Probing the degradation of pharmaceuticals in urine using MFC and studying their removal efficiency by UPLC-MS/MS
2021-07-20PriyShrmDevendrKumrSriknthMutnuri
Priy Shrm,Devendr Kumr,Sriknth Mutnuri,*
aApplied Environmental Biotechnology Laboratory,Birla Institute of Technology&Science(BITS),Pilani,403726,Zuarinagar,India
bCentral Sophisticated Instrumentation Facility,Birla Institute of Technology&Science(BITS),Pilani,403726,Zuarinagar,India
cDepartment of Pharmaceutical Sciences,College of Pharmacy,University of Nebraska Medical Center,Omaha,NE,68198-602,USA
Keywords:
Pharmaceuticals
Urine
MFC
Biodegradation
Liquid chromatography mass spectroscopy
A B S T R A C T
Nutrient recovery from source-separated human urine has attracted interest as it is rich in nitrogen and phosphorus that can be utilized as fertilizer.However,urine also contains pharmaceuticals,steroid hormones,etc.and their removal is crucial as they have detrimental effects on the environment and human health.The current study focuses on investigating the degradation of pharmaceuticals using a double-chamber microbial fuel cell(MFC).Urine was spiked with four pharmaceuticals(trimethoprim,lamivudine,levofloxacin,and estrone)at a concentration of 2μg/mL.The MFC was operated for 7 months in batch mode with this spiked urine as feed.The degradation efficiency of the MFC was studied,for which a selective liquid chromatography-tandem mass-spectrometric method was developed for the quantitation of compounds used in the spiking experiments and was validated with a lower limit of quantification of 0.39 ng/mL.The maximum removal rate achieved was 96%±2%.The degradation mechanism involved processes like sorption and anoxic biodegradation.The voltage curve obtained showed that the presence of pharmaceuticals had an initial negative impact on power generation along with increased organic content;however,after the reactor acclimatization,increased power output was achieved with maximum organics removal at 30 h of retention time.This work opens a new perspective for the anoxic biodegradation of pharmaceuticals and can be useful in future bioremediation studies.
1.Introduction
The current worldwide population is more than 8 billion.The daily production of urine per person is approximately 1.2 L,which varies in composition.The urine,which is considered as waste,comprises nutrients like nitrogen(N)and phosphorus(P)in a form that can be directly taken up by plants[1].Therefore,separating urine from the main wastewater stream to utilize these nutrients(as fertilizer)makes it a valuable resource.The use of sourceseparated urine as a fertilizer is also energy efficient and makes up for the chemical fertilizers.It also reduces the N and P overloading at wastewater treatment plants[2].However,along with these nutrients,urine also contains some enzymes,organic substances,heavy metals,pharmaceuticals,and hormones.The existence of pharmaceutical compounds and hormones is associated with environmental pollution,as these compounds can be transferred to the food chain by infiltrating into groundwater[3].They are termed as micropollutants as the concentration of these compounds is usually very low in the environment(mostly in μg/L to ng/L).
The pharmaceuticals are used to prevent and treat various diseases;however,a significant fraction of the consumed pharmaceutical passes through the human body,mostly in unchanged form.These are excreted in the urine,reach the sewage system,and are frequently detected in the environment.The use of such urine as fertilizer can lead to their potential transfer to the environment,crops,and, finally,humans.Therefore,the fate of these pharmaceuticals must be addressed as these compounds have endocrinedisrupting properties that can severely affect wildlife[4].
There are different classes of pharmaceuticals,such as antipyretics,analgesics,antidepressants,antibiotics,antivirals,and hormones,which are mostly excreted in the urine.Among these,three classes,antibiotics,antivirals,and hormones,have been listed as environmentally relevant in earlier studies[5].Antivirals interfere with the biological system of microorganisms by inhibiting their replication process,while antibiotics have the potential of causing bacterial toxicity.Hormones,on the other hand,have more tendencies to be persistent in the environment and can increase the severity of infections.Hence,it has become essential to analyze these pharmaceutical residues in urine and to act appropriately for their removal.
The biodegradation of these compounds through conventional wastewater treatment technologies is difficult due to the presence of high strength and complex compounds[6].Various methods,like chlorination,advanced oxidation processes,ozonation,electrochemical,and biological ones,have been studied for micropollutants degradation.These methods,however,require more investment and were more energy and time consuming,as mentioned in previous studies[7-10].Hence,there is a need to explore other treatment technologies to remove them effectively.
Microbial fuel cells(MFC)have recently gained interest in this area of research[11,12].MFC harnesses the ability of microorganisms to convert the organic matter present in wastewater into electrical energy.It transforms chemical energy stored in organics into electricity by the microbially catalyzed oxidoreductase reactions.The efficiency of MFCs in degrading micropollutants was successfully reported in some of the studies conducted with sulphamethoxazole and ciprofloxacin(CIP)[13,14].
Earlier pharmaceuticals were typically analyzed in biological samples like human serum,plasma,and urine using techniques based on UV and HPLC[15].However,these detection methods were limited for a concentration up to mg/L.Therefore,a method that combines ultra-performance liquid chromatography(UPLC)with mass spectrometry(MS)has been employed to determine the analytes in the MFC effluent at a quite low concentration(ng/L).
In principle,combining the bioelectrochemical system and biodegradation could be highly advantageous for treating urinecontaining pharmaceuticals.In our study,MFC was operated with urine spiked with four pharmaceuticals of different classes and evaluated for their degradation in the reactor.The pharmaceuticals used were levofloxacin (LFL, fluoroquinolones,antibiotic),trimethoprim(TRI,dihydrofolate reductase inhibitor),lamivudine(3TC,antiviral compound)and estradiol(EST,hormone).All these pharmaceuticals are excreted in urine at different concentrations and have been detected in wastewater and surface water[16].They are persistent,recalcitrant in the environment,and show a varied range of half-life.
Therefore,this work intends to focus on the development of the UPLC-MS/MS analysis method to study the rates of pharmaceutical degradation in MFC.Besides,the ability of MFC to treat urine spiked with pharmaceuticals was investigated by monitoring the voltage produced by the microorganisms and the rate of chemical oxygen demand(COD)removal.Based on the results,the relationship between additions of pharmaceuticals and MFC performance was established.
2.Materials and methods
2.1.Chemicals and reagents
TRI,3TC,LFL,EST,dexamethasone(DXM),carbamazepine(CBZ),and CIP were ordered from Sigma-Aldrich(Germany).Acetonitrile(ACN),methanol(MeOH),formic acid(FA),and ethyl acetate,all of LC-MS grade,were purchased from Merck(Germany)while ammonium acetate was procured from Sigma-Aldrich(Germany).Ultrapure water was obtained from a purification unit(Millipore,Merck).The urine was collected from a group of healthy individuals/volunteers and was filtered through a 0.22μm filter(Millipore,Germany)and stored at 4°C for use in experiments.The stock solutions(1 mg/mL)of LFL and CIP were prepared by dissolving the standard compounds in 0.5% FA in Milli-Q water.The stock solutions of 3TC,TRI,CBZ,EST,and DXM were prepared in MeOH.The internal standard(IS)used for LFL was CIP,CBZ for 3TC,and TRI and DXM for EST.
Potassium chloride(KCl),ammonium chloride(NH4Cl),sodium dihydrogen phosphate(NaH2PO4),sodium hydrogen carbonate(NaHCO3)and sodium acetate(CH3COONa)were purchased from HIMEDIA.
2.2.MFC reactor operation
Two-chamber MFC reactor,made of Plexiglas,with a total working volume of 230mL,was used in this study.A cation exchange membrane(8 cm×8 cm,Ultrex CMI7000,Membranes International Inc.)was used to separate anode and cathode chamber.Stainless steel mesh(SS-304)served as both anode and cathode.The reactor was operated at room temperature with an external fixed load of 100Ω resistance.
The anode chamber(anaerobic)of MFC was fed with simulated media(composition per liter;(KCl 0.1g,NH4Cl 1.5 g,NaH2PO40.6 g,NaHCO32.5 g,CH3COONa 0.82 g,vitamin solution 10 mL,trace element solution 10 mL)mixed with microbial inoculum to activate the MFC.The inoculum was obtained from a biogas plant running on food waste(Birla Institute of Technology&Science,Pilani,KK Birla Goa Campus,Zuarinagar,India),and it was mixed with activated sludge.The anolyte was replaced once a week during inoculation.The cathode chamber contained a phosphate buffer solution(20 mM)and replaced twice a week.The cathode chamber was aerobic and supplied with oxygen that acts as the electron acceptor.After a month of inoculation,the voltage was maintained at a steady level,and the feed was changed to urine.The voltage was measured manually using a digital multimeter.
2.3.Pharmaceuticals spiked batch experiments
After the batch operation of MFC with urine for two months,pharmaceuticals were added at a final concentration of 2mg/L.The analysis was carried out in 5 phases,i.e.,Phase 1:fresh urine without any pharmaceutical,Phase 2:EST spiked urine,Phase 3:TRI and EST spiked urine,Phase 4:3TC,TRI,and EST spiked urine and Phase 5:LFL,3TC,TRI,and EST spiked urine.In each phase,the feed to the MFC varied.The MFC was operated in batch mode during all the phases,and each phase was conducted for a month.The stock solutions were injected into the reactor and changed twice a week.The MFC operation was changed from fed-batch to continuous flow after the acclimatization of the reactor in batch studies.
The effect of the addition of pharmaceuticals on MFC was studied by monitoring voltage produced and analyzing the COD behavior during the experiment.The COD was measured by the closed reflux colorimetric method,as mentioned in previous report[17].Other parameters like phosphorus(Ortho-P),ammoniacal nitrogen(NH4+),and nitrates(NO3-)were also measured as mentioned earlier[18].
2.4.UPLC-MS/MS method development and validation
To enable the determination of all the four pharmaceuticals in urine samples and study their degradation potential in MFC,UPLCMS/MS method was developed and validated.The samples were collected and stored at 4°C until analysis.They were later quantified using mass spectrometer.
2.4.1.LC system and MS
The UPLC-MS/MS analysis of pharmaceuticals was conducted on a system that comprises Series-1290 infinity-11 HPLC system(Agilent Technologies,Inc.)with a flexible pump,autosampler,and a thermostatted column oven.The sample vials were kept at 4°C in the autosampler.
The chromatography for LFL was performed using a Zorbex eclipse C18column(100 mm×2.1 mm,1.8μm)(Agilent Technologies,Inc.,USA).The column temperature was set to 30°C.The mobile phase was a gradient elution type and consisted of 0.5% FA in 1mM ammonium acetate(mobile phase A)and MeOH(mobile phase B)as used earlier[13].
3TC and TRI were determined using a C8column(150 mm×4.6 mm,5μm)from Agilent Technologies,Inc.The elution was carried out using mobile phase A(0.1% FA in MeOH(90%))and mobile phase B(0.1% FA in Milli-Q water(10%)).The same column was used to perform an analysis of EST in an isocratic mode.10mM of ammonium acetate with 0.01% FA was used as mobile phase A,while mobile phase B was MeOH with 0.01% FA.Table 1 presents all the chromatographic conditions used.
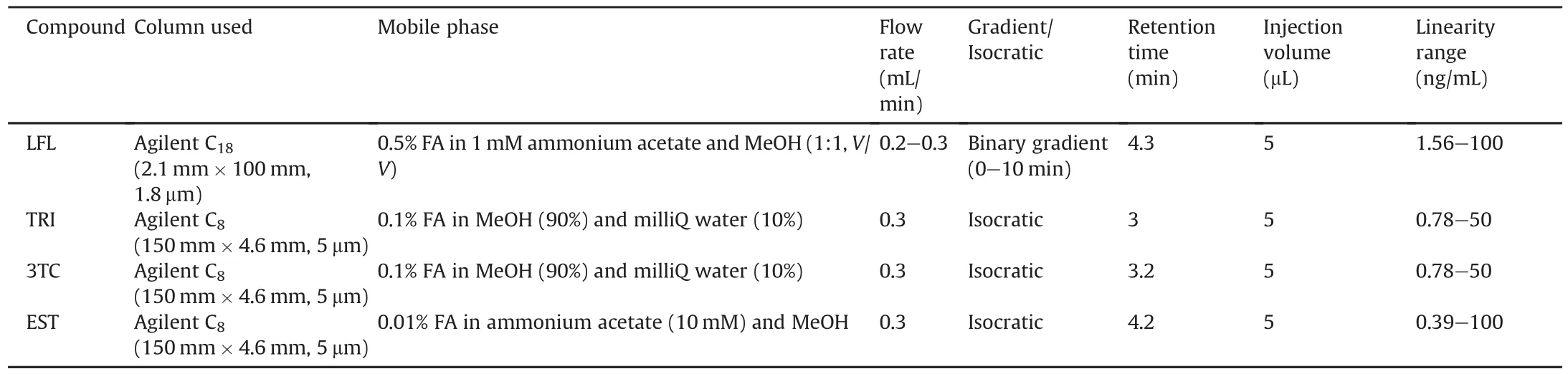
Table 1 Chromatography conditions maintained for UPLC-MS/MS analysis of pharmaceuticals.
6460 Triple Quad UPLC-MS/MS mass spectrometer from Agilent Technologies,Inc.(Santa Clara,CA,USA)with an electrospray interface(ESI)in the positive mode and ion spray voltage of 4kV was used for the detection and quantification of all the analytes.The quantification was performed in a multiple reaction monitoring(MRM)mode. The optimized parameters,elemental composition,and mass measurement of ions obtained from standard solutions for all compounds are summarized in Table 2.A triple quad system was set on the unit resolution.The peak area of all the components was integrated using Mass hunter software version B.08(Agilent Technologies,Inc.).
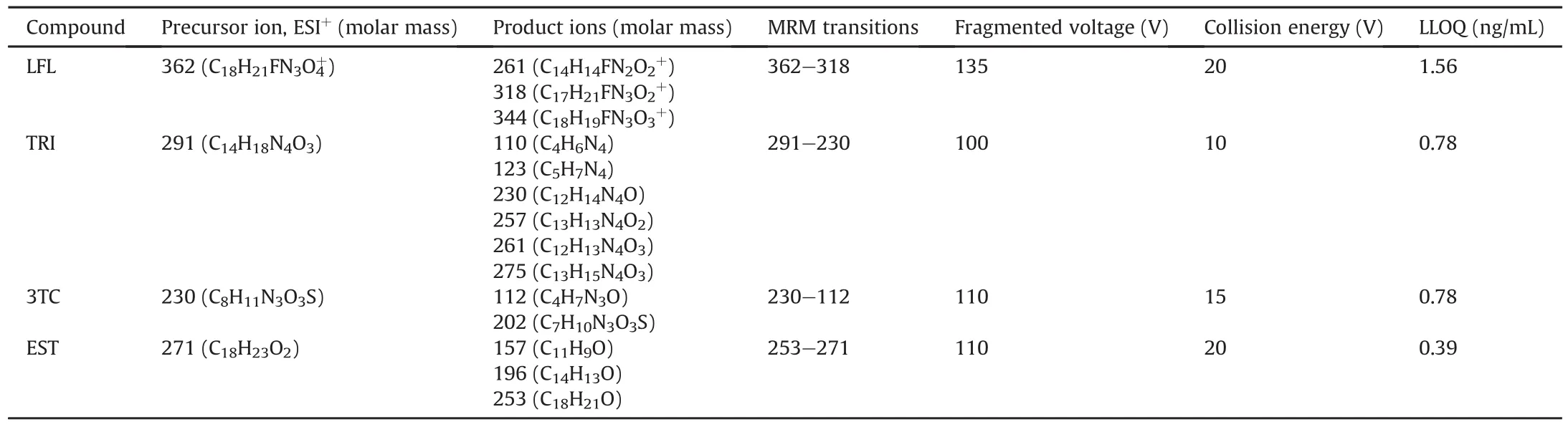
Table 2 MRM parameters and LLOQ concentration for the tested pharmaceuticals.
2.4.2.Preparation of calibration standards and quality control(QC)samples
Working stock solution of LFL was prepared by diluting the stock solution with mixed buffer containing 0.5% FA in ammonium acetate(pH 4.7;1mM)and MeOH(1:1,V/V).The working stock solution of 3TC,TRI,and EST were prepared in a range of 0.039-10μg/mL using MeOH.
The calibration standards and QC samples were prepared by spiking blank urine with a working stock solution of the corresponding concentration.Calibration curve standards of the following concentrations were made:0.39,0.78,1.56,3.12,6.25,12.5,25,50 and 100ng/mL.
The QC samples were prepared in triplicates at four concentration levels.The low-low quality control for LFL and EST was 0.39ng/mL while it was 0.78 ng/mL for 3TC and TRI.The low-quality control for LFL and EST was 0.78ng/mL,and it was 1.56 ng/mL for 3TC and TRI.The middle-quality control was 6.25ng/mL,while the highquality control was 100ng/mL for all the pharmaceuticals.20μL of respective IS(5μg/mL)was added to all the spiked urine samples.All the solutions and samples were kept at 4°C until use.
2.4.3.Sample preparation
A simple dilute and shoot method was used for the extraction of LFL from urine,while liquid-liquid extraction was used for the removal of 3TC,TRI,and EST.
To an aliquot of 500μL of blank urine sample with LFL,20μL of respective IS(5μg/mL)was added and vortexed for 20 s.To this,1 mL of mixed buffer was added to precipitate out the proteins from urine.All the samples were then vortexed vigorously for 3min and centrifuged at 13,000 rpm for 15 min.The supernatant was transferred to an autosampler vial and was injected into UPLC-MS/MS.
For 3TC,TRI,and EST,100μL of urine sample was mixed with 3 mL of ethyl acetate and 0.1% FA.20μL of respective IS(5μg/mL)was added in all the three pharmaceutical samples.The samples were then vortexed for 10 min and centrifuged at 5,000 rpm for 30 min.After centrifugation,the upper organic layer was collected in fresh vial and dried under a vacuum.The samples were reconstituted in 200μL of MeOH/H2O(2:1,V/V).The samples were then injected into UPLC-MS/MS via autosampler.
All the calibration standards,QC samples,and the MFC samples(urine spiked with LFL,3TC,TRI,and EST)were prepared similarly.
2.4.4.Method validation and quantification
The method was validated for selectivity,linearity,accuracy,and precision.The analytes were identified according to peak obtained at their retention time.The calibration standards for all the analytes were made in the range of 0.39ng/mL to 100 ng/mL from their working stock solutions.
The calibration curves were developed daily(by comparing the calculated peak area ratio of CIP to the IS vs.the nominal concentration)for all the working concentrations by injecting each concentration thrice.The results thus generated were analyzed using the regression equation.The quadratic regression value obtained from calibration curves was used to evaluate the squared coefficient of determination for all pharmaceuticals(r2>0.998).
The selectivity of the method was studied by comparing chromatograms of blank urine,with the chromatogram for urine samples spiked with different pharmaceuticals.The blank urine sample was tested for interference,and any carryover effect was reduced by optimizing the runtime of the analytes and by the MRM method.
Linearity was tested for a concentration between 0.39-100ng/mL.It was assessed for five consecutive days.The calibration curves were generated by plotting peak area ratios(analyte/IS)vs.concentration and processed by using the weighing factor(1/x2).
The precision(presented as relative standard deviation,% RSD)and accuracy(% bias)of the developed chromatographic method were assessed by analyzing QC samples at four concentrations.It was obtained from the repeated injections of the blank urine spiked with different concentrations(n=5)during the same day(repeatability)and in five days(reproducibility).The accuracy(inter-day and intra-day)should be within±15% standard deviation.The precision was calculated by using one-way analysis of variance(ANOVA)and should be within±15% RSD[19].
The lower limit of quantification(LLOQ)of the method was determined by injecting spiked urine samples(n=3)and calculated with a signal to noise ratio of 3:1 and 10:1.The LLOQ was in accordance with RSD calculated for the intercept and the slope obtained for the calibration curve.
3.Results and discussion
3.1.Reactor startup and operation
The microbial inoculum mixed with synthetic media was injected into the anode chamber.Microorganisms can extract electrons from organic materials(oxidation)and feed them into an electrical circuit to generate power.Therefore,the inoculation of the anode is a crucial step during the MFC reactor startup.The microorganisms multiply,grow in number,and accumulate at anode surface,forming a biofilm.These bacterial communities utilize the components of the media for survival and growth and hence get the reactor started.The reactor was monitored for voltage production during the inoculation period.The open-circuit voltage produced during the end of the first day of inoculation was(57±4)mV.After that,the reactor was operated as a closed-circuit system with 100Ω external load.The voltage recorded at the end of the first week was(234±17)mV.The feed was changed to urine after a month of inoculation,and fresh urine was added,once the voltage dropped down to 50 mV.After three operating cycles(one week each),the maximum voltage of(480±22)mV was produced.This voltage remained steady for two months of operation.The reproducibility of the voltage indicates the attachment of bacteria to the anode and electrochemically active biofilm.Fig.1 shows the reactor’s voltage generation curve during the startup process.
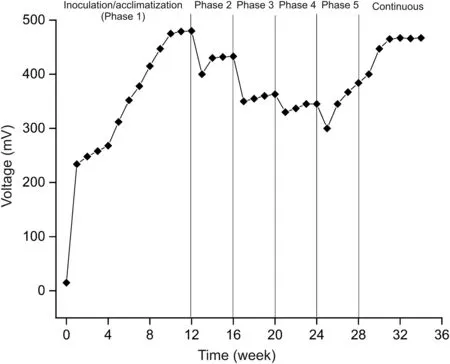
Fig.1.Voltage curve of microbial fuel cell(MFC)reactor at different phases of pharmaceuticals addition.Phase 1:fresh urine without any pharmaceutical,Phase 2:EST spiked urine,Phase 3:TRI and EST spiked urine,Phase 4:3TC,TRI,and EST spiked urine and Phase 5:LFL,3TC,TRI,and EST spiked urine.
After three months of reactor start-up,urine spiked with EST was added at a concentration of 2μg/mL.The concentration was selected based on data presenting the trace amounts of pharmaceuticals quantified from environmental samples[20-22].When EST spiked urine was added into the reactor,the voltage dropped,indicating the inhibitory effects(bactericidal)of EST on the biofilm.For Phase 2 on EST addition,the voltage decreased to(400±14)mV.Once the voltage was stabilized,TRI was added(after a month),and the voltage further decreased to(350±11)mV.The addition of 3TC further reduced the voltage to(330±15)mV.LFL was added at the final Phase 5,and the voltage dropped to 300 mV.
From the voltage curve,it can be seen that with the addition of each pharmaceutical,there was a voltage drop at every phase.Fig.1 shows that EST has a maximum inhibiting effect on MFC performance,while the least is shown by LFL,the reason being longer half-life of EST and structure that is more complex.After 7 months of operation,the voltage started to increase slowly and reached(467±12)mV,which was reproducible and further remained constant.This showed that the biofilm got adapted to the added pharmaceuticals,and the microorganisms could utilize the organic matter effectively.The maximum power output obtained was 2.18mW with the current of 4.67 mA at 100Ω.The power density was 0.88W/m2,with a current density of 1.89 A/m2.
MFC performance was also evaluated in terms of COD removal.The change in COD was attributed to the oxidation of organic matter by microorganisms present in urine.The initial COD of the urine influent at Phase 1 was(7892±205)μg/mL that reduced to(1736±113)μg/mL after 2 months of reactor operation.The average COD removal efficiency of 78% was observed in the absence of pharmaceuticals.The addition of EST increased the COD enormously to(9345±402)μg/mL.Further,the reactor showed variation in COD as well as voltage produced with the addition of each pharmaceutical initially.However,after steady operation of the MFC for 7-8 months,COD decreased to(1682±104)μg/mL.The maximum COD removal rate of 93% was seen at Phase 5 when the reactor acclimatized with all the pharmaceuticals.The reduction in COD and stabilized voltage shows that with time,the biofilm’s microbial community has been adapted to the pharmaceuticals,and hence they can degrade them.The effect of pharmaceuticals on COD removal is shown in fig.2.To quantify the percentage reduction in the concentration of each pharmaceutical,the samples were analyzed using UPLC-MS/MS.
3.2.UPLC-MS/MS method development
To determine the effect of added pharmaceuticals on MFC performance,UPLC-MS/MS was used to quantify the variation of pharmaceuticals and observe their removal at the same time.
3.2.1.Chromatography and MS
The method optimization was started with repetitive injections of individual standards into the mass system.All the compounds were analyzed in ESI mode,both negative and positive.However,the positive mode was selected as it gave better sensitivity.
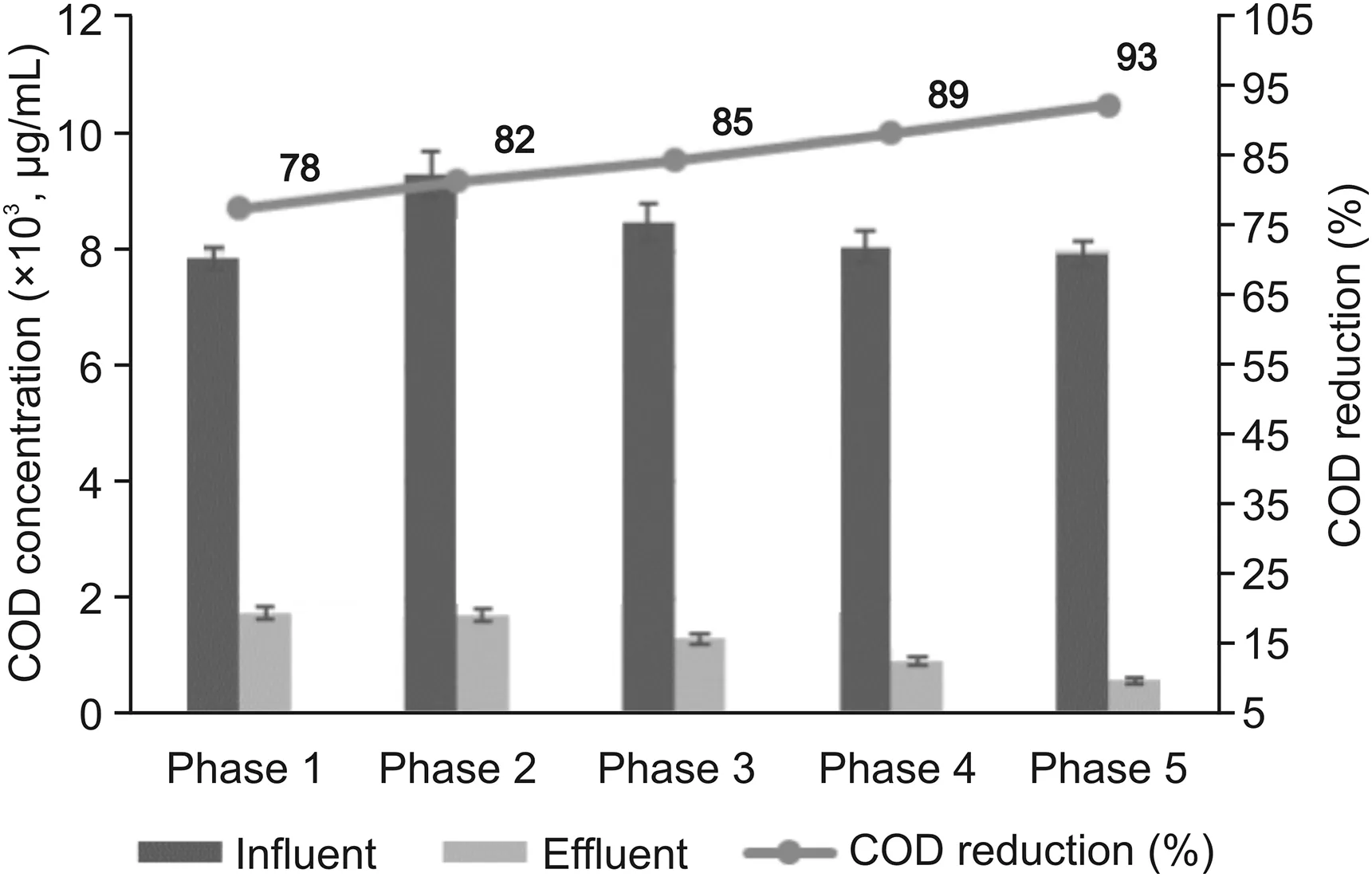
Fig.2.Change in chemical oxygen demand(COD)in MFC at different phases with different pharmaceuticals addition.Phase 1:fresh urine without any pharmaceutical,Phase 2:EST spiked urine,Phase 3:TRI and EST spiked urine,Phase 4:3TC,TRI,and EST spiked urine and Phase 5:LFL,3TC,TRI,and EST spiked urine.
The choice of mobile phase is an important factor in achieving better separation of analytes.Different solvents such as MeOH,FA,Ammonium acetate,and ACN were tested as eluents.For LFL,the mobile phase was selected,as mentioned in our previous research[13].The FA addition at a concentration of 0.1%(3TC,TRM),0.01%(EST)and 0.5%(LFL)gave better sensitivity and sharp peaks.EST was eluted in isocratic mode with ammonium acetate and MeOH as mobile phases.Both 3TC and TRI were eluted using MeOH and Milli-Q water as mobile phases.Different columns,such as Agilent Eclipse plus C18(2.1 mm × 150 mm,3.5μm),Agilent C18(2.1 mm×100 mm,1.8μm),Agilent C100(2.1 mm×50 mm,1.0μm)and Agilent C8(150 mm ×4.6 mm,5μm),were tested for the analytical separation.The Agilent C18(2.1 mm×100 mm,1.8μm)Zorbex Eclipse plus column for LFL,Agilent C8(150 mm×4.6 mm,5μm)for TRM,3TC and EST,gave better separation and peak shape.The sensitivity of the method was enhanced,and the interference in the peaks was reduced by using MRM mode.The[M+H]+ion was used as a precursor ion,while induced fragmentation gave product-ions(Table 2).The MRM spectrum and positive ion spectrum for all the analytes are shown in figs.3-5.
Due to the presence of complex components in biological samples,the extraction of the drugs can be a difficult step.Solidphase extraction(SPE)is generally used in extraction to remove matrix effects from human serum and plasma.However,the cost associated makes it uneconomical to use.SPE has not been used in this study,as urine is less complicated than plasma.Therefore,simple preparation methods like protein precipitation,liquid-liquid extraction,dilute,and shoot extraction,as reported in previous studies[23],have been investigated for efficient extraction of pharmaceuticals from urine.For the extraction of LFL,the dilute and shoot method has been used while other drugs(3TC,TRI,EST)were extracted by using liquid-liquid extraction,as mentioned earlier[23].
3.2.2.Method validation
The developed method was validated according to the FDA Bioanalytical Method Validation guidelines[24].The method showed a linear response over the calibration range of(0.39-100)ng/mL for EST,(0.78-50)ng/mL for 3TC and TRI,(1.56-100)ng/mL for LFL with an r2value of 0.998 for all analytes.The lack of interfering peaks in the urine sample(blank)corresponding to the retention time of all analytes with respect to their IS shows the specificity of the method.The compounds were well separated from the respective IS under the applied chromatographic conditions with retention time mentioned in Table 2.The MRM spectrum shows good separation of all the compounds.
The rate of elution of the compound tells about the matrix effect shown by individual compounds.The compounds,which elute early,show a lower matrix effect.The matrix effect on the quantification of all compounds was determined by finding out the accuracy of QC samples prepared in fresh urine against the calibration curve.For all the studied compounds,the matrix effect was found to be insignificant for the consideration(<10%).
The accuracy and precision for all the analytes were conducted at four fortification levels using five replicates of each concentration level.This was repeated for three consecutive days.The accuracy of the method was based on the percentage bias of the QC samples.The precision was evaluated by using a one-way ANOVA on the RSD data.The inter-day and intra-day accuracy and precision for all the compounds are shown in Table 3,respectively.
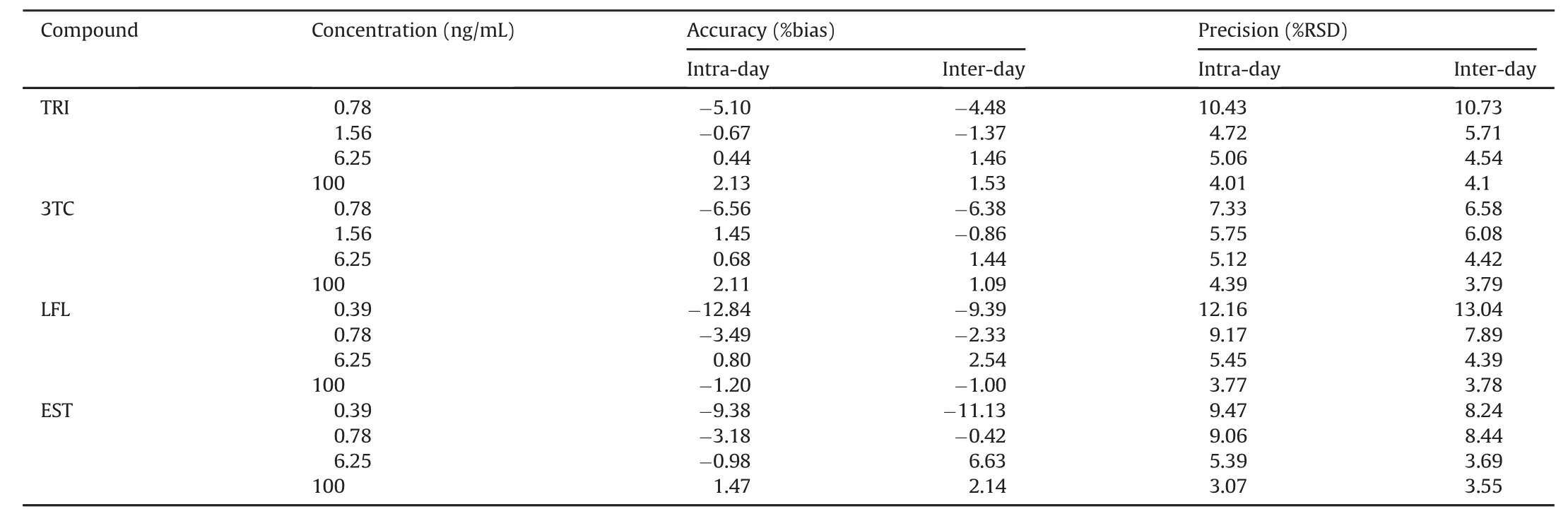
Table 3 Intra-day and inter-day assay precision and accuracy of used pharmaceuticals.
The results obtained were analyzed by linear regression equation acquired in the calibration curve.The achieved r2=0.998 value with y=21.28x(TRI),y=50.11x(3TC),y=41.71x(LFL)and y=32.46x(EST)defines the linearity of the method.The validation of the method was Confirmed by the values achieved for the intercept and the slope with their standard deviation.The LLOQ for EST was 0.39 ng/mL,for 3TC and TRI was 0.78ng/mL and 1.56 ng/mL for LFL.
3.3.Analysis of MFC treated urine samples
Urine is rich in N and P;however,at the anode,microbes utilize very less concentration of these compounds for their growth(5±2)%[18].Therefore,there is a wide scope of recovering these N and P.Ureases are the enzymes that convert N into NH4+/NH3.In MFC,NH4+and other cations migrate from anode to cathode through the membrane that leads to a concentration gradient between two chambers with time.The pH in the cathode chamber increases due to the accumulation of hydroxyl ions.Phosphates and NH4+in the cathode chamber at alkaline pH can be precipitated by adding a magnesium source.
The existence of different kinds of pharmaceuticals in the environment(mostly excreted in urine)has recently gained interest due to the risks associated with their persistent nature(environment and health risks).Therefore,screening of urine and characterization of the recovered product(N,P as struvite)is essential to ensuring that it does not contain any pharmaceutical residue that poses a risk to the environment and human health.The physicochemical characteristics of neat urine and percentage reductions in various parameters on pharmaceutical/hormone addition are shown in Table 4.To analyze,quantify,and monitor the fate of all pharmaceuticals in MFC,UPLC-MS/MS method was developed and validated.This method was employed in studying the behavior of collected samples from MFC at specific time intervals.
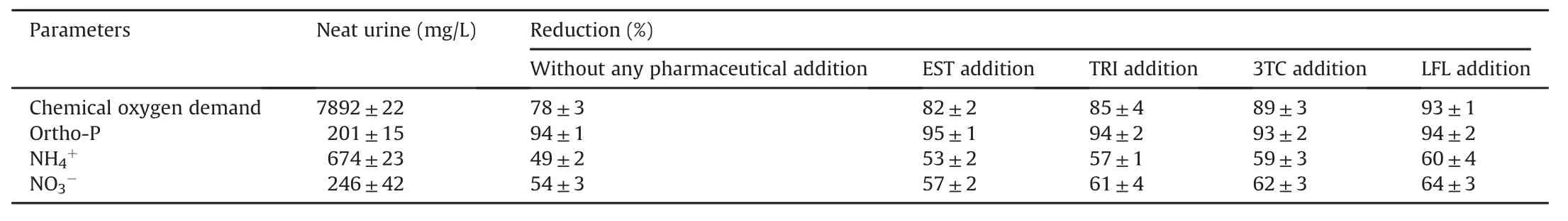
Table 4 Physicochemical parameters analysis of neat urine and reduction percentage on pharmaceuticals addition.
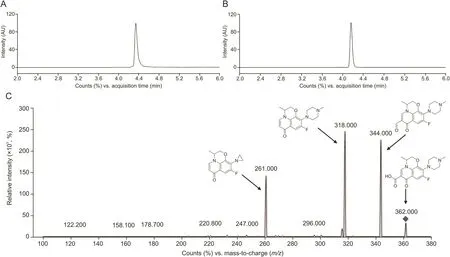
Fig.3.Chromatogram and positive ion electrospray mass spectra of LFL;(A)MRM of CIP(retention time:4.4 min),(B)MRM of LFL(retention time:4.1min),(C)product ion spectra of LFL.LFL:levofloxacin;MRM:multiple reaction monitoring;CIP:ciprofloxacin.
Degradation of antibiotics using MFC has been explored due to its low operational costs[12].MFC has been used in our study to recover nutrients from urine in the form of struvite(MgNH4PO4·6H2O),which is a slow phosphorus release fertilizer.Urine spiked with four different pharmaceuticals was used to study their degradation in MFC.The voltage generation during the inoculation period indicated successful attachment of microorganisms at the anode.The change in COD showed the microbial oxidation of organic matter present in urine.
The performance of MFC declines on the addition of pharmaceuticals as increased organic load affects the metabolic functioning of microorganisms.The pharmaceuticals affect the microbial community by slowing down their metabolism.The continuous operation of MFC with spiked urine slowly acclimatizes the biofilm to the pharmaceuticals.
The initial effluent samples showed a higher concentration of the added pharmaceuticals.However,operating MFC for several cycles in batch mode resulted in reactor acclimatization to the added pharmaceutical.At the end of 7 months,the anolyte was changed after every 2 days,and samples were collected at different time intervals and analyzed.The change in concentration with time is shown in fig.6.The analysis showed that with the retention time of 25-30 h,the MFC was able to biodegrade the pharmaceuticals.During the experiment,the initial concentration was 2000 ng/mL(2μg/mL),which declined to 78.58 ng/mL for EST,58.39 ng/mL for TRI,70.15 ng/mL for 3TC and 37.29 ng/mL for LFL,equivalent to(96±2)% cumulative reduction.
Previously,MFC used in treating tetracycline wastewater resulted in a 90% COD reduction (initial concentration of(850±50)μg/mL)and 99% tetracycline degradation[25].However,with COD as high as(7000±200)μg/mL,the maximum COD reduction of 93% was achieved in our work.The reduction in COD could be attributed to the microorganisms as they are responsible for the direct oxidation of these pharmaceuticals by adsorbing them at anode surface[26].The electron transfer mechanisms(ATP level)in microbes are strong enough to degrade antibiotics using MFC.Antibiotics have also been degraded using electrochemical processes along with osmosis[25].
Due to the presence of pharmaceuticals,there was a change in bacterial communities,and thus,the weakened electron transfer from microorganisms.The presented results showed a decrease in voltage from 480 mV to 467mV.However,the COD removal and degradation of pollutants were more significantly increased with the addition of pharmaceuticals.Therefore,MFC provides a platform for the reduction of the organic matter and pharmaceuticals by integrating the microbial oxidation and electrochemical transformation.
3.4.Biodegradation mechanism
Biodegradation is an eco-friendly and cost-effective method that plays a significant role in the cleaning environment by degrading contaminants.Microorganisms mineralize the organic pollutants into less harmful intermediates that are further degraded by natural metabolic reactions.However,the microbial biodegradation pathways for most of the pharmaceuticals and hormones have not been characterized,which makes it challenging to identify the genes responsible in the mineralization process.The removal of organic compounds is studied by using two processes,biosorption and biodegradation.The latter one is majorly used and considered more favorable.Microbial degradation of pharmaceuticals/hormones can be either metabolism-based or co-metabolism based.Most of the biodegradation processes are metabolism-based in which microbes utilize these compounds as carbon or energy source for their growth.The initial characterization of the biofilm,inoculum,and urine is presented in our previous work[18].The biofilm comprises bacterial species mainly from Proteobacteria and Firmicutes.The major species identified belong to Pseudomonas,Escherichia,Bacillus,Proteus,Tissierellia,Alcaligens,etc.
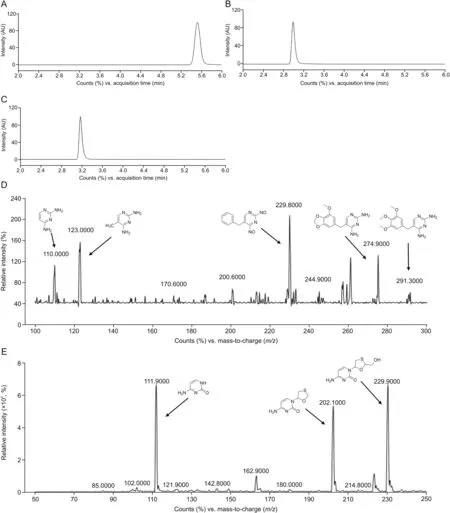
Fig.4.Chromatogram and positive ion electrospray mass spectra of TRI and 3TC;(A)MRM of CBZ(retention time:5.5 min),(B)MRM of TRI(retention time:2.9 min),(C)MRM of 3TC(retention time:3.1 min),(D)product ion spectra of TRI,(E)product ion spectra of 3TC.TRI:trimethoprim;3TC:lamivudine;CBZ:carbamazepine.
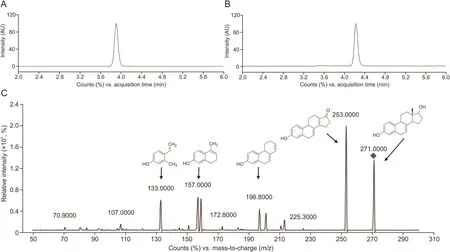
Fig.5.Chromatogram and positive ion electrospray mass spectra of EST.(A)MRM of DXM(RT=3.8 min),(B)MRM of EST(RT=4.25min),(C)Product ion spectra of EST.
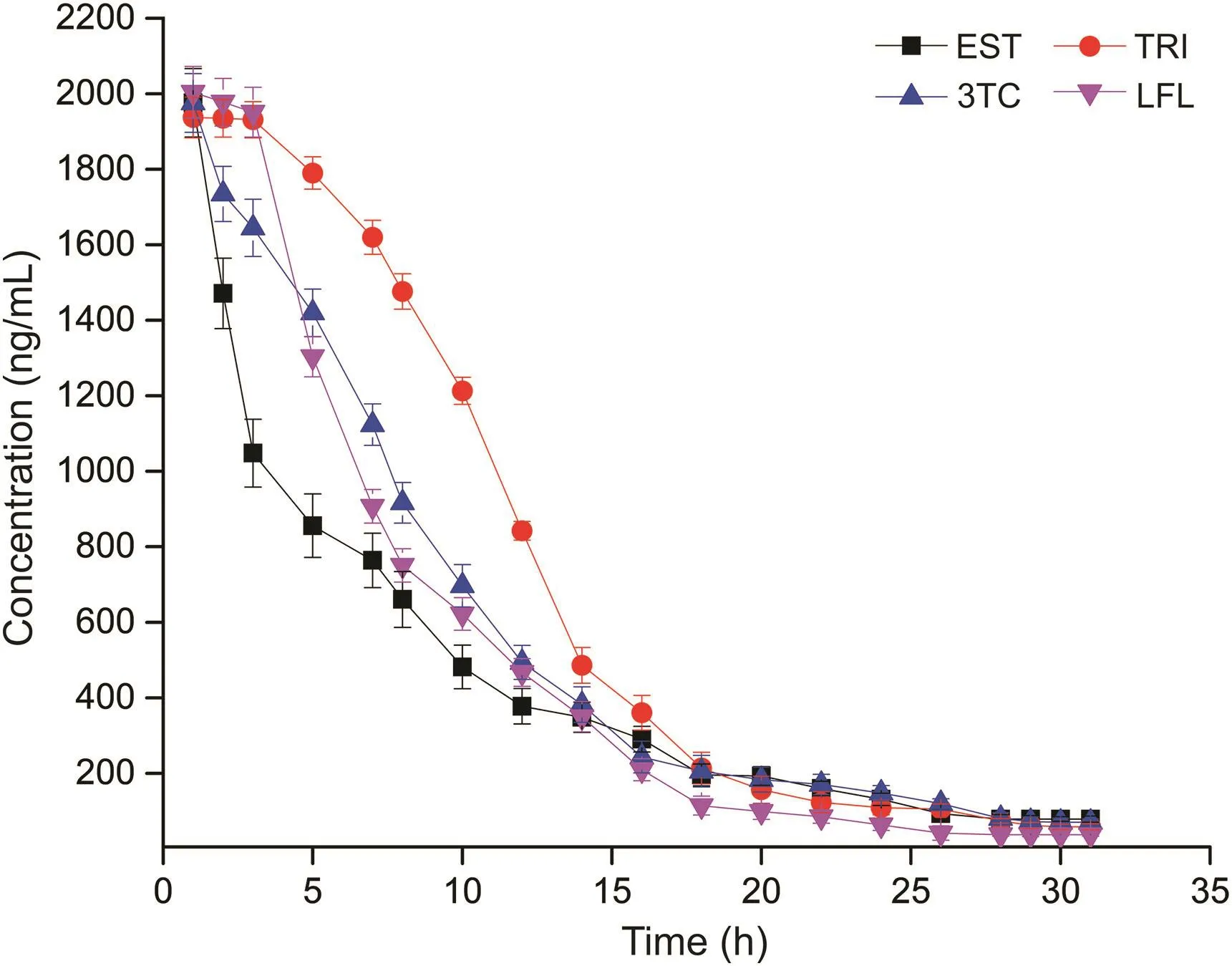
Fig.6.Variation in effluent concentration of spiked pharmaceuticals with time.
Earlier studies have shown the role of human gut bacteria and intestinal bacteria in degrading organic pollutants.E.coli,Alcaligens,and Pseudomonas species have been reported in EST degradation using growth linked reactions[27].The degradation pathway involved:hydroxylation of ring A(aromatic ring)at C-4;hydroxylation of saturated ring;dehydration of ring D(cyclopentane ring)at C-17;and dehydrogenation of ring D at C-17.Members of the Rhodococcus genus have been widely studied in biodegradation of TRI.Later it was found that the same degradation mechanism is followed by some Pseudomonas and Bacillus species,and thus they can be involved in degrading sulfonamides too.These bacteria produce some enzymes that help in the biodegradation process.The primary enzyme involved is acrylamine N-acetyltransferase that has specificity for aromatic amines and uses them as substrate[28].
The microbial degradation of fluorinated compounds like LFL can be either aerobic or anaerobic metabolism.Mono-or dioxygenase/Reductase enzymes attack on the aromatic compound and convert them to dihydroxy aromatic intermediates.These intermediates are further cleaved by intradiol/estradiol dioxygenases or used as substrate for corresponding dearomatizing reductases.The metabolites generated are channeled to central metabolic pathways where they are further degraded into very simple compounds and are utilized for microbial growth[29].The role of the above-mentioned microorganisms in degrading 3TC has not been reported.According to one of the reported work,3TC is listed as non-biodegradable and inhibitory to the bacterial consortia obtained from activated sludge[5].In our study,the degradation observed could mainly be because of biosorption on the anode surface.However,more research is required for 3TC removal.Thus,it can be concluded that species belonging to Pseudomonas,Escherichia,Bacillus,and Alcaligens are involved in biodegradation,and they are still present in the biofilm(after pharmaceuticals addition experiment).The reduced performance of MFC on addition of pharmaceuticals may be attributed to the hindrance in metabolic activity of other microorganisms present in the biofilm that participated in co-metabolism.
4.Conclusion
The chemical precipitation in MFC through pH elevation can be used to recover N and P as struvite from urine.Struvite can be used as a slow phosphorus release fertilizer in agriculture.The struvite was precipitated by adding the necessary quantity of magnesium to the MFC effluent.However,before considering the suitability of the struvite in agriculture,it must be analyzed for micropollutants like pharmaceuticals and hormones.
The described UPLC-MS/MS method serves as a useful tool for analyzing four different pharmaceuticals in the urine samples collected from MFC.This method provides good linearity over a concentration range of 0.39-100ng/mL.The degradation of pharmaceuticals in MFC was achieved by operating the MFC for seven months with spiked urine under anoxic conditions with an initial concentration of 2mg/L(2000 ng/mL).The reactor was operated in batch mode,and the pharmaceuticals were added after three months of inoculation period.During this period,the COD behavior of the reactor was evaluated to get an insight into the microbial biodegradation of organic compounds.The change in the concentration only seen after acclimatization of the biofilm to the respective added compound,indicating the microbial acclimatization in MFC.The biofilm utilizes these pharmaceuticals as a carbon and nitrogen source along with secreting some enzymes that can help with degradation.It was seen that with a hydraulic retention time of 30 h in MFC,96%±2% biodegradation could be achieved.The decomposition of pharmaceuticals may be attributed to the microbial biodegradation,oxygen diffusion at the cathode,and the power generated in the MFC system.Hence,MFC can be employed in bioremediation processes for environment-deteriorating pollutants.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
