A DNA-based nanocarrier for efficient cancer therapy
2021-07-20MuhmmdAsMirzMuhmmdFrnAshrfBigYlingZhngYuShunYngSongyuWuYiqioHuZhongChngWngHiLingZhu
Muhmmd As,Mirz Muhmmd Frn Ashrf Big,Yling Zhng,Yu-Shun Yng,Songyu Wu,Yiqio Hu,c,*,Zhong-Chng Wng,**,Hi-Ling Zhu,***
aState Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing,210023,PR China
bState Key Laboratory of Analytical Chemistry for Life Sciences,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing,210023,PR China
cInstitute of Drug Research and Development,Medical School of Nanjing University,Nanjing,210093,PR China
Keywords:
DNA-nanothread
Cisplatin
Drug delivery
Scavenger receptors
Caveolae
HeLa cell line
A B S T R A C T
The study aimed to achieve enhanced targeted cytotoxicity and cell-internalization of cisplatin-loaded deoxyribonucleic acid-nanothread(CPT-DNA-NT),mediated by scavenger receptors into HeLa cells.DNA-NT was developed with stiff-topology utilizing circular-scaffold to encapsulate CPT.Atomic force microscopy(AFM)characterization of the DNA-NT showed uniformity in the structure with a diameter of 50-150 nm and length of 300-600 nm.The successful fabrication of the DNA-NT was Confirmed through native-polyacrylamide gel electrophoresis analysis,as large the molecular-weight(polymeric)DNA-NT did not split into constituting strands under applied current and voltage.The results of cell viability Confirmed that blank DNA-NT had the least cytotoxicity at the highest concentration(512 nM)with a viability of 92% as evidence of its biocompatibility for drug delivery.MTT assay showed superior cytotoxicity of CPT-DNA-NT than that of the free CPT due to the depot release of CPT after DNA-NT internalization.The DNA-NT exhibited targeted cell internalizations with the controlled intracellular release of CPT(from DNA-NT),as illustrated in confocal images.Therefore,in vitro cytotoxicity assessment through flow cytometry showed enhanced apoptosis(72.7%)with CPT-DNA-NT(compared to free CPT;64.4%).CPT-DNA-NT,being poly-anionic,showed enhanced endocytosis via scavenger receptors.
1.Introduction
Cancer is among the top causes of mortality and morbidity throughout the world,with millions of new cases reported each year[1].There are several methods used to treat cancer,each one presenting with unique benefits and side effects.Platinum-based therapies,such as cisplatin(CPT),are frequently used in various types of cancers[2-4].CPT inhibits cancer cell growth by interfering with the transcription of the DNA in the cell.This inhibition of cellular DNA transcription causes the cell to undergo apoptosis,also known as cellular death[5].CPT is often used as a first or second-line drug to treat testicular,bladder,lung,cervical,and ovarian cancers[6-9].However,its use is severely limited by systemic toxicity,and this is mostly due to kidney damage[10].Since CPT is a mainstay in therapies for multiple types of malignancy,it is worthwhile enhancing its efficacy while decreasing its toxic side effects[11].
Most of the approved dosage forms lack robust targeting,exhibiting passive delivery of active ingredients into the tumor cells,reducing the therapeutic efficacy,and increasing toxicity.However,the development of effective and safe vehicles for cytotoxic drug delivery into targeted tumor cells remains a major challenge in modern medicine.Over the past 20 years,the nanoparticle-based delivery system has gained significance,and various materials like liposomes,inorganic materials,and organic polymers have been explored to formulate nanoparticles[12,13].One such way to enable active transportation and decrease systemic toxicity is improving the route of drug delivery to the tumor site.If the body is exposed to less of the drug while the tumor site is specifically exposed more to the drug,this will both increase efficacy and decrease toxicity.
DNA nanotechnology was first introduced by Tørring and Gothelf[14],and it has served as a robust platform for different applications.DNA is an excellent material to attain the desired shapes of nanoparticles due to its complementarity based on the Watson-Crick model and ease of fabrication.Different DNA strands can be combined and self-assembled into predesigned shapes[14].So the DNA-based materials can be engineered to attain specific properties for various potential applications,including biosensing,drug delivery to targeted sites,as well as in nano-and microelectronics[14-16].
There have been many nanoscale systems used to develop drug delivery systems,but the DNA-nanothread(DNA-NT)based construction has several advantages in comparison with other methods.The DNA-NT based structure has the same physical properties,including size,shape,and charge for each particle[2,17-25].DNA-NT is used to surround the drug and protect it from the environment in the cell culture medium(and body fluids),allowing the drug more time to be delivered into the cancer cells(and tumor site)[6,26,27].
Recently,Zhao et al.[19]reported that doxorubicin-loaded DNA origami nanostructure presents an efficient delivery system with a high level of internalization and excessive programmed cell death in breast cancer cells.
To the best of our knowledge,our group is the first to report the delivery of CPT via DNA-NT.This DNA-NT technology uses base pairing to design the delivery carrier for CPT loading.DNA-NT,as polyanionic ligand,activates the scavenger receptors(SRs)on the cell surface,resulting in the endocytosis of the DNA-NT through clathrin/caveolin endocytic pathway as illustrated in fig.1.We were able to increase the efficacy of the drug and to minimize the cytotoxicity.
2.Materials and methods
2.1.Chemicals
DNA oligomers were purchased from Sangon Biotech Co.,Ltd.(Shanghai,China).CPT(99% purity)was purchased from Aladdin,China.3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide(MTT)(98% purity)was bought from Sigma-Aldrich Co.,Ltd.(St.Louis,MO,USA).Human cervical cancer(HeLa)cells were obtained from Nanjing University,China.
2.2.Instruments
Instruments used were FastScan AFM(Bruker USA),FACS Calibur flow cytometer(Becton Dickinson,San Jose,CA,USA),A microtiter plate reader(ELx 800,BioTek,USA),Nanodrop 1000c(Thermo Fischer Scientific,USA),UV-spectroscopy/instrument(Thermo Fischer Scientific,USA),Gel apparatus(Thermo Fischer Scientific,USA),and polyacrylamide gel electrophoresis(PAGE)apparatus(BioRad,Hercules,CA,USA).

Fig.1.Schematic illustration showing the cellular uptake of cisplatin-loaded DNA-nanothread(CPT-DNA-NT)towards the tumor cells(HeLa)in vitro;(A)Binding of DNA-NT on the cell surface.(B)Internalization of CPT-DNA-NT by scavenger receptors.(C)Intracellular CPT delivery.
2.3.Preparation and characterization
2.3.1.Synthesis of DNA triangles
Designed DNA oligomers sequences were bought from Sangon Biotech Co.,Ltd.(Shanghai,China),and were analyzed for purity using PAGE and concentration was measured by Nanodrop 1000 as per ssDNA protocol[28].The sequence of designed DNA oligonucleotides strands used in this study is detailed in Table 1.A schematic illustration of this process is shown in fig.2.

Table 1 Sequences of oligonucleotides used in this study.
2.3.2.Preparation of circularized DNA
As seen in fig.2,both 3.5 μM circularizable 5′-phosphorylated oligonucleotide(35μL)and 4.5μM splint oligonucleotide(45μL)were brought from 95°C to room temperature over a 3h period.10 μL of T4 DNA ligase(350U/μL)which contained 66 mM Tris-HCl(pH 7.6),6.6 mM MgCl2,10mM dithiothreitol,0.1 mM ATP and 3.3μM of[32P]-Na4P2O7were added,and the mixture was incubated at 16°C for 16h.This was then heated to 65°C for 10 min to inactivate the T4 DNA ligase.The mixture was then cooled in an ice bath for 5 min.10 μL of exonuclease-I(5 U/μL)was then added to the mixture and incubated at 37°C for 30 min,allowing for the breakdown of both the linear splint and unligated oligonucleotides.Exonuclease-I was inactivated by heating at 80°C for 5min and cooled rapidly using an ice bath for 5 min[29].50μL of deionized formamide was added before loading the sample to denaturing polyacrylamide gel[30].
2.3.3.Purification of circularized DNA via denaturing PAGE
The DNA strands underwent 12% denaturing polyacrylamide gel(19:1 acrylamide/bisacrylamide,4.2 M urea)electrophoresis in 1×Tris-borate-EDTA buffer(89mM Tris-HCl,89 mM boric acid,2 mM EDTA,pH 8.0)at room temperature.Electrophoresis was then carried out at a constant voltage of 75V for 2 h.The denaturing gel was separated from the bands and cut finely and eluted in a Tris-EDTA(TE)buffer,consisting of 500 mM ammonium acetate,10 mM Tris-HCl,and 1mM EDTA(pH 7.6).This purified DNA was then dissolved in sterilized water.
2.3.4.Synthesis of self-assembled nanostructures
The DNA strands were mixed in stoichiometric amounts of 0.5μM with 1×Tris-acetate-EDTA-Mg2+(TAE-Mg2+) buffer(40 mM Tris-acetate,2 mM EDTA,and 25 mMMgAc2;pH 8.5)until a final volume of 20μL was reached.This mixture was then annealed in a thermocycler that cooled the mixture from 95°C to 4°C.This was done by cooling from 95°C to 65°C by 1°C every 150 s.The mixture was then further cooled to 50°C by 0.1°C every 10 min or every 7min depending on the number of rings.The mixture was then cooled to 5°C by 1°C every 150 s until the mixture reached 4°C and was maintained at that temperature[31,32].
2.3.5.Stability and integrity of DNA-NT
A native PAGE was used to determine the integrity of the DNANT.Samples were run under TAE-Mg2+buffer for 3 h while being held in a cold-water bath to reduce heating.Samples(15μL)were loaded into wells to assess the electrophoretic mobility of the DNANT using native PAGE(without urea)experiment that may not show the downward movement along with the gel due to the large molecular size.Due to the polymerization of the triangular tiles and large size of the resulting DNA-NT,it could not travel down the gel after applying a voltage of 80V and a current of 25 mA,indicating the DNA-NT was stable.
2.3.6.Qualitative statistics of production yield of DNA-NT
Atomic force microscopy(AFM)testing was used to determine the imaging of DNA-NT production.For AFM imaging,6μm×6μm area was selected after sample preparation on the mica surface using Bruker FastScan AFM(Bruker Scientific,USA)and FastScan A probes(Bruker Scientific,USA).Each sample was analyzed at 4 to 6 different locations on the mica surface[33].
2.3.7.Sample preparation for AFM
5μL of the annealed mixture was placed into a freshly cleaved mica for 2min,which would give us enough time for the DNA-NT to adhere to the surface electrostatically[33].After 2 min,the mica was then washed using 100μL of sterilized water twice,and the filter paper was used to get a wholly dried surface.The image was then captured at room temperature within 3h of placement.
2.3.8.Evaluation of CPT binding to DNA-NT
CPT binding to the DNA-NT was Confirmed by UV spectrophotometric analysis.The aqueous solution of CPT(0.1μg/mL)was incubated with the DNA-NT(20μL,0.25μM)for 4h at 37°C in TE buffer(pH 8.4).The binding of CPT was related to the shift and pattern of the UV-Vis spectra of DNA-NT and CPT.Individual UV-Vis spectra of CPT and DNA were compared to the spectra after their binding to each other.Unbound CPT was evaluated in the supernatant after centrifuging the CPT-DNA-NT at 14,000rpm and 4°C for 15 min.The concentration of unbound CPT in the supernatant sample was evaluated through UV analysis by the standard curve method.
2.3.9.Drug encapsulation efficiency(EE)and drug loading(DL)
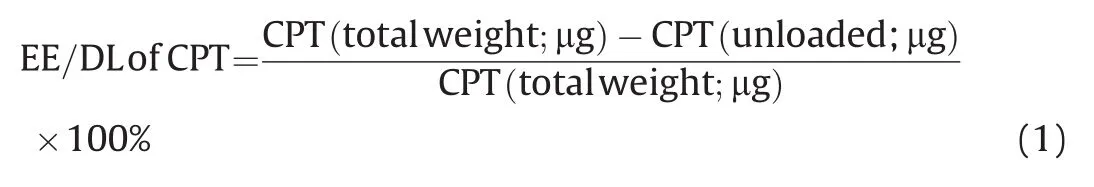
The drug EE and DL were determined by centrifuging the resultant CPT-DNA-NT at 14,000 rpm at 4°C for 15min.The unloaded CPT that remained in the supernatant was removed and estimated for CPT concentration(unbound)through UV analysis by the standard curve method.The CPT-DNA-NT was re-dispersed by adding 20μL of fresh buffer.This process was repeated twice to find the final unbound amount of CPT.The encapsulation or loading efficiency of the CPT was quantified as the percentage loaded on to the DNA-NT compared to the free CPT calculated in the supernatant employing the following equation(Eq.(1)):
2.3.10.UV quantification of the DNA-NT and CPT concentration
The concentration of CPT was determined by using UV-spectrophotometric Nanodrop 2000c(Thermo Fisher Scientific,USA)by adjusting UV-Vis mode at λmax215 nm.DNA-NT was also quantified by using ssDNA protocol at DNA λmax260 nm.2μL drop of the dissolved sample was taken and carefully applied on the optical surface of Nanodrop for accurate measurement after running the blank sample[34].
2.4.Molecular docking of CPT binding to DNA-NT
We performed a molecular docking study of the binding of CPT to DNA-NT using AutoDock Vina software after downloading pdb file from Protein Data Bank(https://www.rcsb.org/).
2.5.In vitro release study
The release of CPT at 37°C using different media(including fetal bovine serum(FBS)and blood plasma)was estimated in an Eppendorf tube set on the agitation apparatus for 36 h.
2.6.Cell culture
2.6.1.Cell line
Human cervical cell line(HeLa)was used.
2.6.2.Growth media
The cell line(HeLa)was cultured using a Dulbecco's Modified Eagle's Medium(DMEM)(Hyclone,Thermo Fisher Scientific,USA).All the media were supplemented with 10% FBS(Biological Industries(BioInd),Israel)100 U/mL penicillin,and 100μg/mL streptomycin,and then incubated in a humidified atmosphere containing 5% CO2at 37°C.
2.7.MTT cell viability assay
2.7.1.In vitro cytotoxicity studies
MTT assay was performed to determine the cytotoxicity of blank DNA-NT.HeLa cells grew on a 96-well plate and were incubated at 37°C for 12 h in DMEM with 10% FBS.After 12h,the HeLa cells were treated with different formulations.Then after 48 h,the media were removed from the plate and cell growth was determined by the addition of MTT reagent(Sigma-Aldrich Co.,Ltd.,St.Louis,MO,USA)5 mg/mL to each well and further incubated for 4h.The unreacted MTT was then carefully withdrawn from the medium.The formed crystals were dissolved in 150μL of DMSO and kept in the dark for 15 min,and the absorbances were measured at 570-630 nm using a microtiter plate reader[35].Cell viability was then determined by Eq.(2).

2.7.2.In vitro anticancer activity
MTT assay was further performed to check the cytotoxicity of the synthesized DNA-NT against the HeLa cells.Brie fly,HeLa cells were placed in a 96-well plate and incubated at 37°C for 12 h.Different concentrations of CPT-DNA-NT and free CPT were added to the medium and incubated in an atmosphere of 5% CO2at 37°C for 48h.After this incubation period,MTT(5 mg/mL in PBS)was added and then incubated for an additional 4h,after which 150μL of DMSO was added to each well and shaken.The absorbances of samples were measured using a microtiter plate reader at 570-630 nm.The IC50value was then calculated by comparing it to the control wells.The assay was performed three times on three duplicate wells for each sample concentration.
2.8.Analysis of cellular apoptosis
HeLa cells were seeded in 6-well plates at the density of 1.0×106cells/well and incubated for 24hat 37°C.The DNA-NT was added in various concentrations with negative control,which was treated with the medium.After the 48h of incubation,trypsindigested cells were washed with PBS twice and then centrifuged at 2,000rpm and 4°C for 5min to collect cells.500μL of the buffer was added to suspend cells,and 5μL of Annexin V-FITC and 5μL of propidium iodide(PI)were added in the absence of light.These were thoroughly mixed,reacted for 15min at room temperature in the dark,and then were analyzed for cell apoptosis using the FACS Calibur flow cytometer(Becton Dickinson,San Jose,CA,USA).
2.9.Double photon confocal laser microscopy
HeLa cells were grown in a 5% CO2water-saturated incubator(37°C)in DMEM supplemented with 10% FBS,100 U/mL penicillin and 100μg/mL streptomycin.For the control group,cells were incubated and then treated with blank DNA-NT for 8 h and then treated with 4′,6-diamidino-2-phenylindole(DAPI)and Pi for 10min at 37°C,washed three times with PBS,and then imaged.For the treatment group,CPT-DNA-NT and HeLa cells were incubated for 8h and treated with DAPI and Pi for 10min at 37°C,washed three times with PBS,and then imaged.
2.10.Statistical analysis
We used Origin Pro-9.1 and GraphPad Prism 6(San Diego,CA,USA)for analyzing the data.One-way ANOVA was performed to analyze the variance between the treated and control groups.Student's t-test was also used to calculate the difference between the two groups.The significance level was set at*P<0.05,**P<0.01.All the experiments were repeated at least five times to calculate the mean±standard deviation(SD),and found to be reproducible.
3.Results and discussion
3.1.AFM of DNA-NT
The results of AFM tapping mode in air showed that the designed nanosheets made up of polymerization of triangular tiles underwent self-coiling and were converted into DNA-NT structures due to the presence of natural curvature in the DNA double helix.They were uniform in diameter ranging from 50 nm to 150 nm,with length of 300-600nm,as shown in fig.3.
3.2.DNA-NT integrity
To evaluate the strength and integrity of the DNA-NT,we performed electrophoretic mobility assay on native PAGE by placing the gel apparatus in cold water bath(10°C)to avoid heating due to applied voltage(85V)and current flow(20mA),using TAE-Mg2+buffer for 3h.8μL of the samples including DNA marker(lane 1),linear scaffold strand(lane 2),circularized scaffold strand(lane 3),linear staple strand 2(lane 4)and the DNA-NT solution(lane 5)were loaded into wells,as demonstrated in fig.4A.DNA-NT with stable structure did not split into constituting strands and showed no electrophoretic mobility down the native PAGE gel due to large molecular size after the polymerization of triangular tiles,as shown in fig.4A,lane 5.
DNA nanotechnology has recently emerged as an established approach to synthesizing specially designed nanoscale objects with high efficiency and precision.Due to the Watson-Crick base-pairing phenomenon,different staple strands can be arranged together with designed patterns to make biocompatible nano-architects for potential applications,including nanomedicine and drug delivery.Circularized DNA nanotechnology based on circular templates further allows synthesizing stiffer architects to form rigid nanostructure[14,30,31,36].Herein,we have designed triangular DNA tiles made from 84-NT circular scaffold self-assembled with three different staple strands.Each side of the triangular tile contained 28 bp with five half turns of DNA.Each triangular strand contained 6-NT sticky overhangs at each corner(Fig.2),which made them combine with the other triangular tiles to form DNA nanosheets.However,due to the curvature in the DNA helix,the DNA nanosheet underwent spinning and coiling around its axis to create rigid and stable nanothread structures.Resultant DNA-NT showed uniform size at nanoscale level Confirmed by AFM imaging showing length in the range of 150-200nm(Fig.3),with excellent stability Confirmed by resisted electrophoretic mobility in native PAGE results(Fig.4A)[33,37].
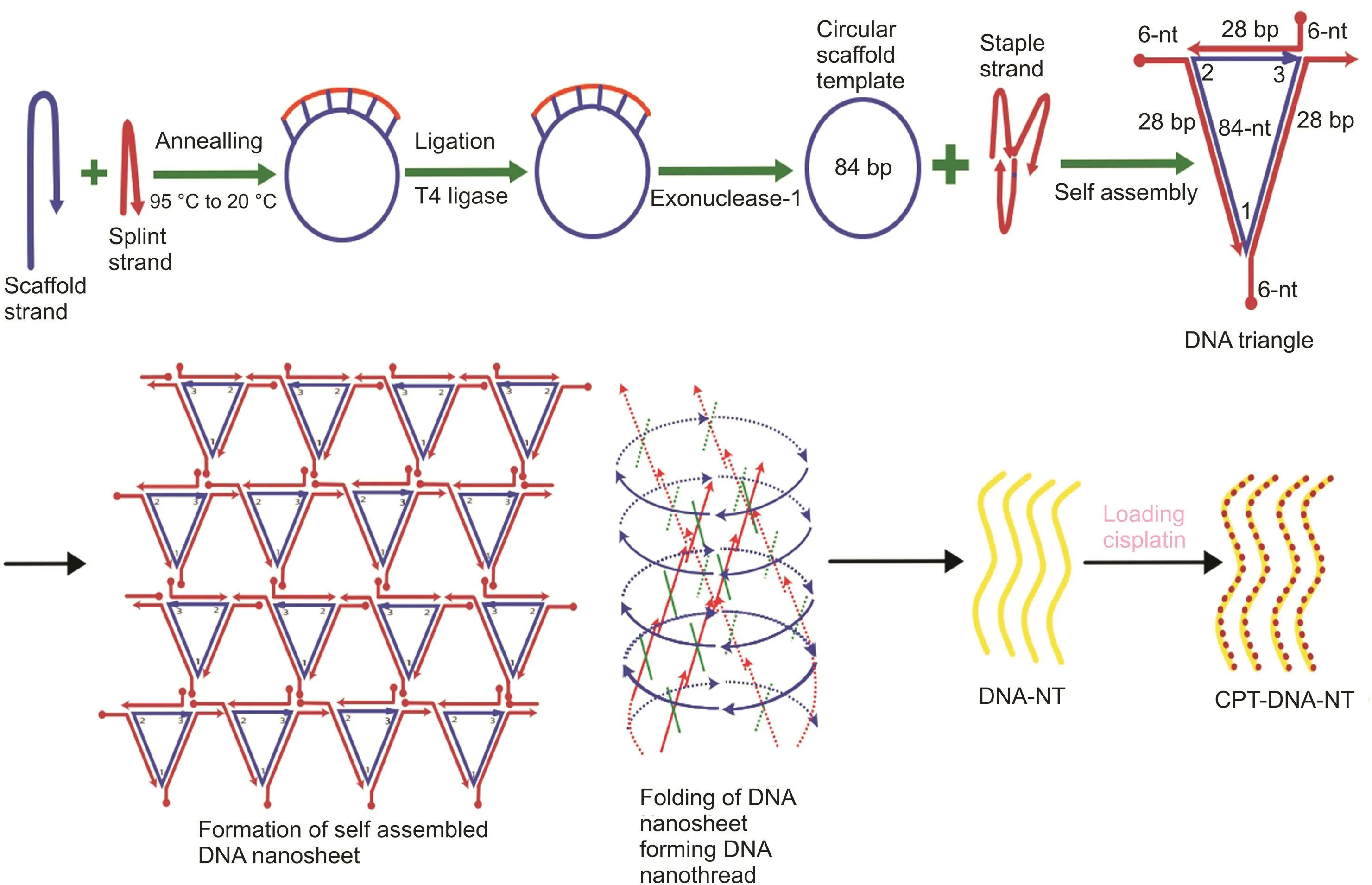
Fig.2.Schematic illustration of the synthesis of CPT-DNA-NT.

Fig.3.AFM images of DNA-NTs(DNA-NT samples scanned through tapping mode in air).

Fig.4.(A)PAGE analysis.(B)Confirmation of CPT binding to DNA-NT by UV-Vis spectrophotometry.(C)Drug loading assay.(D)In vitro CPT release from DNA-NT.
3.3.Confirmation of CPT binding to DNA-NT
The UV-Vis absorption spectrum of pure CPT was observed at 202nm using Nanodrop 2000c,while the DNA-NT also showed fingerprint area of UV absorption at 195-202nm.However,the UV-Vis absorption peak of blank DNA-NT was observed as a broad peak at 256nm.After binding of CPT,the UV-Vis absorption peak of CPT was shifted to 215nm(from 202nm;>10nm shift)with broadening of the peak and increase in the intensity level of the same concentration.The peak of UV-Vis absorption of DNA-NT was also shifted to 268nm(from 256nm;>10nm shift)with further broadening of the peak and increased intensity of the same concentration,as shown in fig.4B,Confirming the binding of CPT with DNA-NT.
CPT was successfully loaded to the DNA-NT by incubating the two solutions together and Confirmed by UV analysis of the supernatant liquid and the pelleted DNA at 210 nm after centrifugation at 10,000rpm for 10 min(two bonds with one base pair)as shown by dotted lines in molecular docking analysis illustrated in fig.5[38,39].Thus,the DNA-NT successfully loaded the CPT due to strong interaction with DNA double helix Confirmed by UV analysis[34].
3.4.Molecular docking study of the binding of CPT to DNA-NT
CPT has two leaving groups,which are made up of two labile chloride ligands in cis-orientation.When the drug reaches the cell,which has a lower concentration of chloride,these ligands are broken down and are replaced by aqua ligands,which form the active complex.This active complex then binds to the necessary parts of the cell,which are responsible for cellular activities such as transcription,thus causing apoptosis.This is achieved by binding to the N7 atom of the purine base.This allows for multiple bindings and cross-linking,although the cis-orientation does lead to a favored binding between adjacent cross-links[38].We performed molecular docking study of the binding of CPT to the DNA-NT using AutoDock Vina software,as shown in fig.5[39].CPT bound to the N7 atom of the purine base.Cis-Configuration of the CPT allowed it to undergo multiple bonds formation and to cross-link between adjacent base pairs.The details of the bond formations by CPT to the adjacent base pairs and cross-links are shown in fig.5.The bond length ranges from CPT 50.CPT1-DG 30.CN7 1.992Å(Fig.5A)to CPT 50.CPT1-DG 31.CN7 2.096 Å(Fig.5B)and CPT 50.CPT1-DG 6.CN72.073 Å (Fig.5C)to CPT 50A.CPT1-DG 7.CN72.108 Å (Fig.5D),respectively.This allows for multiple bonding and cross-linking,although the Cis-orientation does lead to a favored binding between adjacent cross-links.This understanding is necessary because previous experiments showed that there is a preference for closure in the 5’direction[38].
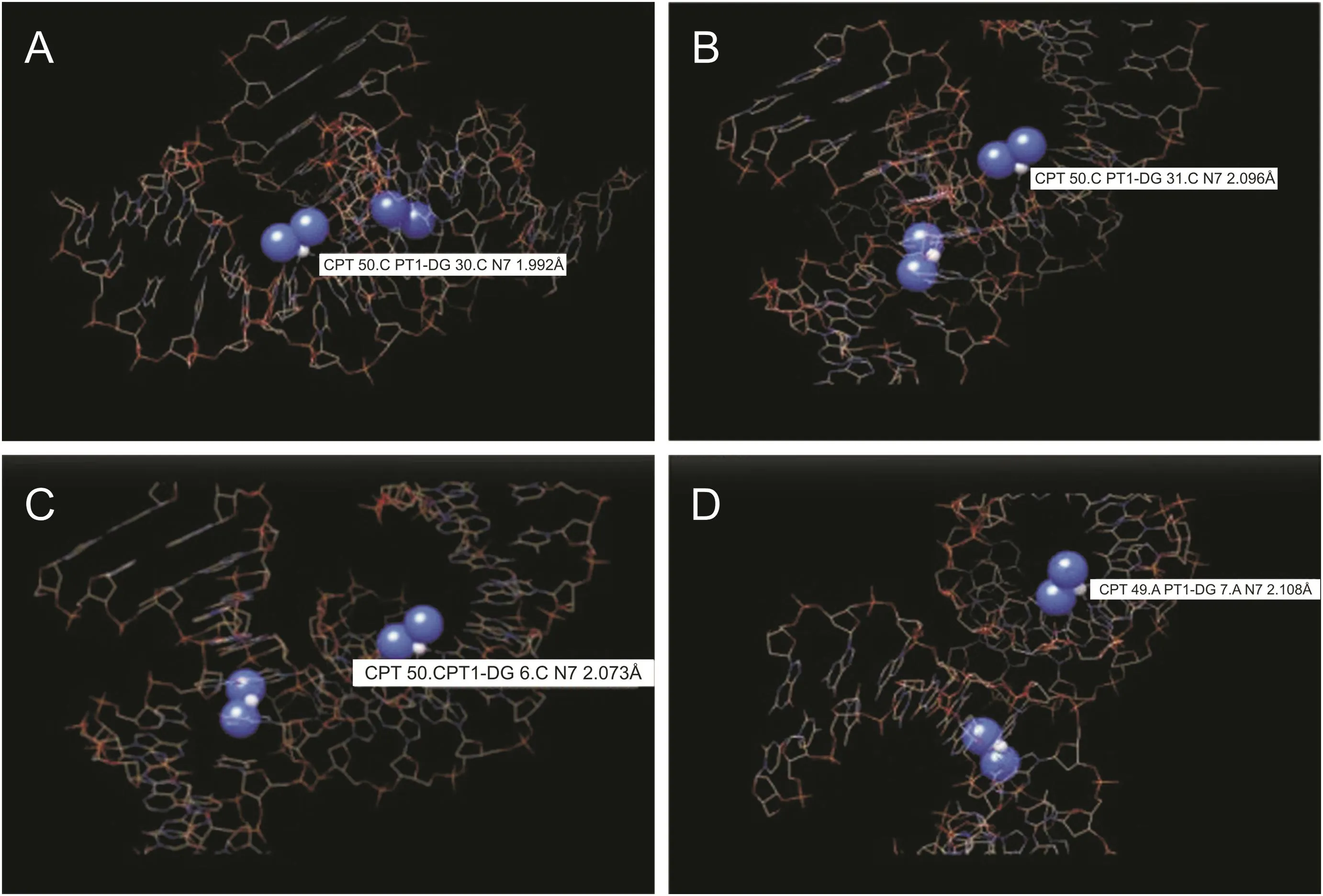
Fig.5.Binding mode of DNA-nanothread with CPT.CPT-DNA-NT complex indicating the across bonding of the drug(Cl2H6N2Pt)with the DNA-NT complex(protein complex structure(3LPV)was obtained from Protein Data Bank.(A)CPT 50.CPT1-DG30.CN7 1.992 Å;(B)CPT 50.CPT1-DG31.CN7 2.096 Å;(C)CPT 50.CPT1-DG6.CN7 2.073 Å;(D)CPT 50.CPT1-DG7.CN7 2.108 Å(Source:https://www.rcsb.org/));Binding mode of CPT(PubChem CID:5702198)with DNA-NT complex in three different dimensional views by AutoDock Vina software.(PubChem CID:5702198).
3.5.CPT EE and its DL
EE was dependent on the relative concentration of the DNA-NT and CPT.As a general rule,with the increase in CPT concentration,the loading capability of DNA-NT could decrease.In this study,we synthesized eight different formulations,C1 to C8,as detailed in Table 2.We observed that with the increase in the concentration of the drug(from 4 to 512nM),and keeping DNA-NT concentration constant(25μM),DL was decreased(from 98% to 59%)as demonstrated in fig.4C.This is because CPT might have bonded to the DNA-NT at specific locations rich in GC content to two consecutive nucleic acids,forming 4 hydrogen bonds,as illustrated in the molecular docking analysis(Fig.5).

Table 2 Details of the different formulations;concentrations used and loading percentage.
3.6.In vitro CPT release from DNA-NT
We evaluated the integrity of the DNA-NTs immediately after assembly.We performed the CPT in vitro release from CPT-DNA-NT in the presence of biological fluids such as DMEM with 5% FBS,DMEM with 10% FBS,blood plasma and blood serum at different time intervals of 4,8,12,20,24,28,32 and 36 h at 37°C.CPT was released slowly from the CPT-DNA-NT reservoir,Confirming the stability and integrity of the DNA-NT in biological media.There was sustained release and targeted intracellular delivery to HeLa cells(Fig.4D)because the free CPT diffused out of the cells at a higher rate due to paxillin-mediated exocytosis.
3.7.Cell viability assay
To Confirm whether there is biocompatibility of DNA-NT in vitro,MTT assay was performed.In this study,the effects of different concentrations of the blank,DNA-NT,CPT-DNA-NT,and free CPT were evaluated for in vitro antiproliferative activity against the HeLa cells for 48 h.The results are shown in Tables 3,4 and Figs.6A and 6B.It can be noticed from Fig.6A that upon increasing the concentration of the blank DNA-NT,the cell viability reduced slightly,though the change is negligible even at the maximum concentration.Hence,the blank DNA-NT was Confirmed to safe,making it biocompatible.On the contrary,CPT-DNA-NT showed apoptosis-inducing effects on the HeLa cells.The DNA-NT showed targeted cell internalization and depot CPT intracellular release and exhibited superior cytotoxic effects compared to free CPT(Fig.6B).The free CPT after cell internalization showed paxillin mediated exocytosis with inferior cytotoxic activity.The half-maximal inhibitory concentration(IC50)of CPT-DNA-NT was 512 nM,which was attributed to the intracellular release of CPT from the CPT-DNANT and the ensuing time-dependent sustained entry into the nucleus of the HeLa cells.

Fig.6.(A)In vitro cytotoxicity of DNA-NT.Blank DNA-NT showed least cytotoxicity at the highest concentration(512 nM)with a viability of 92% as evidence of its biocompatibility for drug delivery.(B)CPT-DNA-NT and free CPT against HeLa cells.CPT-DNA-NT showed superior cytotoxicity than the free CPT due to the depot release of CPT after DNA-NT internalization.
Table 3 In vitro cytotoxicity of DNA-NT.

Table 4 CPT-DNA-NT and free equimolar CPT against HeLa cells.
The blank DNA-NT did not show a considerable decrease in cell viability,as illustrated in fig.6A.The results of cell viability Confirmed that the blank DNA-NT has the least cytotoxicity at the highest concentration with approximately 92% of viable cells,which Confirms its biocompatibility and makes it a suitable nanocarrier for drug delivery.The in vitro cytotoxicity assessment of CPT-DNA-NT displayed similar anticancer activity and also minimized the potential side effects of the free CPT against the HeLa cells.
3.8.CPT-DNA-NT induced cell apoptosis
Cellular apoptosis was determined using Annexin-V/PI double staining assay to detect CPT-DNA-NT induced apoptosis in HeLa cells.As shown in fig.7,after the treatment of HeLa cells with CPTDNA-NT at different concentrations for 12h,the apoptotic rate increased significantly(72.7%)compared with free CPT(64.4%).Thus,it can be concluded that CPT-DNA-NT could induce apoptosis in the HeLa cells.
Our designed DNA-NT after internalization underwent perinuclear localization towards the nucleus where it delivered CPT under the action of hydrolytic enzymes of lysosomes that resulted in increased apoptosis,as evidenced by results of the flow cytometry assay.
3.9.Confirmation of internalization of DNA-NT in the HeLa cells through confocal microscopy
Confocal microscopy was done to inspect the cellular uptake of the DNA-NT with prior staining of the nucleus with DAPI that is permeable to the live cells.We tagged DNA-NT with PI(impermeable to live cells)to visualize the internalization of the DNA-NT in 2h.The results of confocal imaging indicated the presence of DNA-NT inside the HeLa cells,as shown in fig.8.Moreover,CPTDNA-NT seemed to efficiently deliver CPT and cause apoptosis in the HeLa cells after 5 h as evident by the bare nuclei with lysis of the cellular structures,as shown in fig.9.
DNA-NT as polyanionic ligand activates SRs on the cell surface,resulting in the endocytosis of the DNA-NT through clathrin/caveolin endocytic pathway [27,40] as explained by Choi et al., [41].Similarly, Vindigni et al. [42] observed the influence of SRs on theinternalization of octahedral DNA nanocages into COS fibroblasts.In our experiments, the cell internalization assay shown in Fig. 8confirmed the internalization of CPT-DNA-NT through theclathrin/caveolin-dependent uptake process with the involvementof SRs as evidenced by intracellular fluorescence signals in theconfocal microscopy imaging. The clathrin/caveolin-dependent endocytic process of DNA-based materials was previouslyconfirmed by Liang et al. [43] who observed the internalization ofthe DNA tetrahedral nano-architects into HeLa cells. Another studyreported by Yang et al. [44] shows that endocytosis of DNA tetrahedrons duplexes into breast cancer cells via the clathrin/caveolinendocytic pathway.
DNA-NT slowly degrades after internalization into the endosomes,followed by the action of lysosomal enzymes.Thus,CPT gets released at the target sitenear the nucleus overan extended period,with the endosomes acting as local depots.In contrast,free CPT would get transported in quickly,but also diffuse out of the cells at a higher rate due to the paxillin-mediated exocytosis.Therefore, flow cytometry was conducted to investigate the cellular uptake of DNANT(Fig.7).To examine whether this system worked better than the free drug causing more apoptosis,we examined fluorescence-based cell sorting.Using a combination of fITC-labeled annexin V and PI,cells were sorted into populations of live,necrotic,and apoptotic cells.As shown in fig.7,the fluorescent intensity of cells treated with CPT-DNA-NT was significantly higher due to the internalization of PI-DNA-NT,and the appearance of intracellular fluorescence signals of PI in live cells,as compared to those in free CPT group indicated increased cellular uptake of the DNA-NT.A similar result was also found in the confocal microscopy images,as shown in fig.9.
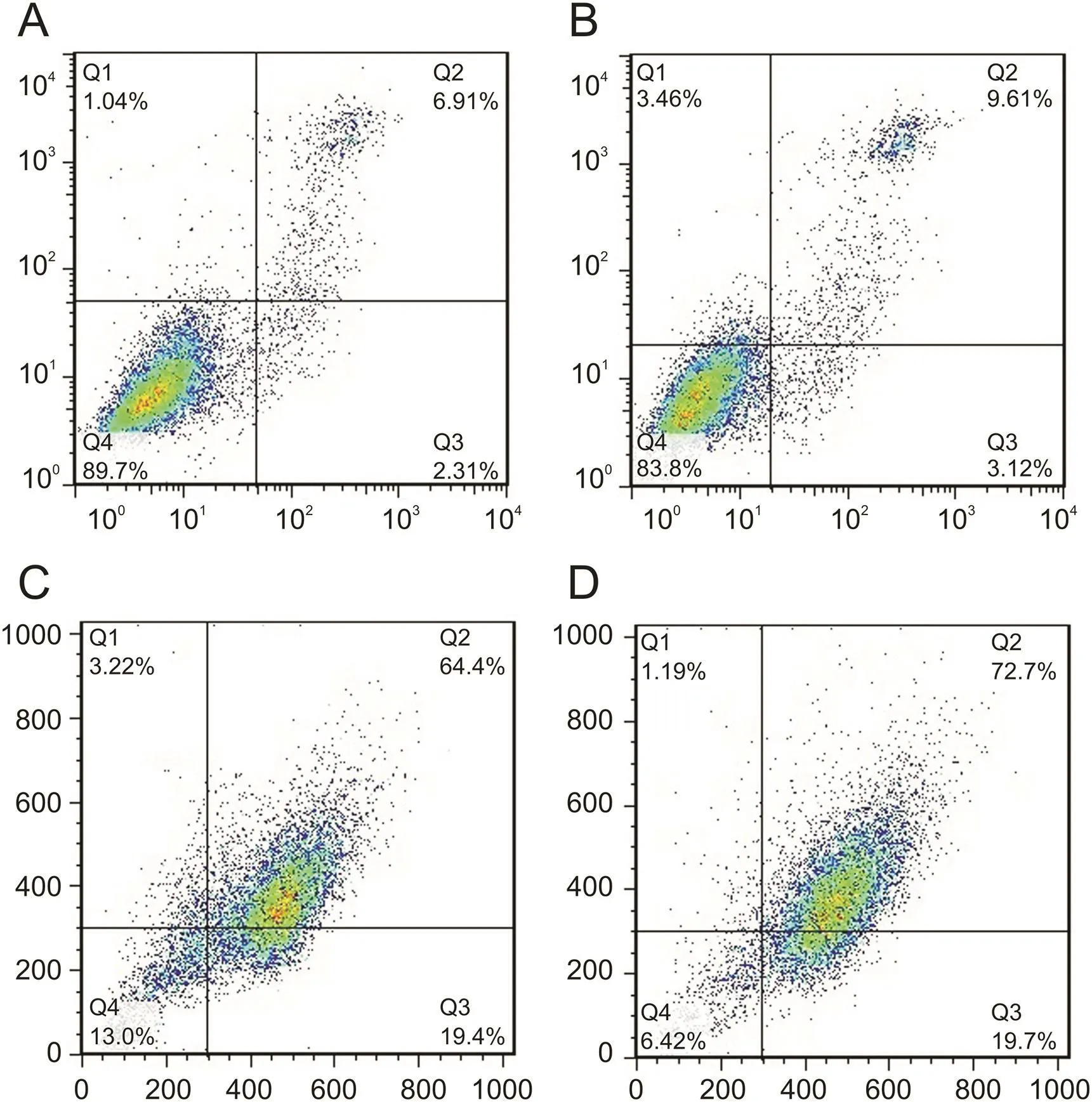
Fig.7.CPT-DNA-NT induces apoptosis in HeLa cell after 12 h.(A)Flow cytometry analysis of control experiment(n=10,000 cells,**P<0.01).(B)Apoptosis estimation of blank DNA-NT to HeLa cell(n=10,000 cells,**P<0.01).(C)Apoptotic analysis of free CPT(n=1,000 cells,*P<0.05).(D)Apoptotic analysis of CPT-DNA-NT against HeLa cells(n=1,000 cells,*P<0.05).In vitro cytotoxicity assessment through flow cytometry showed enhanced apoptosis(72.7%)with CPT-DNA-NT,compared to free CPT(64.4%).
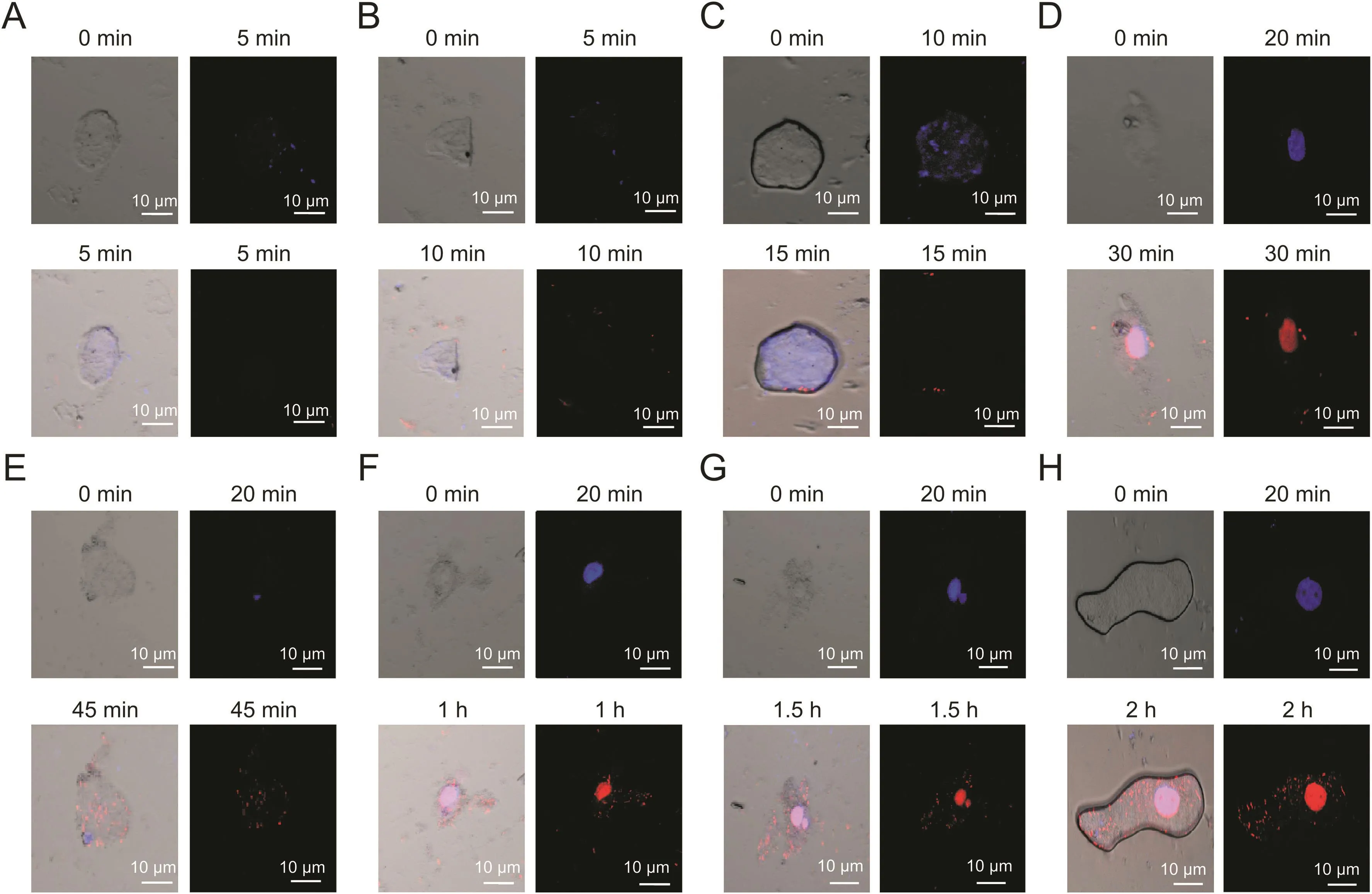
Fig.8.Time-dependent internalization of DNA-NT inside the HeLa cells for 2 h(n=15cells,**P<0.01).Internalization of DNA-NT at different intervals indicated by red fluorescence.(A)5 min,(B)10 min,(C)15 min,(D)30 min,(E)45 min,(F)1 h,(G)1.5 h and(H)2 h.
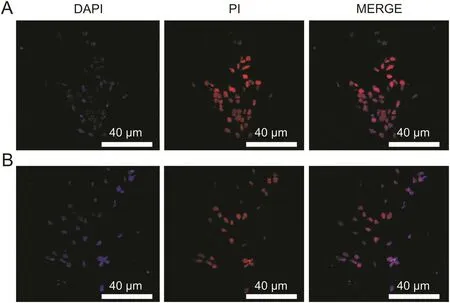
Fig.9.Cellular apoptotic activity by(A)CPT-DNA-NT and(B)free CPT(n=30 cells,**P<0.01).
4.Conclusions
In conclusion,our results showed that DNA-NT represents an efficient delivery system for targeted cancer cell internalization to induce apoptosis in HeLa cells more efficiently than free CPT.We employed circular DNA nanotechnology to fabricate stiff DNA-NT for CPT loading.Circular DNA nanotechnology has been established as a promising tool to design nanoscale architects with specific structure and geometry.Watson-Crick base-pairing enables different strands to self-assemble precisely around the circular DNA template to make DNA-tiles that are arranged linearly into long DNA lattices that have been applied in drug delivery in the present study.We found that CPT-DNA-NT has controlled/prolonged CPT release and enhanced cytotoxic activity through SRs-facilitated endocytosis,making this a promising candidate for anticancer therapy.The sustained CPT in vitro release from CPT-DNA-NT in the presence of biological fluids(in the absence of cells)Confirms the stability and integrity of DNA-NT and indicates in vivo suitability of the DNA-NT.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the China Scholarship Council(CSC)grant(Grant No.20180500458).We also thank the platform for characterization and test at State Key Laboratory of Analytical Chemistry for Life Sciences,School of Chemistry and Chemical Engineering,Nanjing University,China for the assistance with the instrumentation.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
