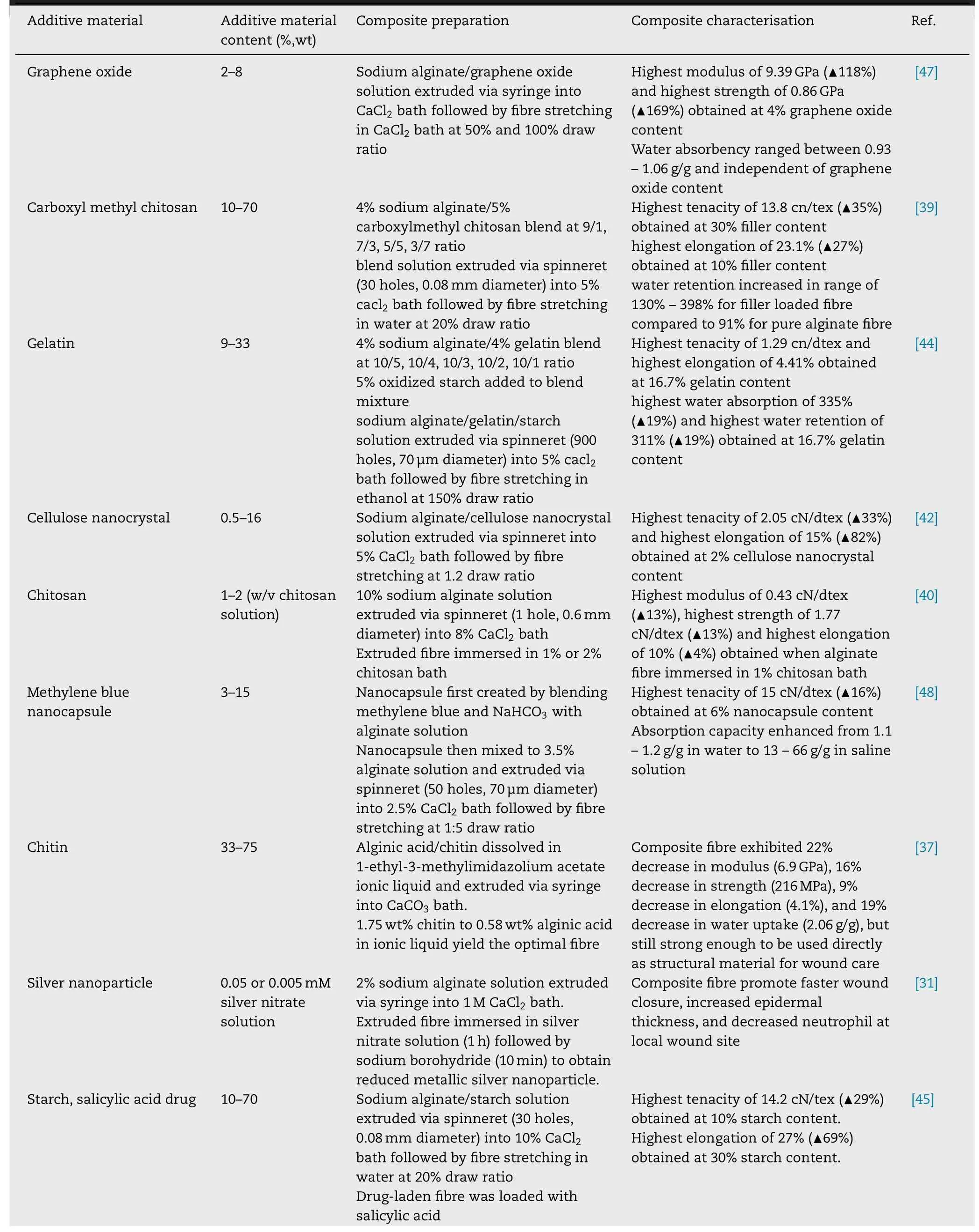
Table 1–Alginate composite fibre prepared via wet spinning technique.
(continued on next page)
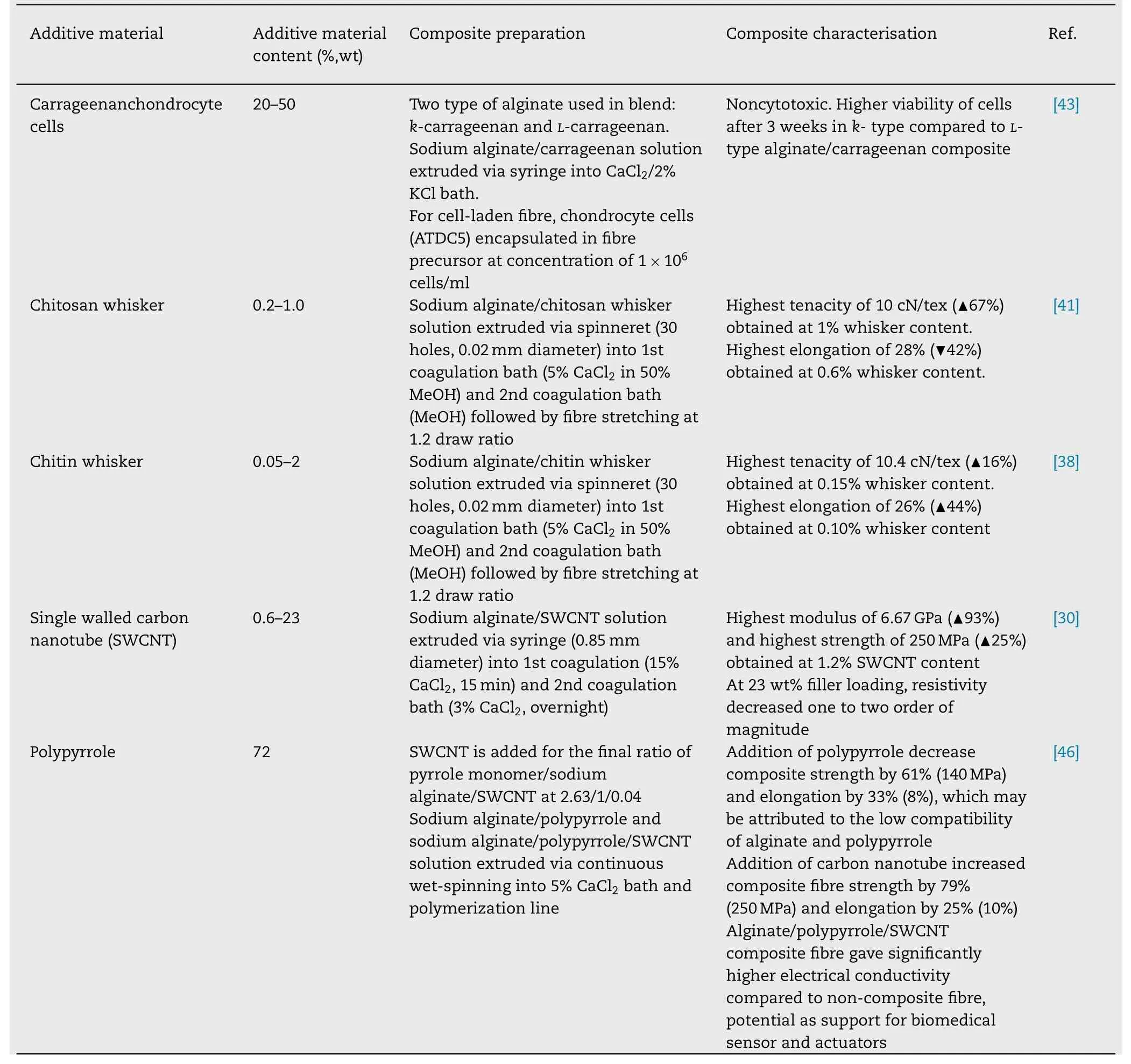
Table 1 (continued)
2.1.2.Fibre formation via electrospinning
Compared to wet spinning,electrospinning allows the fabrication of ultrathin fibre by placing polymer melt inbetween charged electrode.When strong electric field is introduced,typically in the region of kilovolt (kV),the polymer melt will be ejected towards collector electrode,forming a fibrous mat with individual fibre diameter ranging from 10 to hundreds of micrometres [49] .Electrospinning of alginate have found wide application in biomedical field because it can provide higher surface area compared to traditional wet-spun technique [50] .However,it is difficult to obtain continuous and uniform fibrous structure from pure alginate solutions via electrospinning due to lack of molecular entanglement and the rigid structure of alginate.Polymer such as polyvinyl alcohol (PVA) and polyethylene oxide (PEO) often used as spinning aid as they can provide sufficient entanglement with alginate at molecular level.Furthermore,addition of surfactant might reduce the high surface tension of alginate,leading to bead-free and uniform electrospun fibre [51] .Because of the addition of spinning aid,it is safe to say that almost all electrospun alginate fibres are composite fibres.
The morphology of electrospun fibre governed by parameters such as solution feeding flowrate,applied voltage,and distance between electrospinning needle to the target.Table 2 depicts different additive material and processing parameter used during electrospinning of alginate fibre composites.
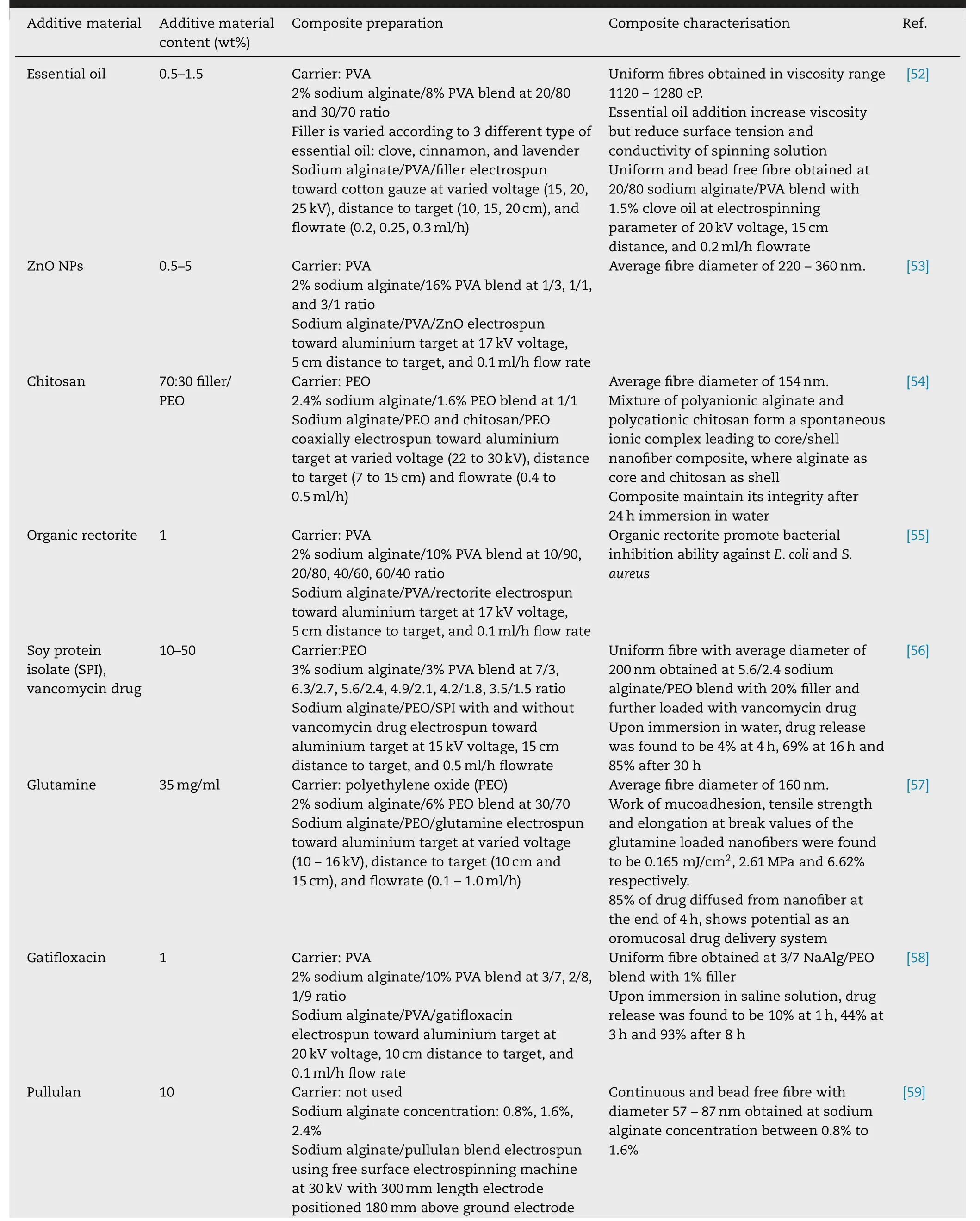
Table 2–Alginate fibre composite prepared via electrospinning technique.
(continued on next page)
Table 2 (continued)
2.1.3. Fibre formation via microfluidic system
Microfluidic system allows precise control of dimensional and morphological characteristic of resulting microfibre due to the formation of stable laminar flow upon injection into microchannel [66] .Pioneering work that produced continuous alginate microfibres using microfluidic system based theirwork on coaxial flow,whereby the sample flow (sodium alginate) and sheath flow (CaCl) met at intersection and leaving the outlet as microfibre [67] .The idea has been further refined to create a hollow fibre by introducing core fluid in the innermost layer which result in the formation of a three-layered coaxial flow that consists of core,sample,and sheath fluid [68] .Fig.2 depicts the concept of coaxial flow in generating alginate microfibre.
Most of alginate composite microfibre fabricated using microfluidic system often carries living cells within it.Compared to wet spinning method where thick fibre can impair cells nutrient and metabolic product exchange,or to electrospinning where high electrical field render cell encapsulation unfeasible,microfluidic system offer gentler and controllable microfibre fabrication,making cell encapsulation within fibre possible.To enhance composite microfibre fabricated using microfluidic system with incorporated cells within it,incorporation of extracellular matrix (ECM)-like constituent is crucial so that suitable cell microenvironment can be provided.Therefore,production of composite alginate microfibers enriched with different ECM constituents such as gelatine [69,70],collagen [71],hyaluronic acid [72],urinary bladder material [69],and chondroitin sulphate [72] has been investigated and successful results had been obtained.Table 3 depicts few microfluidic systems that have been employed to generate cell-laden alginate composite microfibre.
Table 3–Alginate composite fibre prepared via microfluidic technique.
(continued on next page)
Table 3 (continued)
2.2.Bead form
Alginate bead can be produced through extrusion of alginate droplet into a crosslinking bath.This extrusion can beperformed either using syringe or microfluidic system.In biomedical setting,the alginate bead mostly used as immobilisation medium for cells and drug.The bead can also be used as scaffold base for tissue repair.However,its structural integrity can be compromised if sufficient amount of sodium ion presents in bodily fluid diffuse into the bead construct,exchanging itself with Ca— a common ionotropic crosslinking agent used during bead formation.To alleviate this problem,Sarker et al.[76] used partially oxidized alginate which can react covalently with gelatinthrough Schiff’s base formation due to the reaction of free amino group of lysine or hydroxylysine amino acid residues of gelatin and available aldehyde group of oxidized alginate.Mechanical properties of oxidised alginate/gelatin bead can be then tailored by changing the composition,by which higher amount of oxidized moieties lead to stiffer construct [77] .This composite bead also showed higher degradation rate compared to pure alginate bead because of lower molecular weight of oxidized alginate,which is desirable for tissue engineering.The rate of degradation of composite bead can be further regulated by tuning their composition,degree of oxidation,and degree of crosslinking.
Incorporation of inorganic material like nanohydroxylapatite (nHAP) and bioglass to alginate for production of alginate composite bead can stimulate osteoblast cells which in turn create the nanocomposite structure of bone by secreting collagen fibril on which new apatite crystals subsequently form [78] .Apart from cells and inorganic material,incorporation with drug and other materials such as chitosan [79],strontium-substituted HA [80],and PVA[81] have been realized for production alginate composite bead.
2.3.Hydrogel form
Alginate-based hydrogel for biomedical purpose can be either non-injectable or injectable.Non-injectable alginate hydrogel is pre-formed prior its in vivo implantation.On the other hand,injectable hydrogel has wider gelation working windows before its final shape is assumed.This enables defect with irregular shape and size to be filled with minimally invasive procedure [82] .Both kind of hydrogel often incorporated with foreign material whether in particle or fibrous form to produce alginate composite hydrogel with improved overall structural integrity and desirable properties.
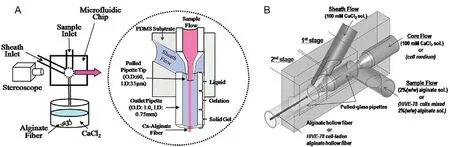
Fig.2–Schematic of microfluidic concept for (A) continuous alginate microfiber generation.Adapted with permission from[67],Copyright (2007) American Chemical Society,and (B) continuous alginate hollow microfiber generation.Adapted with permission from [68] .Copyright (2009),John Wiley and Sons.
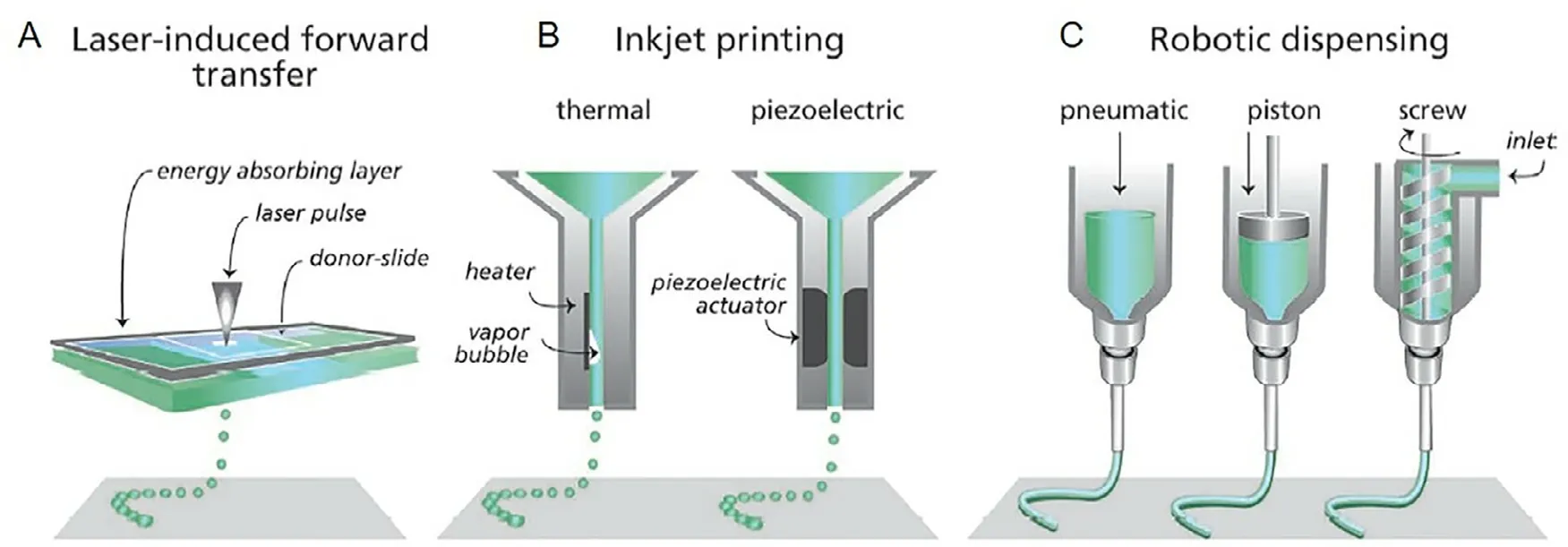
Fig.3–Different type of bioprinting technique.(A) laser-based bioprinter use laser focused on an absorbing substrate to generate pressures that forward transfer cell-containing materials onto a collector substrate.(B) droplet-based bioprinting use either thermal or acoustic mechanism to dispense bioink droplet akin to typical inkjet printer.(C) extrusion-based bioprinting utilize pneumatic of mechanical (piston or screw) to dispense continuous bioink filament.Adapted with permission from [88] .Copyright (2013),John Wiley and Sons.
Cell clustering commonly observed when pure alginate is used as scaffold material.This is to be expected since alginate by itself does not support cell adhesion due to the lack of cell adhesion moieties.Therefore,alginate composite hydrogel incorporated with ECM such as gelatin or bioactive agent such as bioglass and cell adhesive peptides with the RGD sequence (Arg-Gly-Asp) is produced to stimulate cell adhesion and mimic native cell microenvironment to enhance cell growth and proliferation [83,84] .Natural ECM is bestowed with fibre-reinforced composite design,consisting of compliant aqueous matrix reinforced with fibrous collagenous protein.Using this biomimetic concept,the use of fibrous collagen with different arrangement within alginate hydrogel can lead to mechanically robust composite that can be tailored for specific tissue engineering applications [85] .When aligned electrospun gelatin nanofiber used to reinforce alginate hydrogel,up to 541% increase in tensile modulus and 1690%increase in tensile strength over non-composite hydrogel observed,while retaining good transparency at the thickness similar to human cornea [86] .
2.4.3-D printed material
Bottom-up fabrication with bioprinting allows precise deposition of cells and biomaterial components from predefined computer generated design.Alginate found its use as bioink formulation as a result of its biocompatibility.Rastogi et al.[87] recently reviewed the use of alginate-based bioink for tissue engineering.Current bioprinting technologies are based on either extrusion,droplet bioprinting (also known as inkjet bioprinting),or laser mechanism (Fig.3) [88] .For extrusion bioprinting,continuous bioink filament is extruded from printer nozzle using pneumatic or mechanical driven dispensing system.Although the use of nozzle inadvertently generates shear stress to the cell in cell-laden bioink which can lead to low cell viability,it remains the most common bioprinting method as it produces mechanically robust structure compared to inkjet and laser technology.In inkjet bioprinting,picoliter-sized droplets (~50 μm droplet size) are generated using typical printhead from desktop inkjet printer allowing higher resolution construct to be printed.However,the viscosity of bioink must be very low to avoid nozzle clogging.In contrast to nozzle dependant technology,laser bioprinting typically operate based on laser induced forward transfer principle,whereby the laser beam evaporates the focal area of the absorbing layer,generating vapour bubble that pushes the bioink forward [89] .Although highest cell viability is possible for laser-based technology due to shear stress elimination,the associated cost and lack of commercial 3D laser bioprinter slowed down its progress forward.
For extrusion-based bioprinting,the bioink should be formulated at optimal viscosity,which is not too low where printed construct can collapse and not too high to the point the nozzle become clogged.Although cells thrive in soft hydrogel (lower alginate molecular weight),it can be too watery for it to maintain shape for biofabrication purpose.Therefore,other materials such as nanocellulose [90],carrageenan [91] and gelatin [92,93] are mixed with alginate to form sturdy printed alginate composite structure.For inkjetbased bioprinting,collagen [94] and fibrinogen [95] had been added to fabricate printed composite structure and nHAp[96] and with chitosan [97] had been mixed with alginate to be used as bioink for laser bioprinting technology.
3.Biomedical applications of alginate and alginate composites
The good biocompatibility and promising physicochemical properties especially on the capability to form gel and retain the moisture content of alginate have promoted various biomedical applications such as wound dressing,drug delivery and tissue engineering of alginates and alginate composites [98] .The following sections describe the trend and summary of biomedical applications of alginate and alginate composites.More emphasis are given to the alginate composites because of their enhanced properties for biomedical applications.
3.1.Wound dressing application
Dermal wounds,both acute and chronic,represent significant clinical challenges such as poor vascularisation,protease susceptibility and microbial invasion at wound site which affect the early wound closure [99] .Conventionally,cloth dressings comprising of a sterile pad and cloth gauze are used as wound dressing due to their low cost.However,they are prone to bacterial infection and easily stick to the wound,causing suboptimal wound healing process.Thus,the ability of materials,especially natural polymers such as polysaccharides (e.g.,chitosan,chitin,dextran,alginates,chondroitin and heparin),proteoglycans and proteins (e.g.,collagen,gelatin,fibrin,silk fibroin and keratin) have been proposed and studied to accelerate wound healing and to control the infection [32,99-101 ].
Alginate as one of those materials has been attractive due to its good biocompatibility and water content [102] .The water content keeps the moist environment which maintains the optimal pH and accelerates the healing process[103] .For instance,KaltostatAlginate dressing has been a well-known commercial wound dressing based on calcium alginate or sodium alginate in a form of absorbent gelfibre matrices with fluid contact.It can maintain a moist wound environment which facilitates hemostatic effect,atraumatic removal and aids in the control of minor bleeding[99,104] .However,having alginate hydrogel alone as wound dressing is insufficient for preventing bacterial infection and promoting bioactivities,especially in chronic wound healing.Therefore,recently alginate composite dressings have been increasingly developed.The additional components may be functioning for high cell viability,cell adhesion,antibacterial activity,enhanced blood clotting and hemostasis (ability to prevent and stop bleeding).Table 4 summarized some recent studies on alginate composite for wound dressing application.
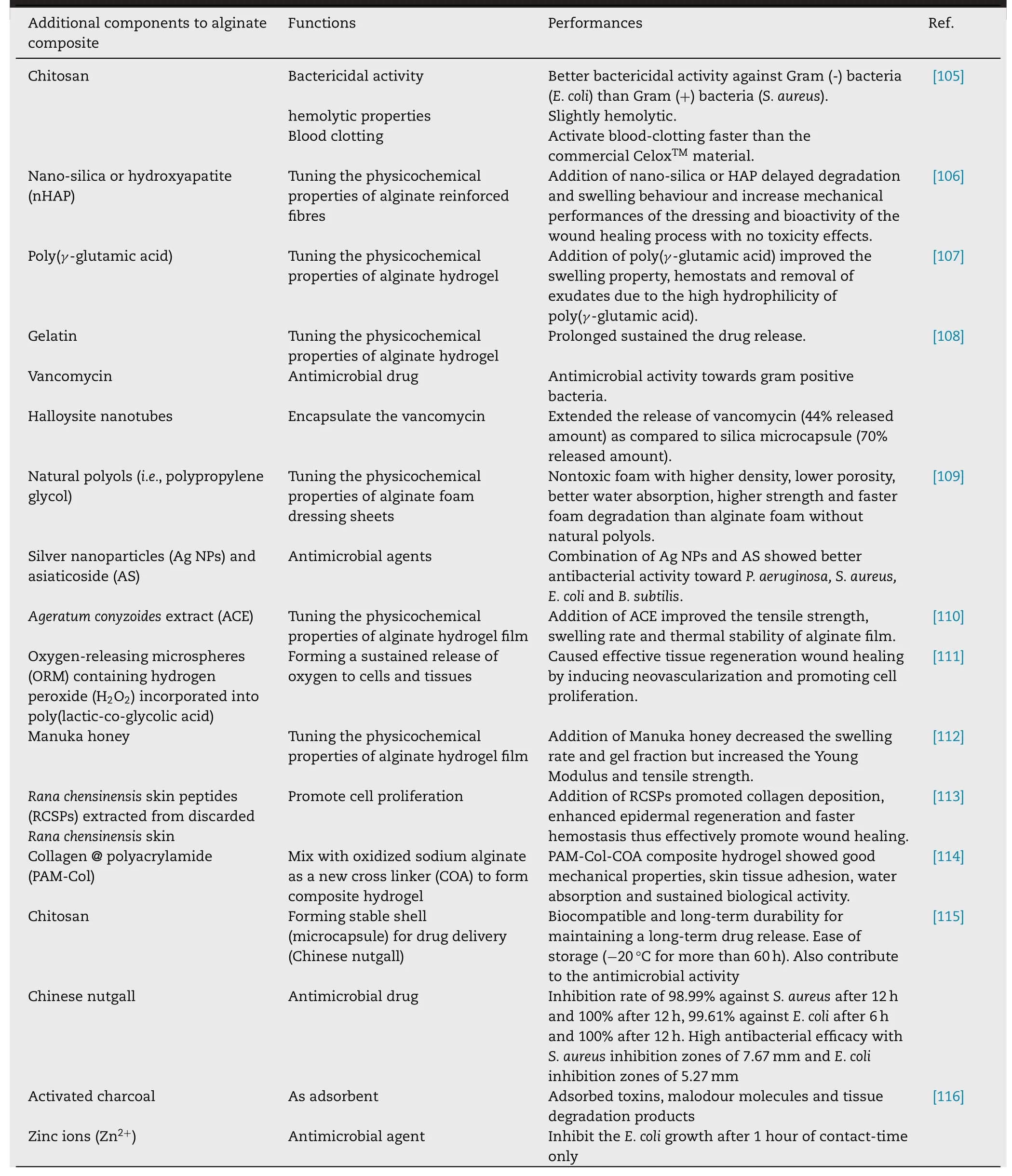
Table 4–Recent studies on the alginate composites for wound dressing application.
In the composites,the alginate is mainly made into hydrogels by cross-linking mechanism using counter ions such as calcium or other multivalent ions (e.g.,calcium and sodium ions) [117] .Although there are several reported making the alginate into fibre mat by electrospinning process [118],sponge [119],and foam sheet [109],the alginate dressings subsequently became hydrogel form when in-contact with water and the moist exudate.In an alginate composite,other materials such as poly(vinyl alcohol)[120,121],cellulose [122],chitosan [105,115,119,123-127 ],and gelatin [108] are also added during the formation of the hydrogel.The presence of poly(vinyl alcohol) affected the physicochemical properties of the hydrogel dressing such as its swelling ratio,tensile strength and elongation [121] .In many cases,the higher content of sodium alginate decreases the gel fraction,maximum strength and break elongation,but increases swelling ability,protein adsorption,hydrolytic degradation and thermal stability [120,121] .On the other hand,the presence of 10% alginate had effectively formed hydrogels with 90% cellulose nanofibrils [122] .The alginate had further increased the swelling of the composite by increasing the amount of charged groups inside the hydrogel and made the internal structure resembled a double network hydrogel with high toughness [122] .
On the other hand,an inhibition of bacterial growth is another important criterion for the development of efficient wound dressing.In some studies,sodium alginate alone has been reported to have antimicrobial activity [120,124],but it is insufficient and less effective during wound healing process.Meanwhile,chitosan is widely used to promote antibacterial properties in biomedical field [128-130] .Usually sodium alginate is mixed with chitosan which has been reported to have antimicrobial activity [124,126,127] .Interestingly,research on this topic keeps increasing and more new materials have been investigated and added to the alginate composite dressing.For instance,a sodium alginate composite hydrogel had been integrated with bioactive hardystonite (HS) bioceramic and was found to inhibit bacterial growth and stimulate angiogenesis for enhanced wound healing as shown in Fig.4 [102] .This alginate composite dressing also stimulated the proliferation and migration of both human dermal fibroblasts and human umbilical vein endothelial cells.
In some reported studies,metallic-based antimicrobial agent (e.g.,silver [109,127,131] and zinc [116] nanoparticles),antibiotic drug (e.g.,clindamycin [121],vancomycin [108],asiaticoside [109],aminoglycoside [132],etc.) or natural products with antimicrobial property (e.g.,curcumin [119],Chinese nutgall [115],lentil seed extract [133],Aloe vera[127],essential oils such as melaleuca,copaiba and lemon oil [134],etc.) are also added into the alginate composite dressings in order to kill and inhibit the bacterial growth.A sustained drugs release has been one of the main targets for such alginate dressings.The high durability of the hydrogel could facilitate a sustained drug release.Recently,Xue et al.had reported that a long-term durable alginate-chitosan composite dressing created a prolonged antimicrobial system(i.e.,a long-term release of Chinese nutgall antimicrobial drugs) for the wound dressing application.The sustained drug release system successfully inhibited the growth of both S.aureus and E. coli with a rate of 100% after 12 h) [115] .In another study,gelatin was added with alginate to form a biocompatible nontoxic hydrogel composite dressing which also prolonged and sustained release of antimicrobial drug(i.e.,vancomycin encapsulated by halloysite nanotubes) [108] .However,the reduction of the drug release rate for alginategelatin hydrogel composite has worsen the antimicrobial effects of the dressing,thus a systematic study on the composition of alginate and gelatin could be performed to get an optimum performance (a sustained drug release with efficient antimicrobial activity).
The moist environment for wound healing does not only come from the water content of the alginate dressing but also from the exudate which contain several growth factors (e.g.,platelet and vascular endothelial growth factors,transforming growth factors and a number of cytokines including interleukins) which regulate the granulation,epithelialization and angiogenesis in the healing process[103] .Recent study reported that sodium alginate composite hydrogel combined with bioglass (BG) and desferrioxamine(DFO) worked synergistically in promoting hypoxia inducible factor-1 (HIF-1 α) and vascular endothelial growth factor(VEGF) expression and subsequently vascularization in the wound site,further facilitated diabetic skin woundhealing [135] .In another reported study,the alginates were combined with human umbilical cord mesenchymal stem cells (hUCMSCs) where the alginates functioned as culture media.The wound dressing model of hUCMSCs-alginate mix had promoted faster wound healing because the hUCMSCs can proliferate well and express massive VEGF [136] .In addition,tissue regeneration for a fast wound healing requires optimum supply of oxygen.Therefore,recently,alginate
composite dressing was also added with oxygen release microspheres (ORMs) which facilitate neovascularization and cell proliferation [111] .The ORMs contain hydrogen peroxide incorporated with poly(lactic-co-glycolic acid),prepared by double emulsion method [111] .
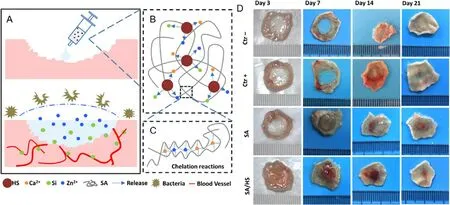
Fig.4–Design and principle of SA/HS composite hydrogel for wound healing.(A) Diagrammatic sketch of wound healing with hydrogel;(B) HS particles release ions into solution;(C) structure of SA/HS composite hydrogel.(D) Wound closure at Days 3,7,14,and 21 after treatment with the composite hydrogel dressing.Adapted with permission from [102] .Copyright(2017),American Chemical Society.
On the other hand,a self-healing ability of a alginate composite hydrogel wound dressing containing dopaminegrafted oxidize sodium alginate and polyacrylamide chains was also reported [137] .Activated charcoal (AC) was also added in another alginate hydrogel as the AC could efficiently adsorb the malodor from the wound and the tissue degeneration products which are commonly associated with chronic wound with heavy exudate [116] .
The presence of alginate-and alginate compositebased wound dressings in the market shows that some research have passed the clinical studies.Clinical studies are necessary and important before the wound dressings can be commercialized and used by patients.In the clinical studies,several aspects related to the performances and adverse effects of the wound dressings on the patients will be evaluated.Many clinical studies were reported on the alginate composite-based dressings with the integration of antimicrobial agent such as silver ions or silver nanoparticles[138-141] .For example,the performance and efficacy of an ionic silver alginate/carboxymethylcellulose (SACMC)dressing had been clinically tested to thirty-six patients with venous or pressure ulcers for over a four-week period[139] .The study found that the presence of silver showed better ability to prevent colonialization of microorganism(biofilm infected) to the wound,thus improved the healing process when compared to non-silver calcium alginate fibre(AF) dressing.The clinical study also reported one adverse effect (AE) of SACMC dressing which was wound maceration,lower than the five AEs from AF dressing (which were wound infection,serious sticking of the wound dressing,over-granulation and rehospitalisation for additional wound care).In another study,an alginate silver wound dressing,commercially named as Askina Calgitrol Agwas clinically tested to 65 patients to compare its performance with the 1%silversulfadiazine (AgSD) in the outpatient management of partial-thickness burn wounds [140] .The results showed that the Askina Calgitrol Agdressing had a lower average pain score,number of wound dressing change and nursing time and faster healing when compared to the AgSD.
3.2.Drug delivery
Ensuring the therapeutic molecules reaching the intended target organ or tissue for maximum effectiveness of the drug has been the main goal of drug delivery [142] .Various materials have been studied and tested for drug delivery.among all,alginate hydrogel has been attractive because the gentle gelation process of making them can be used for cell encapsulation and sustained drug release [98,143] .The drug delivery system of alginate is commonly associated with wound dressing applications.The alginate microcapsules were used for a sustained release of antimicrobial agents or drugs of the wound dressing [108,115] .Recently,a double membrane hydrogel composed of alginate and cellulose nanocrystals had shown a promising potential for a targeted released of antibiotic drugs via controlled swelling mechanisms [144] .As shown in Fig.5,the outer hydrogel(composed of a neat alginate) was responsible for a rapid drug release,while the inner hydrogel (composed of cationic cellulose nanocrystals and anionic alginate,connected via electrostatic interactions) was responsible for a prolong drug release [144] .In addition,a dual-drug delivery system using alginate composite was also reported [145] .For examples,poly(d,l-lactide) (PDLLA) microspheres were embedded in alginate hydrogel beads [145] whereby the microspheres encapsulated glycyrrhetinic acid (GA,a hydrophobic drug)and the alginate hydrogel was loaded with bovine serum albumin (BSA) as a hydrophilic model protein drug [145,146] .
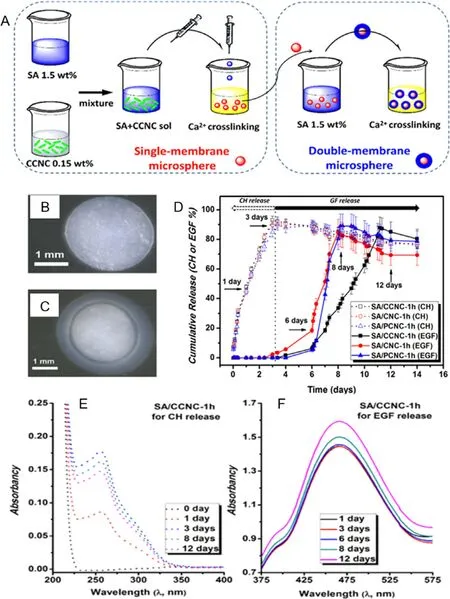
Fig.5–(A) Preparation routine of single-membrane and double-membrane microsphere hydrogels;optical microscope images of (B) the SA/CCNC single-membrane microsphere hydrogel,(C) the SA/CCNC-1 h double-membrane microsphere hydrogel.Complexing drug release study involving ceftazidime hydrate (CH,the open symbols) and epidermal growth factor human (EGF,the solid symbols) of the double-membrane hydrogels in the pH 7.4 buffer solution (D) drug release profiles,(E)UV spectra of CH release for the SA/CCNC-1 h double-membrane hydrogel at the critical times,and (F) UV spectra of EGF release for the SA/CCNC-1 h double membrane hydrogel with the Bradford method at the critical times.Adapted with permission from [144] .Copyright (2016),American Chemical Society.
In another reported study,uniform alginate/CaCOcomposite microparticles (~430 μm) synthesized using microfluidic technology had shown tunable compositions and pH-sensitive for sustainable release of drug (using a model drug of Trypan Blue) in wound healing application[147] .The alginate composite hydrogel can also be embedded with biologically active agents in their water-swollen network.For instance,an alginate sponge dressing which was further formed into hydrogel,incorporated with insulin-loaded poly(D,L-lactide-coglycolide) (PLGA) microparticles [148] has been reported to successfully stabilize and release insulin for up to 21 d,thus effective for a long-term delivery platform of bioactive insulin [148] .According to Omtvedt et al.,βcyclodextrin can also be grafted to alginate hydrogel for a controlled-drug release system in drug delivery application[149] .The study reported that the β-cyclodextrin was grafted to alginate in three-step synthesis using peroxiodate oxidation,reductive amination and copper(I)-catalysed azide-alkyne cycloaddition and the composite hydrogel were stable and able to form inclusion complex with the model drug (methyl orange) [149] .On the other hand,Leppiniemi et al.reported that the functionalization of the alginate composite (mixed with nanocellulose) was made using avidin for the immobilization of bioactive components through biotin −avidin interaction [150] .Interestingly,this alginatenanocellulose composite is 3D-printable for the delivery of therapeutic agents.
In addition,the advancement of nanoscience and nanotechnology has enabled the fabrication and characterisation of alginate into smaller size,nanogels.As the alginate hydrogel is now in nano size,it offers several advantages owing to the high surface area of the nanogels.For example,the high surface area improved the performance of the alginate nanogel for drug delivery.For example,an alginate nanogel co-loaded with cisplatin and gold nanoparticles (AuNPs) has been reported for a combined photothermal therapy and chemotherapy [151] .The hydrodynamic diameter of alginate-cisplastin-AuNPs (ACA)nanogel complex measured by dynamic light scattering (DLS)analysis was in the range of 20—80 nm and it was reported that the combined action of chemo-photothermal therapy using the ACA nanocomplex dramatically suppressed tumour growth up to 95% as compared to control and the nanogel complex prolonged the survival rate of BALB/c mice bearing CT26 colon cancer.The ACA nanogel complex was also tested on U87-MG human glioblastoma cells [152] .In another study,a hybrid alginate nanogel with magnetic and dual responsive properties for a targeted nanotheranostic application was also developed [153] .The hybrid alginate nanogel consists of superparamagnetic iron oxide nanoparticles (SPIONs)which are covalently attached to a disulfide-modified alginate derivative and the nanogels are loaded with anticancer drug doxorubicin.Interestingly,the addition of other materials into the hybrid alginate nanogel also added more functionalities of the alginate drug delivery system such as magnetictargeted characteristics and magnetic resonance imaging(MRI) functions other than improving the drug loading content,co-triggered release behaviour and high toxicity to tumour cells.In addition,the hybrid alginate nanogel was considerably biocompatible based on the low side effects to normal cells [153] .
The clinical study of alginate-and alginate compositebased drug delivery system have been reported as well.However,as compared to wound dressing application the clinical study of the alginate-and alginate composite-based drug delivery system is still low and rare.Some existing reported studies are only at animal testing (preclinical test).For examples,alginate nanoparticles loaded with tuberculosis drugs,isoniazid (INH),rifampicin (RIF) and pyrazinamide(PZA),had been tested with Guinea pigs via inhalation administration [154] .The study was done to compare the inhalable alginate drug delivery system with the oral free drugs (the INH,RIF and PZA drugs were not encapsulated).The findings showed that the alginate-based drug nanocarrier had higher bioavailability when compared with the oral free drugs.The chemotherapeutic efficacy of three doses of the drugs using alginate-based drug nanocarrier nebulised 15 d apart was comparable with 45 daily doses of oral free drugs.Recently,in another study,alginate microsphere (about 95.7 μm in average diameter) had been tested with Chinchilla rabbits [155] .The alginate microspheres were loaded with retinoic acid (RA),a promising drug for the treatment of proliferative vitreoretinopathy.The study found that the alginate microspheres promoted a steady,long-term and effective level of RA.The alginate microspheres also had good biocompatibility as no inflammation and toxic responses were founds in the Hematoxylin and Eosin (HE) staining study [155] .
Apart from drug delivery,alginate also can be used as a mediator in non-viral drug delivery when combined with other polycationic substrates such as chitosan.The cationic nature of chitosan allows it to interact with and easily form complexes with negatively charged DNA [156,157] .Nevertheless,the resulted chitosan-DNA interaction can be too strong,thereby preventing dissociation within the cell and ultimately hinder DNA translation,resulting in low transfection [158] .In order to reduce the strength of interaction between chitosan and DNA,Douglas and co-worker incorporated alginate as secondary polymer to chitosan nanoparticles [159] .It was found that alginatechitosan composites were able to mediate transfection of 293T cells four times than that achieved by chitosan nanoparticles alone and after 48 h,the transfection efficiency yielded by the composites was as high as with positive control,Lipofectamine,but with significantly reduced cytotoxicity.
3.3.Tissue engineering
Tissue engineering is an important tool in creating viable and functional tissue to restore,maintain,or improve defective tissues or organs [160] .In general,it involves propagation of cells that were either seeded onto or encapsulated in suitable material which support the growth and differentiation of the cells.The cell-material complex was then transplanted into the body to form tissue.Alginate has been extensively studied as a material to be used in tissue engineering applications [82] .It had been tested on bone [161,162],cartilage[163],liver [164,165],ocular tissues [166],and other bodyparts [167-170] (Table 5).However,alginate is mechanically poor,possesses uncontrollable mechanical property due to variation in M and G contents and inferior material for cell attachment and proliferation for tissue engineering applications [26] .Thus,alginate is combined with other substances to produce alginate composites with improved features for tissue engineering applications.Both alginate and alginate composite applications in bone,cartilage,liver and other organ tissue engineering are discussed below.
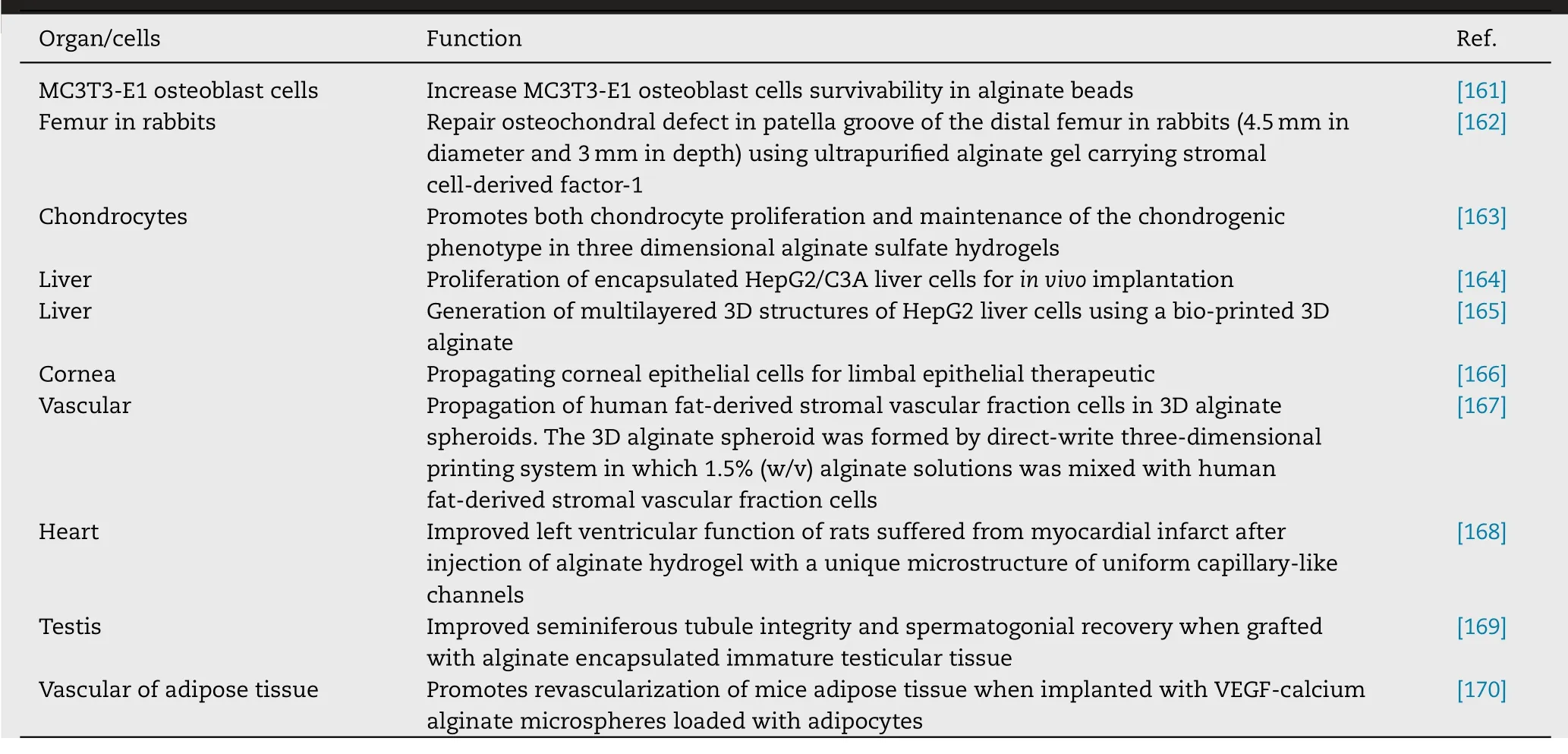
Table 5–Utilization of alginate for tissue engineering of various tissues/organs.
3.3.1.Bone
Bone is a complex and rigid organ that plays a major role in protection and movement of other organs,involves in production of blood and store minerals in human body [171] .Treatments for bone defects normally involve natural grafting from human donor (auto-and allograft) and from other species (xenograft) [16] .Synthetic grafting can also be used and numerous studies had been conducted to replace the natural grafting that has several limitations such as limited availability,risks associated with harvesting either from human or other species and requires additional surgeries that may cause infection to both the receivers and donors [29] .Alginate is one of the materials that was extensively studied for synthetic bone grafting [28] .It can act as scaffolding materials for bone construction and can be used to deliver cells for bone tissue regeneration [28,172] .
Initial studies showed non-modified alginate supported long term growth for 8 months of chick embryo calvarial cells that were encapsulated in the alginate beads [173] .The encapsulated cells were found to be capable of elaboration and mineralization of ECM such as fibronectin,type III collagen and type I collagen.Later,it was demonstrated that alginate also supported the differentiation of murine embryonic stem cells (mESCs) to form bone cells [174] .However,alginate alone is incapable for load-bearing orthopaedic applications because it does not have mechanical strength [175] .Thus,many researches develop ‘stronger’ alginate composites for bone applications.
To make stiffer alginate composites,other materials such as ‘hard’ natural polymer,synthetic polymer and bioglass (combination of glass and ceramic) are mixed with the alginate.Alginate composite that contains ‘hard’natural polymer such as chitosan in the presence of acetic acid demonstrated increase compressive property ranging from 0.79 to 1.41 MPa depending on the concentration of acetic acid [176] .It was observed in this study that the stiffer alginate/chitosan composite showed greater number of MG-63osteoblast-like cells grown on it.Adding synthetic polymer,poly(ethylene glycol) monomethacrylate(PEGmM) and poly(propyleneglycol) monomethacrylate(PPGmM) to methacrylic alginate at different mass fractions produce alginate composites of various charge density and hydrophobicity [177] .While blending alginate with bioglass in the presence of zinc and magnesium,gave alginate composite a greater compressive strength of 1.7 MPa [178] .In this alginate/bioglass/Zn/Mg composite,the presence of zinc and magnesium not only gave strength to the alginate,both of them also provide bacteria-free condition for bone formation.In all these studies above,natural and synthetic polymer,and bioglass which were added to the alginate not only gave strength,it also created a porous composite structure that mimics the bone (Fig.6 A).

Fig.6–Surface morphology of 50/50 alginate/HAP composite scaffolds (3% alginate,crosslinker:0.03 M CaCl 2,freezing temperature:−40 °C) at (A) bottom,(B) top,and (C) midsections of scaffolds.Magnification at 100 ×.Adapted with permission from [180] .Copyright (2004),John Wiley and Sons.
Bone is a rigid structure due to the presence of HA that made 70% of the bone component [179] .Because of this,many studies develop alginate composites combine with HA.It was seen that adding HA to the alginate produced a composite with a well-interconnected porous structure with an average pore size of 150 μm and over 82% porosity[180] (Fig.6).Seeded rat osteosarcoma UMR106 cells attach well onto this composite and propagate better than to the pure alginate scaffold.In more recent study,blending HA with alginate/chitosan composite although result in almost similar average pore size and porosity structure of alginate/HA composite,the HA-alginate/chitosan composite capacity for bone formation is improved in vivo [181] .It was observed that newly formed bone tissue filled the area of 3.0 mm (diameter) mouse calvarial bone defect implanted with HA-alginate/chitosan composite scaffold whereas none was observed when implanted with alginate/chitosan composite.In HA-alginate/chitosan composite scaffold,chitosan promotes cell attachment due to the presence of protonated amino groups on its backbone [182] and HA stimulates new bone cells formation on the composite graft.Based on this result,increasing studies is in trend nowadays to develop alginate composites by mixing alginate with more than one type of material that could provide strength and mimic the microenvironment of the bone to improve the scaffold capacity for bone formation.
In more recent study,Jo et al.[183] showed that grafting of alginate/HA composite that combine with silk fibroin(SF) in Sprague Dawley rats that possess central calvarial bone defects (diameter:8.0 mm) result in higher new calvarial bone formation compared to that of alginate graft only at four weeks post implantation.This is due to the presence of HA and silk fibroin in the composite improved osteoinductivity and bone cell regeneration capacity,respectively.Regeneration of the bone tissue and rapid bone repair was observed as well when dopaminemodified alginate with chitosan/HA composite was implanted in femoral defect (4 mm in diameter and 5 mm deep) of New Zealand rabbits [184] .In this study,treating alginate with dopamine enhance composite to be stable and adhesive in the defected bone.Subcutaneous implantation of encapsulated rat bone marrow derived mesenchymal stem cells (MSC)in alginate dialdehyde/gelatin/nanoscaled bioactive glass(AD/G/nBG) microbeads into rats demonstrated higher cell survival compared to MSC encapsulated in microbeads without nBG,although the difference was not significant [185] .This indicates high compatibility of the multiple-material alginate composite microbeads in 3D environment compared to double-material alginate composite microbeads as multiple materials might better mimic the in vivo environment.
3.3.2.Cartilage
Cartilage is another rigid component in the body.It covers and protects bone ends from friction and made up the structure of ear,nose,bronchial tubes,intervertebral discs and other body components.Cartilage lacks of nerves and blood vessels thus it repairs damage slower than other tissues [186] .It did not heal spontaneously hence causes pain,inflammation and affected limb movement particularly for articular cartilage damage cases.
Grafting of engineered cartilage tissue to the cartilage defectives sites has shown encouraging results in stimulating cartilage growth [187,188] .This could lead to the restoration of the cartilage damage.Therefore,numerous studies were conducted to develop engineered cartilage tissue that involves cells and biodegradable scaffolds.Cells (chondrocytes)isolated from various parts of the body [189,190] and different types of stem cells were studied [191] and various materials to develop the scaffold were investigated [192] .One of the most studied materials to develop the scaffold is alginate.It has been shown in many studies that alginate supported the growth of chondrocytes and retained the typical chondrocytic appearance and properties [8,193] .However,it has also been demonstrated that initial cell loss was observed before alginate could support the chondrocyte growth [194] .Thus,alginate with reinforced hyaluronate was developed to increase the capacity of alginate for chondrocyte proliferation [195] .It was demonstrated that hyaluronate in this composite scaffold forms cross-linked structure with chondrocytes and interacts with CD44 on the cell surface of the chondrocytes hence increase the chondrocytes growth.Another alginate composite made by combining with jellyfish collagen was also shown to have better capacity in supporting human mesenchymal stem cells (hMSCs) chondrogenic differentiation and provide more stable constructs compared to pure alginate [196] .hMSCs chondrogenic differentiates better when seeded into to this polymer composite because jellyfish collagen in the composite is similar to human collagen type II which is the main tissue components of cartilage.During short-term pilot study in which 21 patients with lesion in the knee received alginate/fibrin matrix seeded with the allogenic cartilage cells,the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the Visual Analogue Scale (VAS) scores of the patients improved significantly and histology analysis showed significant repair tissues were present [197] .Twelve months follow-up of the patients showed 41.6% have complete filling or hypertrophy,41.7% have bone-marrow edema and 25% have effusion [198] .
For repairing articular cartilage damage,scaffold with high mechanical performance that can withstand the load-bearing stress is required.Pure alginate has limited mechanical strength and forming alginate composites by adding other material such as chitosan,natural inorganic polyphosphate,and nanocellulose increased alginate mechanical performance [199,200] .Furthermore,adding chitosan for example,upregulates the expression of genes encoding for collagen type II and aggregate of cartilage indicating increased chondrocyte growth [199] .While adding natural inorganic polyphosphate such as polyphosphate,gave flexibility for the composite scaffold to be moulded for replacement of damaged hard tibia bone regions [199] .Adding nanocellulose facilitates better gelation mechanism in hydrogels and enhances the dimensional stability[200] .
Bioprinting is one of the methods used in tissue engineering that provides transplanted tissue with accurate architecture of affected site.Lately,alginate composites were used as bioinks for cartilage bioprinting applications [201,202] .Alginate alone gives weak printed constructs due to its rheological properties (low yield point).Mixing nanocellulose together with alginate retained stiffer printed alginate composite constructs that support chondrocytes proliferation and spreading as well as maintains chondrocyte properties[203] .Composite alginate bioink that contains collagen and polylactide also retain better 3D construct of bioprinting[204,205] .It was observed that when alginate/collagen was used as bioink,the resulting composite printed constructs not only possess enhanced cell adhesion,fasten cell proliferation and preserved chondrocyte phenotypes capacities,the composite constructs also has capabilities to suppressed dedifferentiation of chondrocytes [204] .
3.3.3.Liver
Liver failure could cause approximately 2 million deaths per year worldwide [206,207] .The most effective therapy to treat terminal liver failure is liver transplant.However,problem arises to find liver donor to reduce graft rejection.Liver tissue engineering may be the best option to provide liver mass to replace the defective liver tissues [208] .Liver tissue engineering involves growing hepatocytes or stem cells that can be induced to differentiate to become hepatocyte on scaffold to create liver mass.
Fig.7–Phase contrast image of poly(ethylene glycol)-microencapsulated mesenchymal stem cells for bone tissue engineering.Adapted with permission from[215] .Copyright (2012),Authors.
Extensive studies had been conducted and promising results showed encapsulated and seeded hepatocytes in and onto alginate remain viable and maintain their functional phenotype [209,210] .However,better results were demonstrated when alginate composites were used for hepatocyte growth.For example,alginate/galactosylated chitosan composite demonstrated higher cell attachment and viability than alginate alone [211] .This is owing to the presence of galactose in the composite that acts as specific signal for hepatocyte attachment and chitosan in the composite that mimic the ECM of the liver.Forming alginate composite by adding xyloglucan (XG) in which also act as synthetic ECM enhanced adhesion and proliferation of human hepatoma HepG2 cells with increasing concentration of XG [212] .Transplantation of liver-differentiated hMSCs seeded onto RGD-modified chitosan-alginate composite scaffold to defective rat liver (70% of the liver was removed)demonstrated that seeded cells remain at the transplantation site and function well with the remaining liver tissue [213] .The transdifferentiated hMSCs expressed hepatocyte function of glycogen storage although somewhat less and expressed the human hepatocyte markers such as AFP,CK19,CK18,albumin,HNF-3 β and MRP-2.
Another approach to create liver mass for the liver transplant is formation of microcapsules.Microcapsules formation involves entrapment of cells within microbeads in which multiple microbeads were later enveloped by semipermeable membrane to produce microcapsules[214,215] (Fig.7).Having the membrane surrounding microbead-containing cells provides the cells 3D microenvironment that recapitulates various aspects of the in vivo microenvironment in vitro [216] .The membrane also gave immuno-protection to the cells from the outside environment once the microcapsules transplanted inside the body and at the same time selectively allows diffusion of oxygen,nutrients and waste metabolites.Encapsulated HepG2 cells in alginate microbeads surrounded by chitosan membrane were shown to be viable,able to proliferate and secrete proteins for at least 12 d in the microcapsules[217] .Later,it was demonstrated that galactose/alginate composite microbeads have increased viability,proliferability and liver-specific functions of human hepatocyte,LO2 similar to the seeded hepatocytes onto galactosylated alginate scaffold although the former were wrapped with the chitosan membrane [218] .HepG2 cells were also observed to be viable and maintain hepatocyte function in sericin/alginate microbeads that were enveloped with chitosan shell [219] .Sericin is a protein that promotes proliferation as well as cell attachment.The cells were uniformly distributed within the sericin—alginate—chitosan microcapsules indicating that the microcapsules maintain favourable microenvironment for the cells.
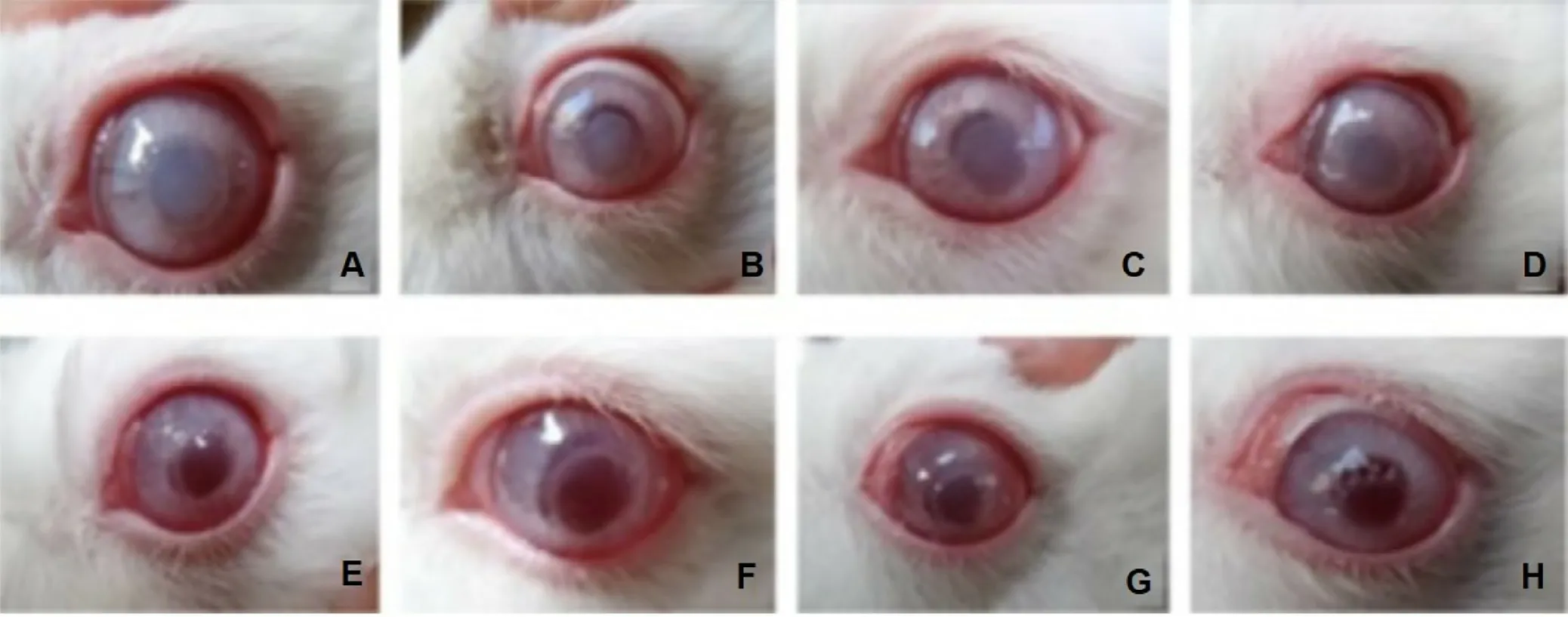
Fig.8–Typical eyeball images after operation.(A-D) Negative control group on the 15th,30th,45th and 60th day;(E-F)experimental group receiving encapsulated corneal endothelial cells (CECs) in hydroxypropyl chitosan/oxidized alginate on 15th,30th,45th and 60th day.Adapted with permission from [229] .Copyright (2011),Elsevier.
3.3.4.Other tissues and organs
Alginate and alginate composites had also been used for tissue engineering of other tissues and organs such as nerve,muscle,eye and heart.Earlier studies showed scientists used non-modified alginate or alginate only for nerve,muscle,eye and heart tissue engineering as scaffold and cellencapsulated materials [19,26,82] .But later,many utilized alginate composites in their studies because the presence of various biological and synthetic materials in the composites allows formation of materials with desired properties that mimic the environment of these tissues and organs hence enable cells regeneration.
The following are the alginate composites that had been tested to be used as scaffold to regenerate the neural cells in vitro .It was demonstrated that alginate/HA composite supported the growth of mESCs and assisted differentiation of the cells towards neuronal lineages [220] .Electrically conductive polypyrrole/alginate (PPy/Alg) composites had also been shown to promote hMSCs cell adhesion,growth and differentiation to become neural cells when the cells were seeded onto the composite scaffold [221] .PPy is a conductive biomaterial that can be used to influence responses of various cells,including electrically excitable cells such as neurons.In more recent study,a novel microgel alginate composite,methacrylic anhydride modifiedcollagen/hyaluronic acid/alginate was observed to support the growth of induced pluripotent stem cells (iPSCs).The iPSCs capability to differentiate become neuron was enhanced when bioactive GRGDSP/Ln5-P4 was combined with the gel[222] .Other than using the alginate composites as scaffold for neural cell regeneration,it could also be utilized as nerve guidance scaffold for axonal regeneration.Freeze-dried alginate gel bar covered by polyglycolic acid mesh had been proven to guide axonal regeneration when cats with 50-mm gap sciatic nerve recovered motor and sensory nerves after 13 weeks of the implantation [223] .Alginate-polyornithine encapsulated porcine choroid plexus cells (NTCELL) have shown a suggestive signal of efficacy in neurodegenerative Parkinson’s disease patients when NTCELLwas implanted in the patients’ putamen during phase IIb clinical trial[224] .
Successful muscle regeneration to repair and provide tissues for muscle defects depends on the specific tissue-like mechanical behaviours of the scaffold materials which could be appropriately provided by alginate composite compared to alginate.Alginate composite such as alginate/polyacrylamide and oxidised alginate/gelatin after refinement had been shown to have promising mechanical properties to be scaffold for muscle regeneration [225,226] .Recent study showed human skeletal muscle progenitor cells (hSMPCs) seeded onto skeletal muscle ECM treated-alginate/gelatin/heparin(Alg/G/H) scaffolds proliferated,differentiated,and matured[227] .It was also shown that the seeded hSMPCs cells onto the treated composite scaffold demonstrated better results than the seeded cells onto individual scaffold (i.e.gelatin,Matrigel,or ECM alone).This might be due to the combination of alginate with gelatin is a better matrix for cell adherence and it has proven that heparin provides growth factor retention and release in an extended manner for MPC proliferation and differentiation [228] .
For ocular system,there is high interest in using alginate composites for tissue engineering to repair cornea.Attempts to repair cornea damage due to corneal endothelial disorders by growing in situ encapsulated corneal endothelial cells(CECs) in hydroxypropyl chitosan/oxidized alginate onto Descemet’s membranes has been made [229] .After 60 d,rabbit corneas with transplanted encapsulated CECs resumed well and retained full transparency while rabbits that are not receiving polymer-cell complex became opaque,edema and thicken (Fig.8).Repairing cornea damage due to alkali burn with transplantation of limbal stem cells (LSCs) encapsulated in carboxymethyl chitosan/oxidized alginate also produced successful results [230] .28 d postburn,the rabbit corneal opacity completely recovered and was comparable to that in a normal cornea.
Utilization of alginate and its composites in treating heart problems had been intensively investigated and few are in the advance clinical trial [231,232] .However,injection of acellular alginate implant rather than alginate-embedded with stem cells implant into the heart wall showed clinical translation success.During clinical trials,injection of alginate based material,IK-5001 (Bellerophon Therapeutics,USA) and Algisyl(LoneStar Heart,Inc.,USA) into left ventricle (LV) wall of acute and chronic heart failure patients prevent further remodelling of LV,increase ejection fraction and showed New York Heart Association functional class improvement [233,234] .Despite disadvantages of implantation of alginate-embeddedcells due to complex regulatory pathway,uncertainty of shelf availability and high costs,many recent studies were conducted to increase the efficacy of the cell/matrix-based technologies.One of the strategies is to improve the matrix used for entrapping the cells.Because alginate lack of appropriate motifs for adherent cells to attach,blending of natural ECM such as gelatin together with alginate enhances the cells to attach and proliferate [235] .gelatin is a denatured form of collagen type I that possess essential motifs required for cell proliferation and growth.It was observed that rat cardiomycetes encapsulated in alginate/gelatin showed increased cell viability after 7 d compared to when cardiomycetes were encapsulated by alginate alone [236] .The encapsulated cells in the blended polymer also able to retain stemness feature and cell multipotentiality compared to non-encapsulated cells.Other study showed different type of needle used to inject the encapsulated cells also play important role in maintaining the viability of the injected encapsulated cells.Curley et al.[237] found that encapsulated hMSCs metabolic activity reduced into half of control and had a significant decline in cell viability when helical needle was used.
4.Conclusion and future outlook
Gel forming capability and biocompatibility of alginate have made it become a go-to biomaterial in biomedical and pharmaceutical field.It provides moist healing environment during wound dressing,serve as scaffold during tissue regeneration,and function as carrier for drug or cell during targeted delivery process.Despite these advantages,native alginate gel suffers from the lack of mechanical integrity which can lead to distortion of scaffold structure or leakage of encapsulated substance.This requires incorporation of compatible reinforcing agent within alginate structure in order to improve alginate mechanical integrity especially in challenging psychological environment.
When the concern is solely about strength improvement,natural polymer (e.g. cellulose and chitin),synthetic polymer(e.g. polylactic acid and polycaprolactone),and carbonbased material (e.g. graphene and carbon nanotube) are generally used as reinforcing agent.Chitosan and metal nanoparticle,on the other hand,can provide respectable improvement in mechanical properties while simultaneously impart additional antibacterial properties.Inorganic material(e.g. nano-hydroxyapatite (nHAP) and bioglass) also provide additional strength reinforcement to alginate while being able to stimulate mineralized cell (e.g. osteoblast) growth and viability.However,if the concern of cell proliferation outweighs the need of mechanical stability,alginate can reservedly act as reinforcing agent instead.For example,during tissue regeneration,incorporation of ECM-like constituent (e.g. gelatin,collagen,and hyaluronic acid)is crucial so that suitable cell microenvironment can be provided.ECM itself lacked sufficient mechanical strength to be further manipulated,while alginate-based hydrogel,albeit being structurally stronger than ECM,does not possess receptor that can be recognized by cell for them to proliferate and differentiate.The symbiotic relationship of alginate as matrix to be reinforced or as reinforcing material itself,truly mark the versatility of this material.Adding to the fact that alginate composite can also be processed into various form(fibre,bead,hydrogel,and 3D-printed matrices) allows further fine-tuning the function-tailoring for specific biomedical applications.
Control of crosslinking agent densities and reinforcing material during alginate composite preparation allows different range of drug encapsulation medium with tuneable release kinetic to be developed.However,instantaneous external gelation of alginate upon contact with crosslinking agent during preparation process may limit it usage to premade tissue scaffold which requires surgeries for the scaffold to be embedded later in vivo .For this reason,research related to the formulation of injectable hydrogel,which relies on wider gelation working windows are gaining momentum nowadays.Typically,the use of low water solubility species relative to standard alginate crosslinking agent,CaCl 2,allows more uniform distribution in alginate solution and provide longer gelation time.However,if the gelation occurs too late,the uncured injected gel might be flushed away and it can unnecessarily prolonged patient waiting time.Hence,control over gelation rate is crucial if injectable alginate based hydrogel is to be used in real clinical setting.If this can be achieved,not only alginate-based scaffold can be injected to harder-to-reach region,but also can effectively improve the efficacy of drug delivery targeting.
Research on bottom-up biofabrication using alginatebased bioink also trending recently.As natural microenvironment and tissue arrangement in vivo is typically complex,the ability to create intricate scaffold structure that simultaneously laden with living cells is biomimeticaly inspired.However,it should be noted that the use of alginate itself as scaffold can actually reduce cell-to-cell connection and residual scaffold polymer can disrupt normal cells organization,especially when cells need to be tightly interconnected such as in myocardial tissue.Although the strategies such as scaffold-free tissue construct had been reported recently,whereby alginate served as outer mould for tissue growth,it is limited to simple geometric construct which seems counterintuitive given the hallmark of bottomup biofabrication is its ability to form complex construct.We believe that in order for tissue engineering to reach its fullest potential is by the preservation of complex and robust cell-only structure,mimicking the way Mother Natures intended.
As for now,alginate has successfully shows its credibility as wound dressing material,and continues to become material of choice for simple scaffold in tissue engineering and encapsulation medium for drug delivery.In the future,we envision more processing innovation towards intricate cell-laden alginate construct that can offer gentle and targeted alginate degradation without affecting embedded cells viability.
Conflict of interest
The authors declare that there is no conflict of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors acknowledge the financial support received from Ministry of Education Malaysia(FRGS/1/2018/TK05/UIAM/03/3).Writing on fibre preparation,chitin,and chitosan are directly related to the said grant.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- The application of label-free imaging technologies in transdermal research for deeper mechanism revealing
- Drug-drug cocrystals:Opportunities and challenges
- Recent advances of sorafenib nanoformulations for cancer therapy:Smart nanosystem and combination therapy
- Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies
- In vitro - in vivo - in silico approach in the development of inhaled drug products:Nanocrystal-based formulations with budesonide as a model drug
- pH-sensitive micelles self-assembled from star-shaped TPGS copolymers with ortho ester linkages for enhanced MDR reversal and chemotherapy
