Pharmacokinetic comparison with different assays for simultaneous determination of cis-,trans-cefprozil diastereomers in human plasma
2021-07-20SeungHyunJeongJiHunJangHeaYoungChoYongBokLee
Seung-Hyun Jeong,Ji-Hun Jang,Hea-Young Cho,Yong-Bok Lee
aCollege of Pharmacy,Chonnam National University,77 Yongbong-ro,Buk-gu,Gwangju,61186,Republic of Korea
bCollege of Pharmacy,CHA University,335 Pangyo-ro,Bundang-gu,Seongnam-si,Gyeonggi-Do,13488,Republic of Korea
Keywords:
Cefprozil diastereomers
Comparison
HPLC-UV
UPLC-ESI-MS/MS
Pharmacokinetic parameter
Biochemical parameters
A B S T R A C T
The purpose of this study was to compare pharmacokinetic(PK)parameters obtained using two newly developed assays,HPLC-UV and UPLC-ESI-MS/MS.Selection of assay and results obtained therefrom are very important in PK studies and can have a major impact on the PK-based clinical dose and usage settings.For this study,we developed two new methods that are most commonly used in biosample analysis and focused on PK parameters obtained from them.By HPLC-UV equipped with a Luna-C8 column using UV detector,cefprozil diastereomers were separated using water containing 2%(V/V)acetic acid and acetonitrile as a mobile phase.By UPLC-ESI-MS/MS equipped with a HALO-C18column,cefprozil diastereomers were separated using 0.5%(V/V)aqueous formic acid containing 5 mM ammoniumformate buffer and methanol as a mobile phase.Chromatograms showed high resolution,sensitivity,and selectivity without interference by plasma constituents.Both intra-and inter-day precisions(CV,%)were within 8.88% for HPLC-UV and UPLC-ESI-MS/MS.Accuracy of both methods was 95.67%-107.50%.These two analytical methods satisfied the criteria of international guidance and could be successfully applied to PK study.Comparison of PK parameters between two assays Confirmed that there is a difference in the predicted minimum plasma concentrations at steady state,which may affect clinical dose and usage settings.Furthermore,we Confirmed possible correlation between PK parameters and various biochemical parameters after oral administration of 1000 mg cefprozil to humans.
1.Introduction
Cefprozil is an orally active second-generation cephalosporin type antibiotic[1].It works by weakening the cell walls of the bacteria causing the infection,thereby killing the bacteria.Various studies have verified that cefprozil has a broad antibacterial range[2].Cefprozil is particularly active against gram-positive organisms such as Streptococcus pyogenes,S.pneumoniae,S.agalactiae,and methicillin-susceptible Staphylococcus aureus.Cefprozil is also moderately active against gram-negative organisms such as Haemophilus influenzae,Moraxella catarrhalis,Neisseria gonorrhoeae,many Enterobacteriaceae,and certain anaerobic organisms[3].One of the reasons for its broad antibacterial range is that cefprozil is relatively stable to hydrolysis by a large number of beta-lactamases[4].Cefprozil can be used to treat mild to moderate upper and lower respiratory tract infections including sinusitis,otitis media,pharyngitis/tonsillitis,secondary bacterial infection of acute bronchitis,acute bacterial exacerbations of chronic bronchitis,and skin or skin structure infections[1,4,5].Cefprozil consists of cis-isomer and trans-isomer with a ratio of approximately 90:10[6].In terms of drug efficacy,these two isomers of cefprozil show similar antibacterial activity against gram-positive organisms.However,they have different antibacterial activities against gram-negative organisms.It has been reported that cis-cefprozil is at least six times more potent than trans-cefprozil for gram-negative organisms[3].Therefore,it is necessary to quantify blood concentrations and calculate in vivo pharmacokinetic(PK)parameters separately for each isomer of cefprozil.
Several studies have reported HPLC-UV and LC-MS/MS methods for quantitative analysis of cefprozil.However,each of the reported methods had limitations that could be used as PK study or directly applied in vivo assays.Cefprozil quantitation methods for bulk and pharmaceutical formulations are unsuitable for analysis of cefprozil in biological samples.In addition,these methods did not separate two isomers of cefprozil[7,8].Although the methods of separating the two isomers of cefprozil using HPLC-UV have been reported[9,10],they were methods for formulation content analysis,not for in vivo content analysis.Most of these formulation assays have low sensitivity,and because of the completely different sample preparation method used in biological samples,there are limitations to apply it directly to PK study like ours.In human plasma using HPLCUV,separation assays for two isomers of cefprozil have been reported[6,11,12].However,the lower limit of quantitation(LLOQ)for cefprozil was as high as 5000 ng/mL and the analysis time was as long as 30 min[11].In addition,only PK values for eight[6],nine[11],and ten[12]subjects were presented.It was also reported that plasma concentration of cefprozil in humans was quantified using HPLC-UV method,but cis-and trans-isomer were not separated by the method[13].HPLC-MS/MS has also been used to simultaneously quantify two isomers of cefprozil in human plasma[14].Unfortunately,it also had high LLOQ values(125 ng/mL for cis-isomer and 40.3 ng/mL for trans-isomer).And because of the high flow rates of the mobile phase,the consumption of organic solvents used in analysis was significant.In addition,the most recently reported method was also HPLC-MS/MS analysis[15].However,in the presented chromatograms,the analyte peaks were not completely separated at high concentrations and the peak was suspected to be splited.It was applied to PK study in only four healthy Chinese.Therefore,new analytical methods that can simultaneously quantify both diastereomers of cefprozil in a biological sample more sensitively,quickly,and accurately need to be developed.In this regard,we focused on the development of improved UPLC-MS/MS method in terms of sensitivity,efficiency and applicability to a larger number of biological samples than previously reported methods.
In addition,studies that compare differences in analytical methods such as HPLC-UV and UPLC-ESI-MS/MS in biological samples for cefprozil have not been reported yet.In general,UPLCESI-MS/MS methods have the advantages of higher sensitivity,higher selectivity,and higher throughput than HPLC-UV methods[16].However,since the MS detector is usually expensive and difficult to maintain and manage,analyzing biological samples using relatively inexpensive UV detector will be a great economic advantage.Roth et al.[17]reported that the relatively inexpensive LC-UV/DAD is more widely available than LC-MS/MS in hospital laboratories performing many clinical studies including therapeutic drug monitoring(TDM).Our research material,cefprozil,requires TDM when there is concomitant medication and kidney disease.Numerous studies comparing LC-UV and LC-MS methods in the analysis of biological samples for other drugs have been reported[16-23].The purpose of the inter-comparison of these methods is perhaps to ensure that the results are identical even if one of the two methods is used.In other words,by choosing the appropriate analytical method for the specific situation,it will be possible to derive the optimal PK result.In essence where plasma concentrations are high and rapid turn round of data is not required,the use of the LC-UV is more than adequate.However,if sensitivity is an issue,limited amounts of plasma are available and/or where tight deadlines are pivotal,LC-MS is the method of choice[19].
Therefore,our final purpose of this study was to compare performances of newly developed UPLC-ESI-MS/MS and HPLC-UV methods for simultaneous quantification of cis-and trans-cefprozil in human plasma samples.There may be differences in the quantitative values depending on the analytical method,which may cause differences in the PK parameter values.Drug dose and usage settings based on the individual parameters for which the difference occurred may produce different results in clinic.It is very important to compare the changes of PK parameters according to the analytical methods.
Additionally,we tried to Confirm the association between the various biochemical parameters(total proteins,albumin,alkaline phosphatase(ALP),aspartate aminotransferase(AST),alanine transaminase(ALT),total bilirubin,cholesterol,blood urea nitrogen(BUN)and creatinine)and PK parameter values including area under the curve(AUC),half-life and clearance obtained after oral administration of cefprozil tablets to humans.This relationship study between PK parameters and biochemical parameters could be useful for future population PK studies of cefprozil.
2.Experimental
2.1.Reagents and chemicals
Chemical standards of cis-and trans-cefprozil(purity≥98%;CAS No.92665-29-7)were obtained from Hanmi Pharma,Co.(Seoul,Korea).Cefaclor(purity≥99%;CAS No.53994-73-3)was supplied by Sigma-Aldrich,Inc.(St.Louis,MO,USA).Acetonitrile(ACN),dichloromethane(DCM),methanol,and water(18.2 mΩ)of LC-MS/MS grade were also purchased from Sigma-Aldrich,Inc.(St.Louis,MO,USA).Ethyl acetate of HPLC grade was obtained from Fisher Scientific,Inc.(Hampton,NH,USA).In addition,LC-MS grade formic acid,acetic acid,and trichloroacetic acid(TCA)were purchased from Thermo Fisher Scientific,Inc.(Waltham,MA,USA).All chemicals used in this study had the highest HPLC grade or better quality available.Cefaclor was used as an internal standard(IS)in both methods.Fig.1 shows structures of cis-cefprozil,trans-cefprozil,and cefaclor.
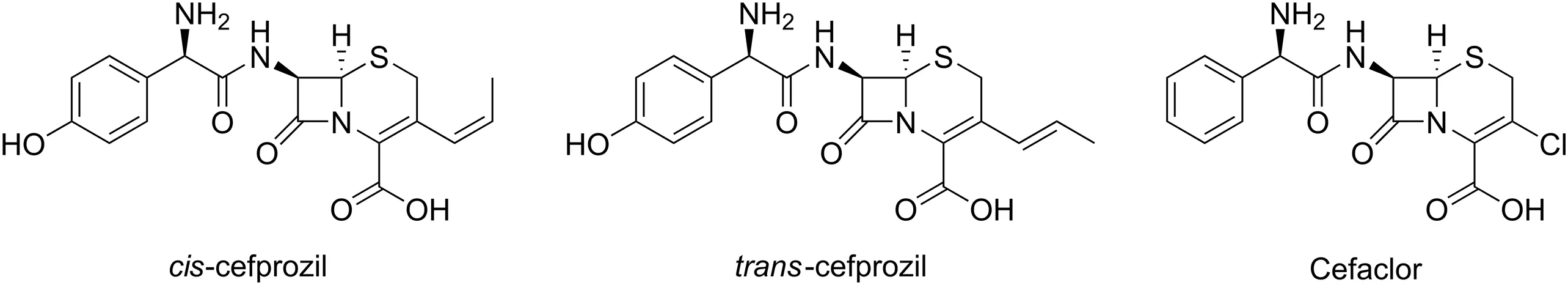
Fig.1.Chemical structures of cefprozil diastereomers and cefaclor(internal standard).
2.2.Instrumental and analytical conditions
2.2.1.UPLC-ESI-MS/MS method
The newly developed UPLC-ESI-MS/MS system consisted of a Shimadzu Nexera-X2 Series UPLC system(Shimadzu,Kyoto,Japan)equipped with a Shimadzu-8040 mass spectrometer with a DGU-20A degassing unit and an SIL-30AC autosampler.Various conditions were tested to obtain the best chromatographic condition,including mobile phase pH(0.1%(V/V)aqueous formic acid(pH 2.5),0.2%(V/V)aqueous formic acid(pH 2.3),0.5%(V/V)aqueous formic acid(pH 2.0),containing 5%(V/V)of 5 mM ammonium formate(pH 3.0)buffer),type of mobile phase organic solvent(ACN,methanol),and column(Acquity UPLCⓇBEH C18(50 mm × 2.1 mm,1.7μm),Inertsil C8-3(100 mm ×2.1 mm,2μm),Phenomenex KINETEX core-shell C18(50 mm × 2.1 mm,1.7μm),and HALO-C18(100 mm×2.1 mm,2.7μm)column).Optimized chromatographic separation of cefprozil diastereomers was conducted with a HALOC18column at an oven temperature of 40°C.The mobile phase consisted of 0.5% aqueous formic acid containing 5%(V/V)of 5 mM ammonium formate(pH 3.0)buffer(mobile phase A;pH 2.0)and methanol(mobile phase B).Analysis was performed with a gradient elution and a flow rate of 0.3 mL/min.The elution program was as follows:0-0.5 min(5%B),0.5-2.0 min(5%-60%B),2.0-3.2 min(60%B),and 3.21-4.0 min(5%B).All analytical procedures were evaluated with negative electrospray ionization.And quantification was achieved using multiple reaction monitoring(MRM)modes at m/z 388.00→249.20 for cis-,and trans-cefprozil and m/z 365.90→286.20 for IS.Analysis and acquisition of data were achieved using a LabSolutions program with collision energy of 13 and 21 eV for cefprozil(cis-or trans-)and IS,respectively.The loading volume on column was 5μL.
2.2.2.HPLC-UV method
The newly developed HPLC-UV system consisted of a Shimadzu LC-10AD series(Shimadzu,Kyoto,Japan)equipped with a photodiode array detector with LabSolutions software.A Luna-C8column(150 mm×4.6 mm i.d.,5μm;Phenomenex,USA)was used as a stationary phase.The oven temperature of the column was maintained at 40°C.Furthermore,the mobile phase consisted of 2%(V/V)acetic acid in water(mobile phase A;pH 2.7)and ACN(mobile phase B),and the flow rate was set at 1.0 mL/min.And the ratio of mobile phase composition was 95:5(V/V)for mobile phase A and B.Chromatographic separation was conducted using isocratic elution.The detection wavelength of UV was 280 nm and the injection volume was 20μL using a Rheodyne injector.The total run time per sample was 20 min.Peaks were assigned by spiking samples with standard compounds,followed by comparison of UV spectra and retention times.
2.3.Preparation of standard samples
Individual standard stock solutions of cis-,trans-cefprozil,and IS were prepared by weighing accurately and dissolving in mobile phase solution at 1 mg/mL.They were stored at-20°C prior to making working solutions.Standard working solutions of cis-cefprozil(1,5,10,50,150,and 200μg/mL for HPLC-UV;0.05,0.2,1,5,10,50,and 200μg/mL for UPLC-ESI-MS/MS),trans-cefprozil(0.2,0.5,1,5,15,and 25μg/mL for HPLC-UV;0.15,0.3,0.5,1,5,10,and 25μg/mL for UPLC-ESI-MS/MS),and IS(30μg/mL for HPLC-UV and 5μg/mL for UPLC-ESI-MS/MS)were prepared by diluting the standard stock solutions with mobile phase solution.Calibration working solutions were prepared by adding each diluted working solution into blank human plasma to obtain different final concentrations(cis-cefprozil:ranging from 0.1 to 20μg/mL for HPLCUV and from 0.005 to 20μg/mL for UPLC-ESI-MS/MS;trans-cefprozil:ranging from 0.02 to 2.5μg/mL for HPLC-UV and from 0.015 to 2.5μg/mL for UPLC-ESI-MS/MS).To measure the accuracy and precision of the two methods,quality control(QC)samples of cefprozil at four concentrations were similarly prepared(cis-cefprozil:0.1,0.5,5,and 15μg/mL for HPLC-UV;0.005,0.025,4,and 16μg/mL for UPLC-ESI-MS/MS;trans-cefprozil:0.02,0.05,0.5,and 1.5μg/mL for HPLC-UV;0.015,0.06,0.8,and 2μg/mL for UPLC-ESI-MS/MS).Each QC and calibration sample was prepared on the same day of analysis for both methods.
2.4.Sample preparation
In HPLC-UV method,cefprozil diastereomers were extracted from plasma using protein precipitation(PP)method with ACN and TCA.A 100 μL of IS solution(30 μg/mL of cefaclor in mobile phase solution)was added to 1000μL of each human sample and then 300μL of 10%(V/V)TCA was added to each plasma sample.The mixed sample was added to 2 mL ACN and then vortex-mixed for 5 min.Then an additional 3 mL DCM(to remove ACN)was added and vortex-mixed for 10 min and centrifuged at 3000 g for 10 min.The aqueous supernatant(1 mL)was transferred to a new test tube.It was dried gently with a centrifugal vacuum evaporator under ultra-purity nitrogen gas for 3 h at 40°C.The dried matter was reconstituted with 200μL of mobile phase solution and vortexed for 1 min.After centrifugation at 12,000 g for 5 min,20μL of the supernatant(aliquot)was injected into HPLC-UV system.In UPLCESI-MS/MS method,sample preparation of cefprozil diastereomers was tested using PP method with ACN and methanol as well as liquid-liquid extraction(LLE)method using methylene chloride,di-ethyl ether,methyl tert-butyl ether(MTBE),and ethyl acetate.In the case of ethyl acetate,extraction efficiency was compared by adding formic acid from 0 to 4%(V/V).Finally,samples were extracted by LLE using 3%(V/V)formic acid in ethyl acetate and protein was precipitated by PP using methanol.A 10μL of IS solution(5μg/mL of cefaclor in mobile phase solution)was added to 100μL of each human sample.The mixture was added to 1000μL methanol-3% formic acid in ethyl acetate(60:40,V/V),vortexed for 5 min,and centrifuged at 13,000 g for 5 min.Then 1000μL of the supernatant organic layer was dried gently with a centrifugal vacuum evaporator under ultra-purity nitrogen gas for 3 h at 40°C.The dried matter was reconstituted with 50μL of mobile phase solution and vortexed for 5 min.After centrifugation for 5 min at 13,000 g,5μL of the supernatant(aliquot)was injected into UPLC-ESI-MS/MS system.
2.5.Method validation
Method validation was carried out in accordance with the Guidance for Industry:Bioanalytical Method Validation by Food and Drug Administration[24].Selectivity,sensitivity,linearity,accuracy,precision,recovery,matrix effect,stabilities,carryover,and incurred sample reanalysis(ISR)were then estimated.
2.5.1.Selectivity and sensitivity
Selectivity was determined to Confirm the influence of endogenous compounds located in the closed retention time of analytes.Therefore,blank plasma which was obtained from six different individuals,zero plasma,plasma spiked with cefprozil diastereomers(LLOQ concentration),and plasma samples obtained after oral administration of cefprozil(4 tablets of 250 mg cefprozil)to Korean subjects were used to demonstrate the selectivity of the method.Sensitivity of the method was expressed as LLOQ determined as the lowest concentration of the standard sample with a signal-to-noise ratio of at least 10:1 in accordance with an acceptable accuracy within±20% and precision of less than 20% evaluated with five replicate samples.
2.5.2.Linearity
The calibration curves used to quantify the concentration in plasma samples were prepared from seven calibration points by linear regression with weighting factor of 1/concentration2.The linearity of calibration curves was determined by plotting analyte/IS peak area versus theoretical concentration of analyte.Linear calibration equation with its correlation coefficient(r2)was determined.
2.5.3.Accuracy and precision
The intra-day precision and accuracy were evaluated by analyzing QC samples at five different times on the same day(n=5).In addition,inter-day evaluations were carried out in the same way for five consecutive days(n=5).The concentration of each QC sample was evaluated using freshly prepared calibration standards.In other words,the concentrations of QC samples were quantified with calibration curves obtained with excellent linearity on the day.The precision was evaluated by calculating coefficient of variation(CV)for the analysis of QC samples.The CV of precision for each QC level should not deviate by more than±15% except for LLOQ with a limit of 20%.Furthermore,the accuracy was determined based on the following criteria:the mean value should not exceed 15% of the nominal concentration except for LLOQ,which should not exceed 20%.
2.5.4.Recovery and matrix effect
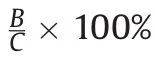
2.5.5.Stabilities
Stabilities of cefprozil diastereomers in human plasma were determined under various physical conditions,including the shortterm stability,long-term stability,and freeze-thaw stability.QC samples at low(cis-cefprozil:0.5μg/mL in HPLC-UV and 0.025 μg/mL in UPLC-ESI-MS/MS;trans-cefprozil:0.05μg/mL in HPLC-UV and 0.06μg/mL in UPLC-ESI-MS/MS)and high(cis-cefprozil:15 μg/mL in HPLC-UV and 16 μg/mL in UPLC-ESI-MS/MS;transcefprozil:1.5 μg/mL in HPLC-UV and 2 μg/mL in UPLC-ESI-MS/MS)concentrations were examined for all stability tests.Short-term stability test was carried out after keeping QC samples at 25°C for 24 h while long-term stability was determined after freezing QC samples at-80°C for 4 weeks.In addition,QC samples were stored at-80°C for 24 h and then thawed completely at 25°C for the freeze and thaw stability test.This cycle was repeated and analysis was conducted after third cycles.Stabilities of stock solutions of cefprozil diastereomers and IS were evaluated after storing them at-20°C for 4 weeks.To determine post-preparative stability,prepared QC samples were placed on table at 25°C for 24 h in HPLC-UV analysis and in an autosampler maintained at 15°C for 24 h in UPLC-ESI-MS/MS assay.As a result,all stabilities were determined as percent ratio of measured drug concentration to the initial drug concentration(n=5).Samples were considered stable if test values at each level were within±15% of the sample nominal concentration and the precision was less than 15%.
2.5.6.Carryover
Carryover test was performed by injecting a blank sample after injecting the highest concentration(20μg/mL for cis-cefprozil and 2.5μg/mL for trans-cefprozil)of the sample used for the standard curve.The acceptance criterion of the carryover was that the peak in the blank sample should be less than 20% of the one in the LLOQ sample.
2.5.7.Incurred sample reanalysis(ISR)
ISR was carried out to ensure the reproducibility of newly developed methods for the analysis of cefprozil diastereomers.Computerized random method was used to select samples(10% of analyzed samples)to be reanalyzed.The selection criteria of the samples for ISR were the samples near the maximum concentration(Cmax)and elimination phase in the PK pro file of the finally obtained cefprozil.As a result,forty-two samples from humans were reanalyzed and compared with initially analyzed values.Results should satisfy the acceptable criteria that variability between mean value of the initial analysis and that of the reanalysis was within±15%.In addition,reanalysis values for 67% of all samples should be within 20% of their initial values.
2.6.Application to the pharmacokinetic study
Thirty- five healthy male Korean subjects(age,24.00±1.51 years;body weight,69.46±10.01 kg;height,174.66±6.64 cm)were recruited for this clinical study.The Institutional Review Board of the Institute of Bioequivalence and Bridging Study,Chonnam National University,Gwangju,South Korea,approved the study protocol(Bioequivalence Test No.611;11.06.2007).This study was conducted in accordance with the revised Declaration of Helsinki for biomedical research involving human subjects and rules of Good Clinical Practice.All subjects provided written informed consent prior to participation.In addition,the subjects received a medical history,physical examination,and laboratory tests.As a result,each participant was healthy enough to participate in the clinical test.All subjects fasted more than 10 h before receiving 1000 mg cefprozil tablets and kept fasting for 4 h thereafter.They avoided beverages and foods containing xanthine.Each subject received cefprozil(4 tablets of 250 mg cefprozil)with 240 mL of water.Blood samples were drawn from forearm vein before administration(0)and at 0.5,0.75,1,1.25,1.5,1.75,2,3,4,8,and 12 h after oral administration of cefprozil.Blood samples were transferred to 10 mL Vacutainer®tubes(Becton,Dickinson and Company,Franklin Lakes,NJ,USA)and immediately centrifuged(10,000 g,10 min,4°C).Each collected plasma sample was placed in a polyethylene tube and stored at-80°C until further analysis.The maximum plasma concentration(Cmax)and the time to reach Cmax(Tmax)were individually calculated using plasma concentrationtime curve.The area under the curve(AUC0-∞)was integrated by a linear trapezoidal rule to the final measured concentration(Clast;AUC0-t)and extrapolated to infinity by adding area from Clastto infinity(Clast/k)(i.e.,AUC0-∞was calculated as the sum of AUC0-tand Clast/k,where k was the elimination rate constant at terminal phase).Half-life(t1/2)was calculated as 0.693/k and the volume of distribution(Vd/F)was calculated as dose/k·AUC0-∞.Clearance(CL/F)was calculated by dividing dose of cefprozil by AUC0-∞,where F was the bioavailability of oral administration.All PK parameters were subjected to noncompartmental analysis using WinNonlin®software version 8.1(Pharsight®,a Certara™ Company,Princeton,NJ,USA).
2.7.Comparison of pharmacokinetic parameters
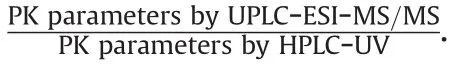
2.8.Determination of biochemical parameters
Plasma samples were used for determination of biochemical parameters including total proteins,albumin,ALP,AST,ALT,total bilirubin,cholesterol,BUN and creatinine.The used plasma samples were those obtained from the forearm vein prior to oral administration(as 0 h)of cefprozil.The determination of the main biochemical parameters in this study was performed in a dry automatic analyzer by microsides VITROS(Ortho Clinical Diagnostics,NJ,USA)operating by reflectance spectrophotometry.
2.9.Statistical analysis
Statistical methods have been used to Confirm the correlation between biochemical and PK parameter values.Pearson correlation is a statistical technique that describes the degree of linear association between two continuous quantitative variables normally distributed.Values between -1 and +1 indicate the strength(interpreting the coefficient value)and the direction(taking the sign of the coefficient)of linear association.The ‘+’symbol indicates a direct proportionality relationship between the correlated values and the ‘-’symbol,an inverse relationship.In addition,statistical analysis was performed on the PK parameters calculated by HPLC-UV and UPLC-ESI-MS/MS methods.All PK parameters determined by each quantification method were analyzed for statistical significance by Student's t-test with P<0.05,indicating a significant difference.The Statistical Package for the Social Sciences(SPSS)software version 23(IBM,NY,USA)was used for the statistical analysis.
3.Results and discussion
3.1.Method development
3.1.1.UPLC-ESI-MS/MS method
In this study,accurate UPLC-MS/MS method was newly developed for simultaneous quantitative analysis of cefprozil diastereomers in human plasma with improved sensitivity.Product ion mass spectra of cefprozil diastereomers resulted from scan mode after injecting individual standard solution into the mass spectrometer.As a result of both positive and negative ionizations,the observed response intensity was larger in negative ionization,similar to the result of a previous study[14].Of course,m/z 390 of cefprozil was Confirmed as a precursor ion even in positive ionization mode as in another report[15],but the sensitivity at negative ionization mode was about 3 times higher in our system.Perhaps it was because of different analytical conditions such as differences in mobile phase composition.Cefprozil diastereomers generated deprotonated molecular ion[M-H]-in negative ion mode.IS performed in scan mode also generated a deprotonated molecule ion[M-H]-like cefprozil diastereomers.Both the cefprozil isomer and cefaclor contained hydroxyl and carboxyl groups in the structure,making it easy to ionize into anions in the negative mode.The most abundant fragment ion for MRM was m/z 388.0→249.2 for cefprozil diastereomers and m/z 365.9→286.2 for IS.In the previous report[14],the product ion of cefprozil was Confirmed to m/z 249.2 and 310.1 as well as 205.0,but the quantification of cefprozil was performed at m/z 388.0→205.0.In this study,we found that cefprozil's product ion had better sensitivity at m/z 249.2 than 205.0,and by choosing it,we were able to significantly improve sensitivity.Fig.S1 presents the relevant mass spectra we obtained as mentioned above.Other ionization parameters that optimized simultaneous determination of cefprozil diastereomers included desolvation temperature of 250°C,collision energy of 13 eV,nebulizing nitrogen gas flow of 3 L/min,and drying nitrogen gas flow of 15 L/min.For cefprozil diastereomers,chromatographic separation was required because the same MRM transition was used to simultaneously analyze these two isomers.Therefore,different columns,various organic solvents(including extraction and precipitation solvents),and mobile phase with varying pH were tested to obtain an efficient peak separation with good sensitivity and high resolution.Acquity UPLCⓇBEH C18,Inertsil C8-3,Phenomenex KINETEX core-shell C18,and HALO-C18columns using 0.5%(V/V)formic acid in water containing 5 mM ammonium formate(pH 3.0)buffer and methanol by gradient elution at a flow rate of 0.3 mL/min were tested to obtain an optimized chromatogram.All these columns mentioned above have the same filling material(octadecyl,C18)as a stationary phase except Inertsil column(octyl,C8).However,the filling technique is slightly different for each manufacturer.There is also difference in the size of filled particles.HALO-C18column(100 mm × 2.1 mm,2.7μm)was employed because it showed relatively good sensitivity,selectivity,and symmetric peak shapes for simultaneous determination of cefprozil diastereomers.Above all,its separation of cefprozil isomeric peaks was perfect.In addition,there was no overlap with IS peak.The test results are shown in fig.S2A.Fig.S3A shows representative MRM chromatogram with moderate retention time of 2.69 min for cis-cefprozil,2.79 min for trans-cefprozil,and 2.39 min for IS.The retention time of trans-cefprozil was longer than that of cis-cefprozil.This might be because of its higher polarity.These results were consistent with the results of other studies[14,15].We attempted a mobile phase A(5% of 5 mM ammonium formate buffer added)pH condition with water containing 0.1%(V/V)formic acid(pH 2.5),0.2%(V/V)formic acid(pH 2.3),and 0.5%(V/V)formic acid(pH 2.0)for simultaneous determination of cefprozil diastereomers with HALO-C18column at a flow rate of 0.3 mL/min by gradient elution.It has been reported that adding formic acid to the mobile phase can improve the sensitivity of the cefprozil peak[14].As a result,a 0.5%(V/V)formic acid in water containing 5%(V/V)of 5 mM ammonium formate(pH 3.0)buffer displayed the best resolution with the highest intensity.The test results are shown in fig.S2B.As the concentration of formic acid in the mobile phase increased,the retention time of cefprozil in the reversed phase column increased,which resulted in the peak separation between the analytes.In addition,mobile phase B composed of methanol exhibited a higher sensitive response and a better resolution than that containing ACN as reported in another research[14].The test results are shown in fig.S2C.Finally,methanol(mobile phase B)and water containing 0.5%(V/V)formic acid containing 5%(V/V)of 5 mM ammonium formate(pH 3.0)buffer(mobile phase A)were optimized as mobile phase using gradient elution.Such gradient elution satisfied retention time,peak shape,and interference peaks for separating cefprozil diastereomers.In addition,5%(V/V)of 5 mM ammonium formate(pH 3.0)buffer(pH 2.0)kept the retention time of cefprozil diastereomers constantly.LLE and PP methods for sample preparation were tested.For LLE,ethyl acetate,MTBE,di-ethyl ether,and methylene chloride were attempted for extraction.For cefprozil diastereomers,ethyl acetate extracted the largest amount compared to methylene chloride,MTBE,and di-ethyl ether.PP method using methanol and ACN was also tested.Methanol showed higher sensitive response with lower noise than ACN.And methanol effectively reduced impurities such as lipids and proteins extracted by LLE,thereby reducing interference with endogenous substances.Thus,it was then chosen as the precipitation solvent for the PP method.Cefprozil diastereomers to be analyzed are acidic substances(pKa 3.5)[25].Thus,1% or 2% or 3%(V/V)formic acid was added to extraction solvent in order to suppress the ionization of cefprozil diastereomers and increase the transfer of them to the organic solvent layer.As a result,the best extraction efficiency was resulted from when 3%(V/V)formic acid was added to the extraction solvent.Therefore,PP method with methanol and LLE method with 3%(V/V)formic acid added to ethyl acetate were optimized for determination of cefprozil diastereomers in human plasma.In this UPLC-ESI-MS/MS method,the supernatant after extraction was evaporated to dryness under a gentle nitrogen stream at 40°C to improve the sensitivity of cefprozil diastereomers.This UPLC-ESIMS/MS method provided an improved sensitivity(an LLOQ of 0.005 μg/mL for cis-cefprozil and 0.015 μg/mL for trans-cefprozil)than that in a previous study(LLOQ value of 0.025-0.125 μg/mL for cis-cefprozil and 0.014-0.0403 μg/mL for trans-cefprozil)[14,15].
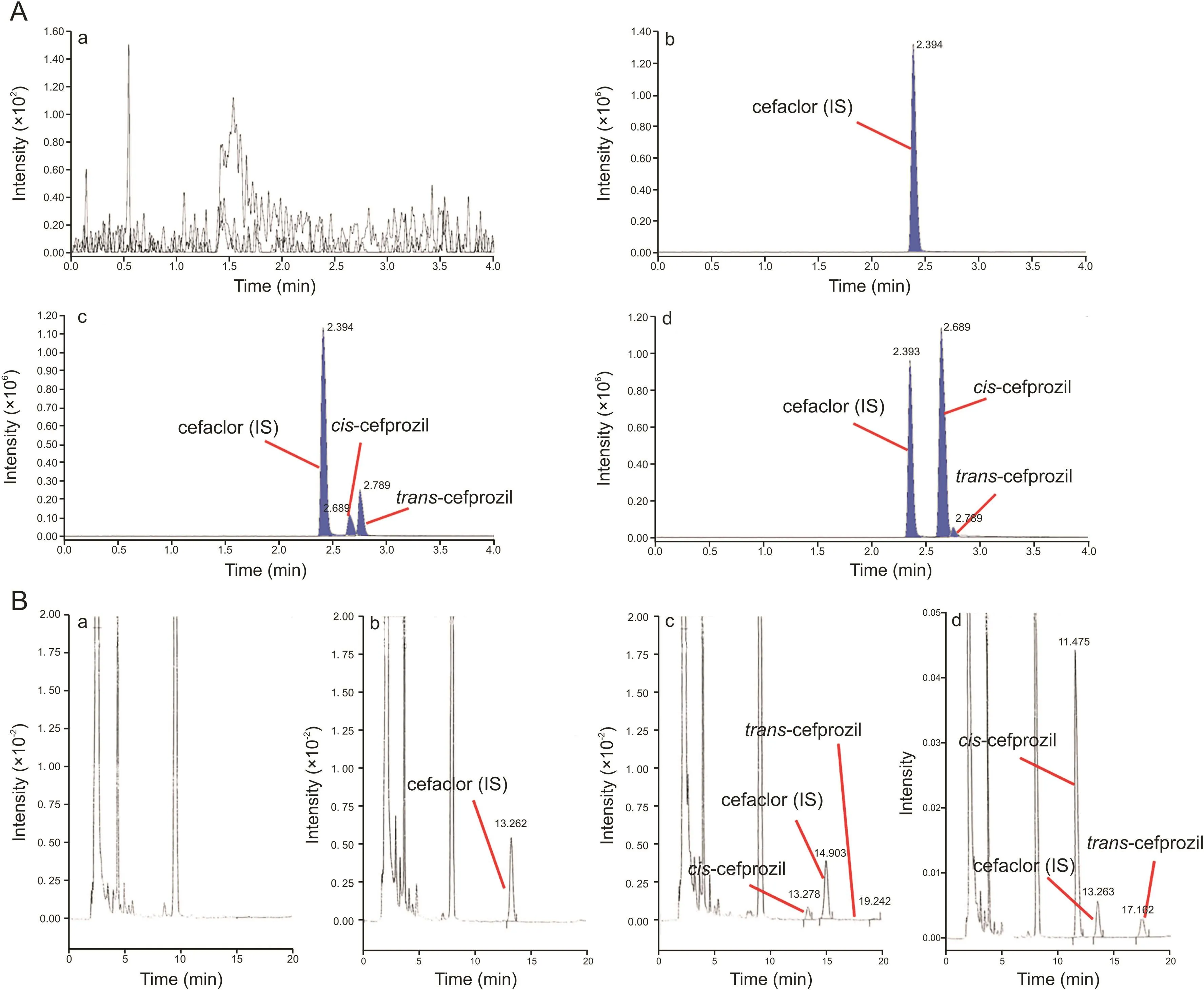
Fig.2.Chromatograms of blank plasma(a),zero plasma containing the IS(b),blank plasma containing LLOQ of cefprozil diastereomers and IS(c),the plasma sample at 1 h after oral administration of 1000 mg cefprozil tablet(d);(A)UPLC-ESI-MS/MS;(B)HPLC-UV.
3.1.2.HPLC-UV method
In HPLC-UV method,chromatographic conditions were obtained on a reversed phase Luna C8column(150 mm×4.6 mm,5μm).Peak shape,selectivity,and symmetry of cefprozil diastereomers in mobile phase consisting of 2%(V/V)aqueous acetic acid and ACN(95:5,V/V)at a flow rate of 1.0 mL/min were then determined.Fig.S3B shows representative HPLC chromatograms with moderate retention times of 11.47 min for cis-cefprozil,17.16 min for trans-cefprozil,and 13.26 min for IS.The HPLC-UV method was conducted using isocratic elution with a total run time of 20 min.Cefaclor was used as IS considering peak shape,retention time,and extraction efficiency from human plasma.PP method with 10%(V/V)TCA and ACN(with DCM)was optimized for the determination of cefprozil diastereomers in sample preparation based on reference related to cefprozil analysis[6].TCA,an acid reagent,was used to increase the extraction efficiency of cefprozil diastereomers(or to increase protein precipitation)in the same way as that for the UPLC-ESI-MS/MS method.The aqueous supernatant of 1 mL was evaporated to dryness under a gentle nitrogen stream at 40°C to improve the sensitivity of cefprozil diastereomers in this HPLC-UV method.
3.2.Quantitative method validation
3.2.1.Selectivity
Selectivity was determined in response of blank plasma,zero plasma containing the IS,blank plasma containing LLOQ cefprozil diastereomers and IS,and plasma sample at 1 h after oral administration of cefprozil tablets.Representative chromatograms are presented in fig.2.In both methods,there were no significant interferences from endogenous substances around retention times of analytes in blank plasma.In addition,in some previous reports,Park et al.[6]and Liu et al.[14]isolated cefprozil isomeric peaks using a reversed phase C8column on HPLC-UV and a C18column on LC-MS/MS,respectively.We also were able to isolate peaks of cefprozil diastereomers on a chromatogram by optimizing analytical conditions such as mobile phase composition,gradient elution,and using an HPLC C8or a UPLC C18column.
3.2.2.Calibration curves
Linearity for cis-cefprozil in human plasma was excellent over concentration range of0.1-20 μg/mL for HPLC-UV and 0.005-20 μg/mL for UPLC-ESI-MS/MS.Trans-cefprozil also showed excellent linearity over concentration range of 0.02-2.5 μg/mL for HPLC-UV and 0.015-2.5μg/mL for UPLC-ESI-MS/MS.All calibration curves fitted well,with correlation coefficient(r2)exceeding 0.99.Linear regression equations of cis-cefprozil in human plasma were as follows:y=(0.5010±0.0231)x+(0.0115±0.0012)for HPLC-UV and y=(0.1043±0.0072)x+(0.0101±0.0043)for UPLC-ESI-MS/MS.For trans-cefprozil in human plasma,they were as follows:y=(0.5992±0.0314)x+ (0.0219±0.0014)for HPLC-UV and y=(0.1482±0.0125)x+(0.0096±0.0010)for UPLC-ESI-MS/MS,where y is peak-area ratio of each cefprozil isomer to IS and x(μg/mL)is plasma concentration of cefprozil isomer.The developed UPLC-ESI-MS/MS and HPLC-UV methods provided LLOQ of 0.005μg/mL and 0.1μg/mL,respectively,for cis-cefprozil.LLOQ for trans-cefprozil by UPLC-ESI-MS/MS and HPLC-UV methods were 0.015 μg/mL and 0.02 μg/mL,respectively.Such LLOQs were sufficient for PK study after oral administration of cefprozil tablets in humans.Although cefprozil dose was as high as 1000 mg and the resulting Cmaxvalue was expected to be sufficiently large,cefprozil was rapidly removed from the blood.Therefore,it is important to lower the LLOQ value sufficiently to obtain a clear PK for postelimination phase.HPLC-UV analysis performed previously[6]has shown that the Cmaxvalue of cefprozil is high(approximately 18.8μg/mL).However,more than 99% of the dose was eliminated from the blood within 12 h.A previous LC-MS/MS analysis[14]showed the same result.It quantified below LLOQ for some samples that corresponded to the early phase of drug absorption and terminal phase of elimination.Thus,it would be necessary to obtain a clear PK pattern in the body by lowering the LLOQ.
3.2.3.Accuracy and precision
During validation,good performance with consistent accuracy and low deviation was investigated using four QC samples.Table 1 presents inter-and intra-batch accuracy and precision for cefprozil diastereomers.Intra-batch accuracies for cis-cefprozil and transcefprozil by HPLC-UV and UPLC-ESI-MS/MS ranged from 95.67% to 104.58% and from 96.17% to 107.50% with precision(CV)of<8.88% and<6.98%,respectively.Inter-batch accuracies for cis-cefprozil and trans-cefprozil by HPLC-UV and UPLC-ESI-MS/MS ranged from 96.67% to 106.75% and from 97.39% to 106.89% with precision(CV)of<6.88% and <7.82%,respectively.All CV values of cefprozil diastereomers ranged from 1.64% to 8.88% and the accuracy ranged from 95.67% to 107.50%,suggesting that both methods were reproducible and accurate for determination of cefprozil diastereomers in human plasma.
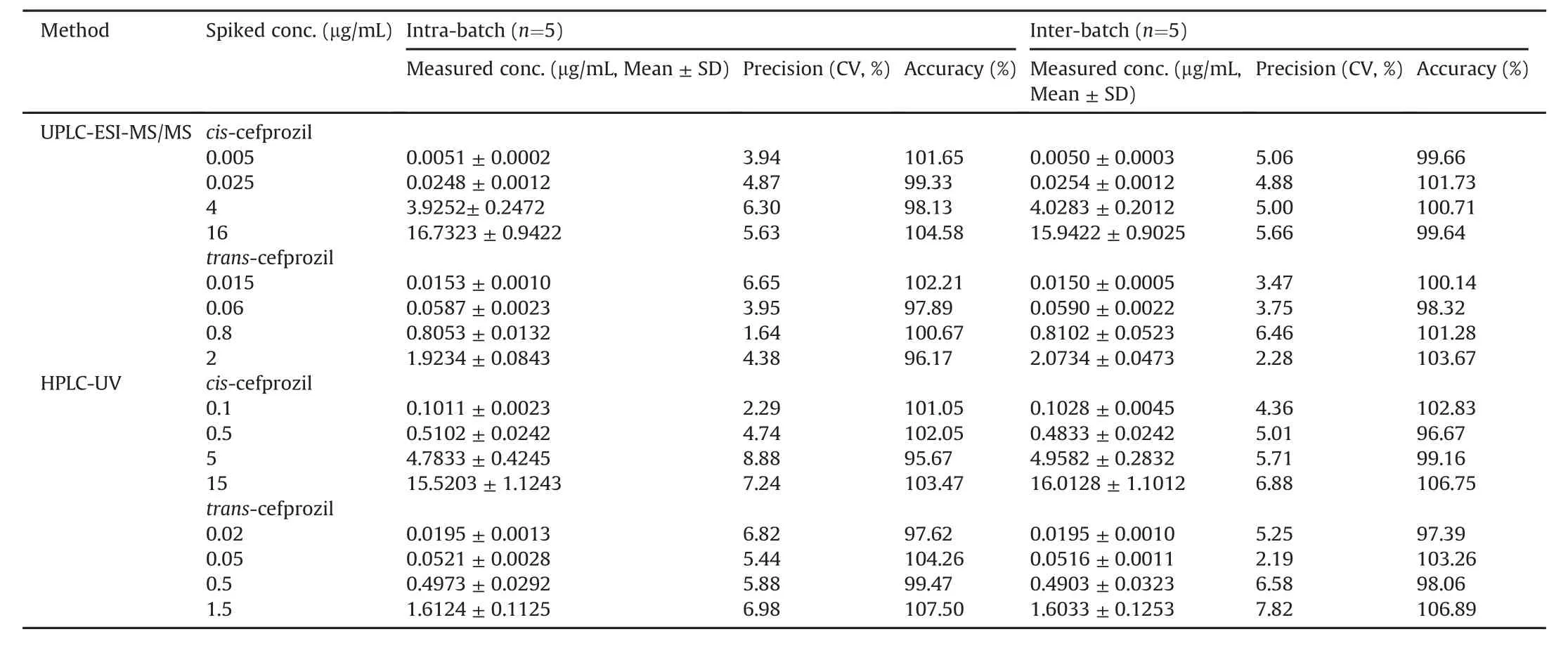
Table 1 Precision and accuracy of UPLC-ESI-MS/MS and HPLC-UV analysis for the determination of cefprozil diastereomers in human plasma(Mean±SD,n=5).
3.2.4.Recovery and matrix effect
Table 2 presents recoveries and/or matrix effect for cefprozil diastereomers in HPLC-UV and UPLC-ESI-MS/MS analyses.Extraction recoveries for cis-cefprozil and trans-cefprozil from human plasma were 88.48%-90.64% and 89.88%-90.30%,respectively,with UPLC-ESI-MS/MS method.They were 87.58%-88.92% and 86.49%-88.27%,respectively,with HPLC-UV method.Recoveries of IS were 90.11%±2.95% and 87.30%±3.11% with UPLC-ESI-MS/MS and HPLC-UV methods,respectively.There were no significant matrix effects in the detection of cefprozil diastereomers(cis-cefprozil:97.99%-100.94%,trans-cefprozil:98.21%-100.01% for UPLCESI-MS/MS)or IS(99.06%±2.58% for UPLC-ESI-MS/MS).These results indicated that the extent of analyte recoveries was consistent,precise,and reproducible.Such simple PP and/or LLE procedures were then successfully applied to the determination of cefprozil diastereomers in human plasma.
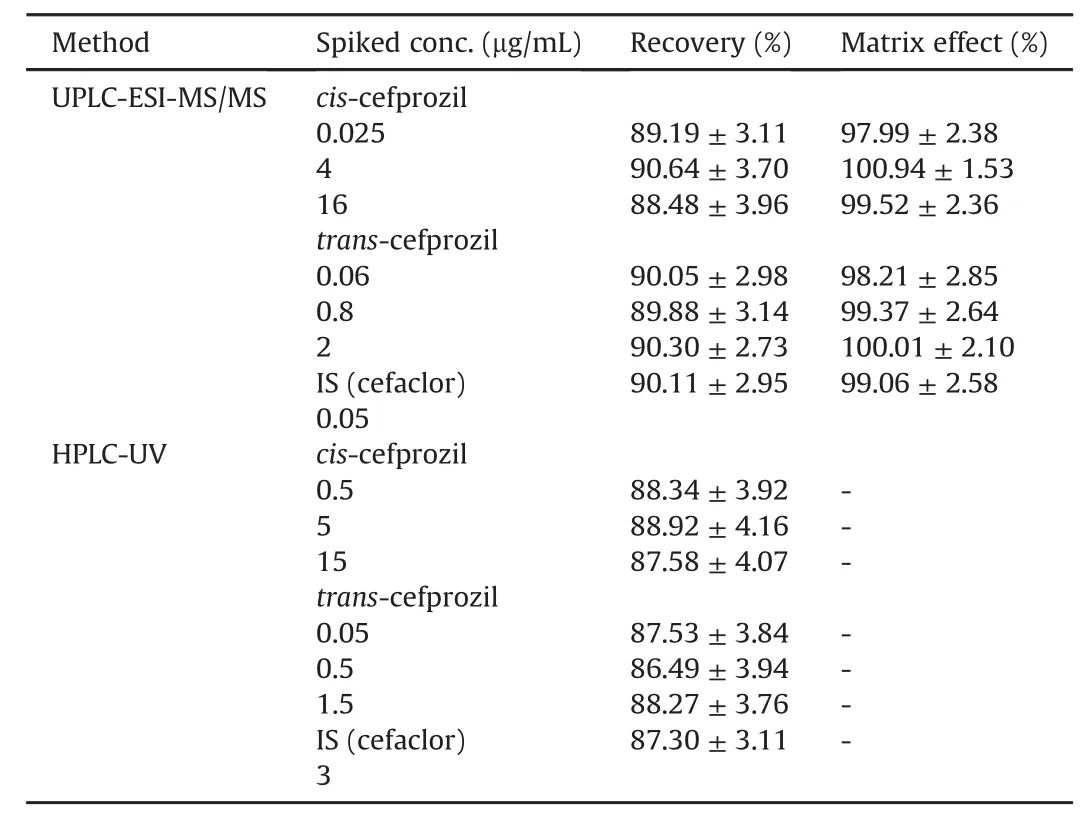
Table 2 Recovery and matrix effect for the determination of cefprozil diastereomers in human plasma by both methods(Mean±SD,n=5).
3.2.5.Stabilities
In both methods,the stability for cefprozil diastereomers was examined using two different levels of QC samples under various conditions.Stabilities assessed included short-and long-term stability,and freeze-thaw stability.Results are presented in Table 3.These cefprozil diastereomers were stable in human plasma at 25°C for 24 h without any significant degradation(stabilities ranged from 99.19% to 100.24% for both methods).In the long-term stability test after storage at-80°C for 4 weeks,cefprozil diastereomers were stable.This guaranteed quantitation quality after sample collection within 4 weeks(stabilities ranged from 97.90% to 100.46% for both methods).In the freeze-thaw cycle test,all analytes were stable after three cycles(stabilities ranged from 98.19% to 100.21% for both methods).In post-preparative stability,cefprozil diastereomers were stable with stability ranging from 98.97% to 100.11% based on the UPLC-ESI-MS/MS method at 15°C for 24 h and from 99.24% to 101.03% based on the HPLC-UV method at 25°C for 24 h.Finally,ranges were within limits of the guidelines of the FDA(±15%)for all stability tests.After assessing the stability of stock solutions for cis-cefprozil,trans-cefprozil,and IS,all were stable in storage concentration at-20°C for 4 weeks.Stabilities of cis-cefprozil and trans-cefprozil stock solutions were 100.22%±3.03% and 99.71%±2.95%,respectively.The stability of IS stock solution was 100.05%±2.14%.These results demonstrated stabilities of cefprozil diastereomers under different types of storage conditions.
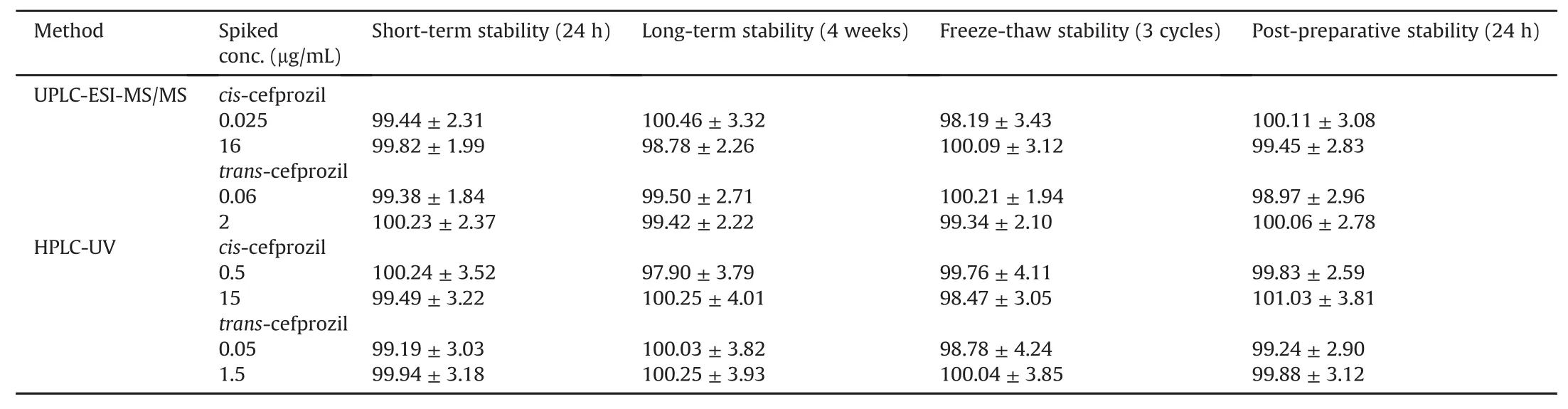
Table 3 Stabilities of cefprozil diastereomers under various conditions using UPLC-ESI-MS/MS and HPLC-UV quantification methods(Mean± SD,n=5).
3.2.6.Carryover
As presented in fig.2(a),there was no clear visible peak of analyte in the blank plasma sample after injecting the highest concentration sample of the standard curve.Thus,carryover would not influence the analysis.
3.2.7.ISR
Forty-two(10% of total analyzed samples)clinical plasma samples were used to evaluate ISR.In both methods,variability of all plasma samples was within 20% between the value of initial analysis and that of the reanalysis.In addition,thirty-six plasma samples were within 10% for UPLC-ESI-MS/MS and thirty-four plasma samples were within 10% for HPLC-UV method.Therefore,these newly developed methods showed reproducibility for their initial analysis results.In other words,our developed methods have been fully validated against ISR,unlike the previous reports,and sufficient reproducibility has been Confirmed.
3.3.Comparison of methods
Individual HPLC-UV and UPLC-ESI-MS/MS methods have been developed for simultaneous quantification of cefprozil diastereomers in human plasma(and for comparison study).Table S1 summarizes the information of both methods.The UPLC-ESI-MS/MS method was found to be 20 times more sensitive than HPLCUV method for quantification of cefprozil diastereomers.Highly sensitive UPLC-ESI-MS/MS method is required for in vivo models in order to quantify very low levels of analytes in large numbers of biological samples.As a result,PK pro file showing absorption phase and elimination phase could be obtained more clearly.In addition,UPLC-ESI-MS/MS method requires less sample volume and much less organic solvent for sample preparation.Moreover,the time required for total sample analysis is much shorter.It is therefore more environmentally friendly and economical than HPLC-UV method.However,UPLC-ESI-MS/MS is usually more expensive than HPLC-UV which has a high operating cost.Although the run time was 20 min and the HPLC-UV method was five times longer,the HPLC-UV method was shorter than the UPLC-ESI-MS/MS method in sample preparation process.Therefore,HPLC-UV method may be more economical if the total number of samples to be analyzed is small.In addition,if plasma concentration levels fall into the range of micrograms,HPLC-UV method can be used with high precision and accuracy[26].The reason for the difference in plasma sample preparation between UPLC-ESI-MS/MS and HPLC-UV methods is that the extraction solvent composition for each analytical instrument was developed and applied.Extraction solvents applied to HPLC-UV suspected significant matrix effects inUPLC-ESI-MS/MS and recovery was not satisfactory.In other words,it was optimized and applied to UPLC-ESI-MS/MS with an extraction solvent different from HPLC-UV in consideration of the matrix effect and recovery for UPLC-ESI-MS/MS.In addition,chromatographic conditions(including columns and mobile phases)are also optimized for each analyzer.That is,since there are already differences between the analyzers of HPLC-UV and UPLC-MS/MS,the differences in the analytical conditions(including columns and mobile phases)were inevitable.Table S2 summarizes the previously reported methods(already mentioned in Section 1.Introduction and included in the reference lists)of cefprozil analysis[6,7,9-15].Regarding the development of UPLC-ESI-MS/MS method for cefprozil diastereomers,which we focused on in this study,we have improved sensitivity over the previous methods and enabled biometric analysis through relatively simple sample preparation.Furthermore,the newly developed methods were finally applied to a large number of biological samples analyzes to obtain reliable PK parameter values.Up to our best knowledge,this was the first time to report the quantification of cefprozil diastereomers by UPLC-ESI-MS/MS in a large number of biological samples.According to Churchwell et al.[27],overall,the UPLC can offer significant improvements in sensitivity,speed,and resolution compared to HPLC.Therefore,development of UPLC-ESI-MS/MS method is expected to increase the utilization in related PK studies and clinical practice in the future.
Fig.3 presents a two-dimensional graph of points taken from the same human plasma samples.The linear regression line of the graph was as follows:y=0.99488x+0.07695 for cis-cefprozil;y=0.99619x+0.02472 for trans-cefprozil;and y=0.99306x+0.08368 for total cefprozil.Slopes of these regression lines were all close to 1 and all correlation coefficients(r2)were more than 0.98,indicating a very reliable and highly correlated relationship.In HPLC-UV method,11 samples(cis-cefprozil,2.62% of total)and 36 samples(trans-cefprozil,8.57% of total)were below LLOQ.However,all plasma samples were quantified in UPLC-ESI-MS/MS method.These results are presented in fig.3 with points where the x-axis is 0 and the y-axis is in the range of 0.005-0.1.Of a total 420 points,412 points for cis-cefprozil and 410 points for transcefprozil were within 95% prediction intervals.This is a statistical value corresponding to 97.62%.Fig.4 presents the difference between the two analytical methods(HPLC-UV and UPLC-ESI-MS/MS)depending on the concentrations measured by UPLC-ESI-MS/MS.The linear regression line of the graph was as follows:y=0.0184x+0.0212 for cis-cefprozil;y=0.0093x+0.0101 for trans-cefprozil;and y=0.0205x+0.0238 for total cefprozil.The slopes of the regression line were close to zero,indicating that the difference between the two methods is not significant.
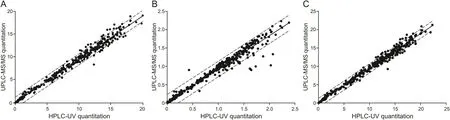
Fig.3.Comparison of sample analysis results using HPLC-UV(x-axis)and UPLC-ESI-MS/MS(y-axis)quantification method.Straight line represents the linear regression line and dashed line shows 95% prediction interval line.(A)cis-cefprozil;(B)trans-cefprozil;(C)total cefprozil.
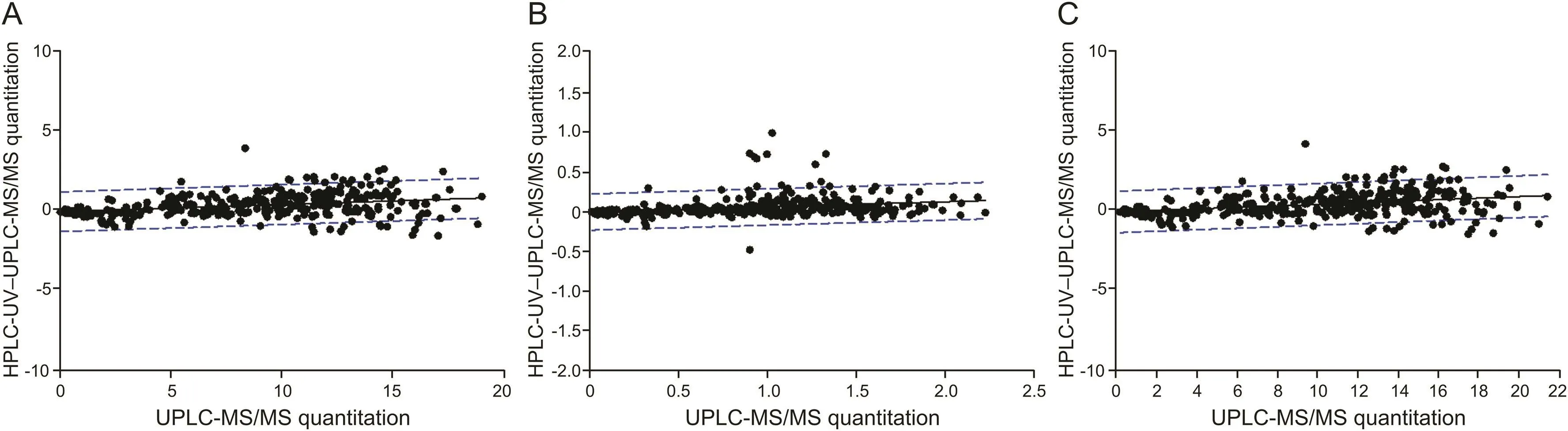
Fig.4.Correlation of the method differences between UPLC-ESI-MS/MS(x-axis)and HPLC-UV-UPLC-ESI-MS/MS(y-axis).Straight line represents the linear regression line and dashed line shows 95% prediction interval line;(A)cis-cefprozil.(B)trans-cefprozil;(C)total cefprozil.
3.4.Pharmacokinetic study
We conducted an effective PK study by quantifying the cefprozil(both cis-and trans-cefprozil)concentrations in the blood by single oral administration of the maximum daily dose(as cefprozil 1000 mg)in humans.As a result,cefprozils(both cis-and transcefprozil)in the blood were quantified at all sampling points(when analyzed by UPLC-ESI-MS/MS method)and clear PK parameter values were calculated.The two newly validated HPLC-UV and UPLC-ESI-MS/MS methods were applied to PK study of cefprozil diastereomers after oral administration of 1000 mg cefprozil tablets in 35 healthy Korean subjects.Plasma concentration-time curves of cefprozil diastereomers in human subjects obtained through HPLCUV and UPLC-ESI-MS/MS quantification methods are presented at Fig.5.The PK parameters(containing CL/F,Vd/F,t1/2,AUC0-∞,AUC0-t,Cmax,and Tmax)obtained from the two assays are listed in Table 4.Here,we used the cis-and trans-cefprozil isomer doses of 900 and 100 mg,respectively,to calculate PK parameters such as CL/F and Vd/F of cefprozil diastereomers.Interestingly,PK values of CL/F,t1/2,and Vd/F were similar between cis-and trans-cefprozil isomers.In addition,difference of Tmaxvalue was within 5%.These results indicated that cis-and trans-cefprozil had similar PK patterns after oral administration,although there are structural differences as diastereomers.These results on PK similarity of cefprozil diastereomers were identical to those reported in previous researches[6,14,28].In addition,PK parameters were also similar to those reported in previous studies[6,14].In particular,when analyzing human plasma samples by the LC-MS/MS method in a previous study[14],Tmaxand CL/F values were 1.5 h and 15.64 L/h,respectively,similar to our results(1.97±0.66 h and 16.41±4.35 L/h,respectively).The value of t1/2was 1.25 h in the previous study[14],similar to our result(1.66±0.12 h).When analyzing human plasma samples by HPLC-UV method in a previous study[6],Tmax,t1/2,AUC0-∞,and Cmaxvalues were similar to our results,with differences<5%.As shown in fig.5,the appearance of two peaks(in both cis-and trans-cefprozil)near Cmaxis thought to be related to the absorption of the formulation.In other words,four tablets(250 mg cefprozil per tablet)were administered orally in this study and are thought to be related to elution from these tablets and double absorption in the gastrointestinal tract.However,to clarify this reason,further research such as in vitro studies may be needed in the future.
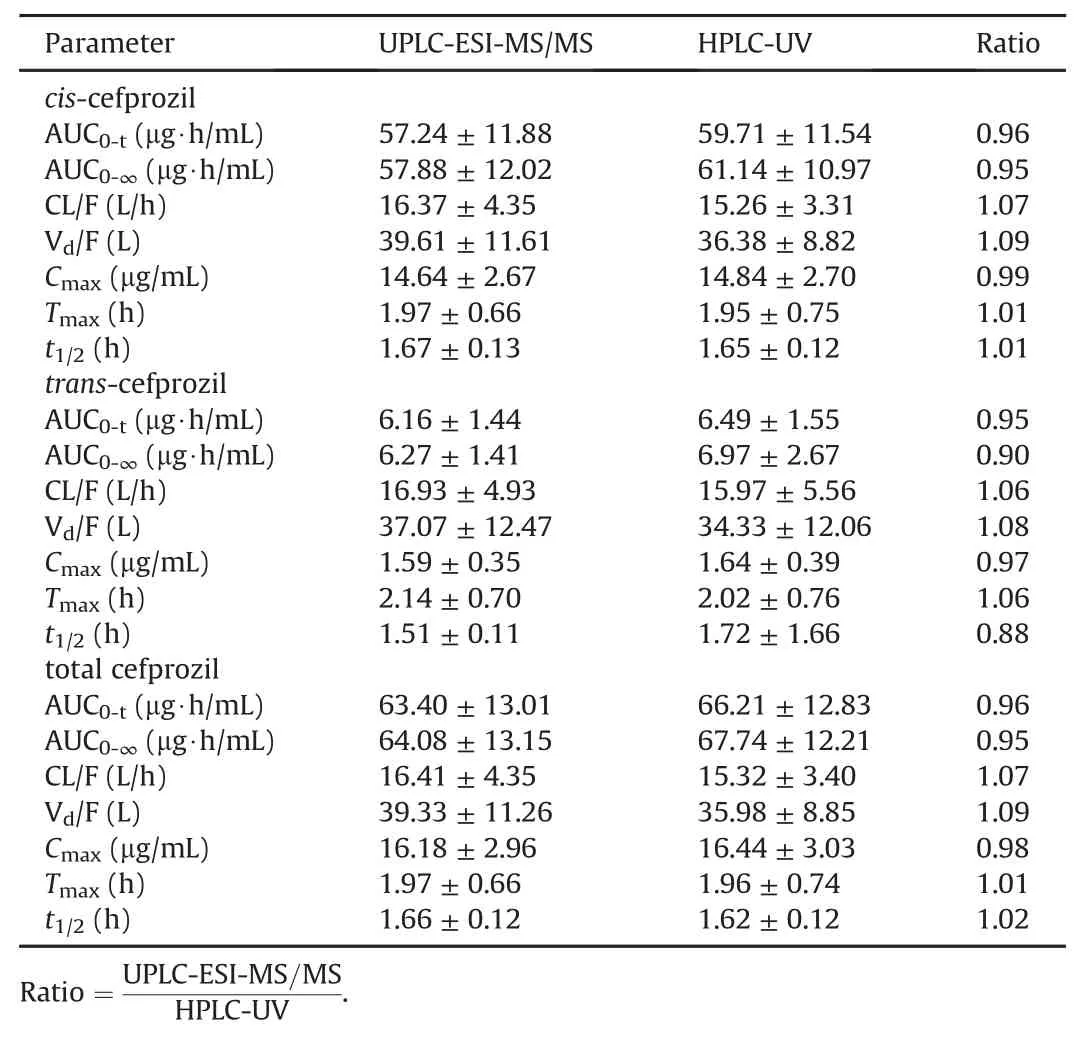
Table 4 Pharmacokinetic parameters for cefprozil diastereomers in humans after oral administration of 1000 mg cefprozil tablet with UPLC-ESI-MS/MS and HPLC-UV quantification methods(Mean±SE,n=35).
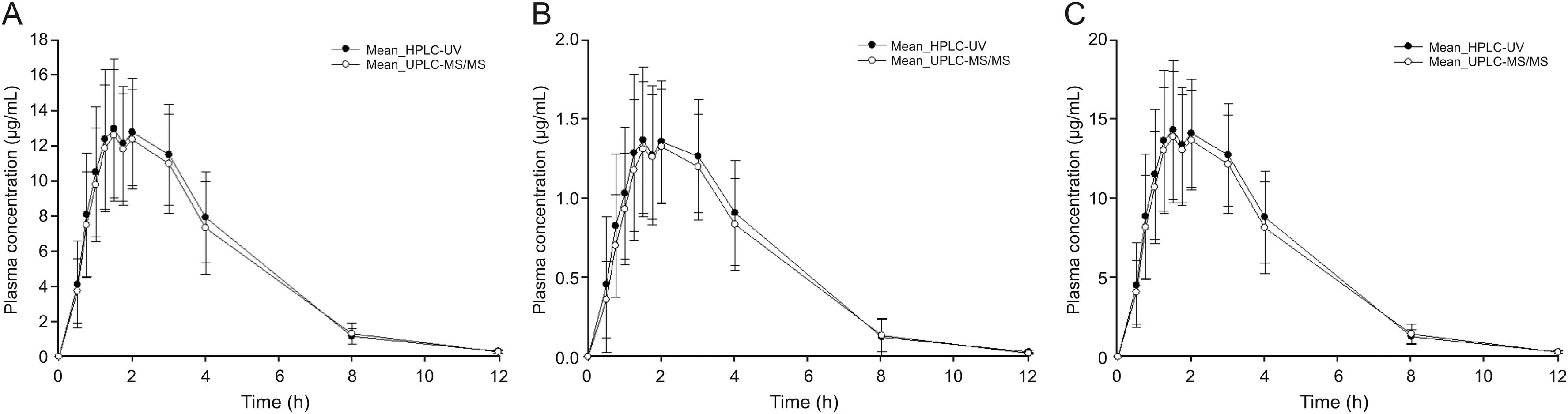
Fig.5.Mean plasma concentration-time pro files of cefprozil diastereomers after oral administration of 1000 mg cefprozil tablet according to UPLC-ESI-MS/MS and HPLC-UV quantification method.(A)cis-cefprozil;(B)trans-cefprozil;(C)total cefprozil.Vertical bars represent standard deviation of the mean(n=35).
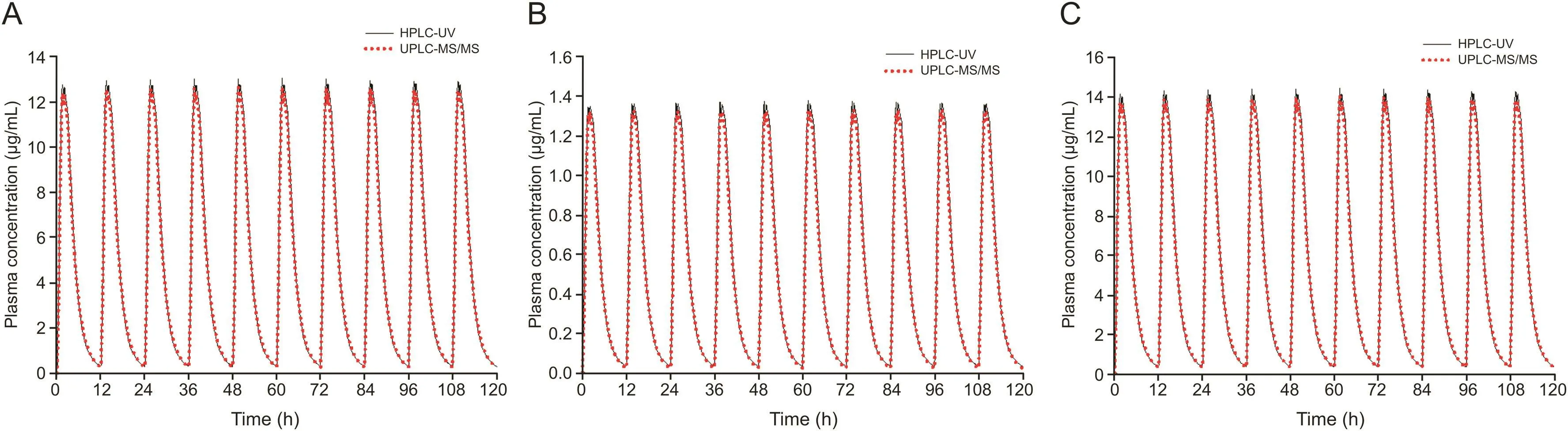
Fig.6.Simulation(mean value)graphs of multiple doses based on single dose(mean)data of cefprozil diastereomers obtained using HPLC-UV and UPLC-ESI-MS/MS.(A)ciscefprozil;(B)trans-cefprozil;(C)total cefprozil.
3.5.Comparison of pharmacokinetic parameters
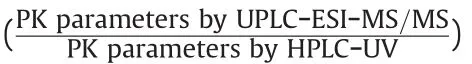
In addition,we simulated cis-,trans-,and total cefprozil plasma concentrations at multiple doses based on single dose data from each assay.This simulation assumed that 900,100,and 1000 mg doses of cis-,trans-,and total cefprozil are administered at 12 h intervals,similar to clinical dosing of twice daily[1].Multiple dose simulations were performed by WinNonlin®software version 8.1.As shown in fig.6,the simulated PK graphs of cis-,trans-,and total cefprozil did not differ significantly.Fig.7 is the estimated PK graphs of cis-,trans-,and total cefprozil from 0 to 12 h at steady state.A double peak pattern in fig.7 may be attributed by the PK results of single dose(Fig.5).Steady-state(mean)plasma concentrations predicted by each assay were approximately 4.82-5.10 μg/mL(for cis-cefprozil),0.52-0.58 μg/mL(for trans-cefprozil),and 5.34-5.65 μg/mL(for total cefprozil)with little difference.Table 5 presents the estimated PK parameters of cis-,trans-,and total cefprozil at steady state after oral multiple administration of 900,100,and 1000 mg doses of cis-,trans-,and total cefprozil.As shown in Table 5,the estimated PK parameters did not differ significantly between the two methods.In both HPLC-UV and UPLC-ESI-MS/MS methods,estimated PK parameter results(excluding the predicted Cminvalue of trans-cefprozil at multiple doses)and graph shapes for cefprozil diastereomers showed similarity.This result will be very important considering the fact that the use of LC-UV/DAD is more widely available in hospital laboratories and PK study sites.However,the(mean)predicted Cminof trans-cefprozil at steady state was 0.05 μg/mL(by HPLC-UV method)and 0.02 μg/mL(by UPLCESI-MS/MS method),which were significantly different(P<0.05).In addition,there was a significant difference(P<0.05)in the ratio of Cmax/Cmin(of trans-cefprozil)to 33.81(mean)and 79.50(mean)for HPLC-UV and UPLC-ESI-MS/MS methods,respectively.The ratio of Cmax/Cminwas about 2 times larger than that of HPLC-UV in the UPLC-ESI-MS/MS method.This suggests that if we use the HPLC-UV method for the calculation of trans-cefprozil PK parameters,we will be apt to underestimate the toxicity or safety of trans-cefprozil.Since HPLC-UV predicts higher Cminof trans-cefprozil than UPLCESI-MS/MS,it can be judged that the current dose is appropriate even though the actual blood drug concentration is not sufficient.The main reason for the difference in PK parameter values of transcefprozil was probably related to the low plasma concentration of trans-cefprozil.In other words,there is a difference in the LLOQ value according to the analytical method,which suggests that it may cause a difference in the PK parameter estimation of multiple doses by affecting the analysis values of the initial absorption phase and elimination phase of the drug.Our findings suggest that the selection of assay for biosamples is critical for PK analysis and interpretation(including clinical dose and regimen setting).
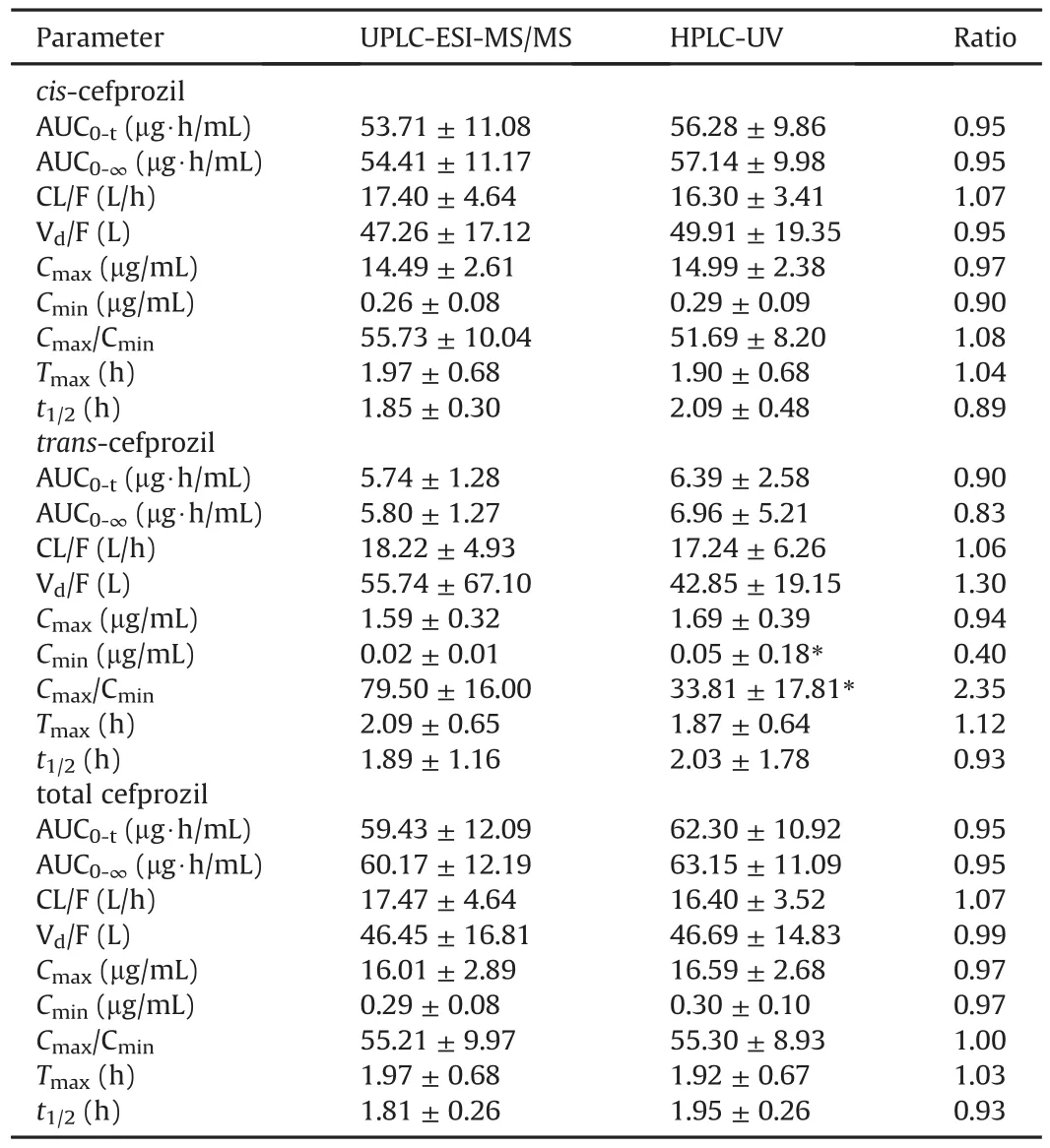
Table 5 Estimated pharmacokinetic parameters of cis-,trans-,and total cefprozil in humans at steady state after oral multiple administration of 900,100,and 1000 mg doses of cis-,trans-,and total cefprozil with UPLC-ESI-MS/MS and HPLC-UV quantification methods(Mean±SE,n=35).
Fig.7.Estimated steady-state(mean value)graphs at multiple doses based on single dose(mean)data of cefprozil diastereomers obtained using HPLC-UV and UPLC-ESI-MS/MS.(A)cis-cefprozil;(B)trans-cefprozil;(C)total cefprozil.
3.6.Analysis of correlation between biochemical and pharmacokinetic parameters
Pearson correlation is a measure of the linear correlation(dependence)between two variables X and Y,giving a value between+1 and-1 inclusive,where 1 is total positive correlation,0 is no correlation,and-1 is total negative correlation.in fig.8,the Pearson correlation coefficients were obtained by calculating using statistical software mentioned above and is a measure of the correlation between the two variables(biochemical and PK parameters).
As a result,we could not Confirm that there is a clear linear relationship between biochemical and PK parameters.This is probably because our studies were only for healthy male subjects and their age was limited to 20-30.However,creatinine,a representative biochemical parameter of renal function,and ALP,ALT,and AST,which are representative biochemical parameters of liver function,showed the same pattern in correlation with PK parameters.In other words,reduction of renal and liver function responsible for elimination and metabolism in the body will contribute to increasing AUC by decreasing clearance and increasing half-life of cefprozil.This was consistent with what was reported in previous studies on metabolism and excretion of cefprozil[4,29,30].Therefore,even if only a small(potential)correlation was Confirmed in this study,it would be necessary to conduct a correlation study between biochemical and PK parameters in more diverse and large population groups in the future.
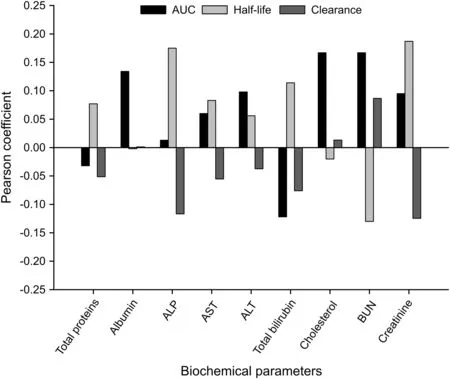
Fig.8.Pearson correlation coefficients between biochemical and pharmacokinetic parameters.
4.Conclusion
For this study,HPLC-UV and UPLC-ESI-MS/MS methods have been newly developed and validated for the simultaneous quantification of cefprozil diastereomers in human plasma.Our results demonstrate that these developed analysis methods are precise,accurate,reproducible,selective,and relatively impervious to endogenous interference.
We compared PK parameters obtained using two newly developed assays and Confirmed the impact and significant correlations between PK parameter values and bioanalytical methods that are the basis of PK studies.Our study is expected to be a guide for judging whether there is a significant difference in PK interpretation of cefprozil as a result of applying HPLC-UV and UPLC-ESI-MS/MS,which are commonly used in PK studies.
In addition,we Confirmed there is no significant correlation,but a potential relationship between some biochemical parameters(creatinine,ALP,ALT,and AST)and PK parameters(AUC,half-life,and clearance).Our results are expected to increase the utilization in related PK studies and clinical in the future.Furthermore,this relationship study between biochemical and PK parameters could be useful for future population PK studies of cefprozil.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.07.001.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
