Constructing 1D/2D Schottky-Based Heterojunctions between Mn0.2Cd0.8S Nanorods and Ti3C2 Nanosheets for Boosted Photocatalytic H2 Evolution
2021-07-13ZhiminJiangQingChenQiaoqingZhengRongchenShenPengZhangXinLi
Zhimin Jiang , Qing Chen , Qiaoqing Zheng , Rongchen Shen , Peng Zhang *, Xin Li ,*
1 Key Laboratory of Energy Plants Resource and Utilization, Ministry of Agriculture and Rural Affairs, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou 510642, China.
2 College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China.
3 State Center for International Cooperation on Designer Low-Carbon & Environmental Materials (CDLCEM), School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China.
Abstract: Sustainable photocatalytic H2 evolution has attracted extensive attention in recent years because it can address both energy shortage and environmental pollution issues. In particular, metal sulfide solid-solution photocatalysts have been widely applied in photocatalytic hydrogen generation owing to their excellent light harvesting properties, narrow enough band gap, and suitable redox potentials of conduction and valance bands. However, it is still challenging to develop low-cost and high-efficiency sulfide solid-solution photocatalysts for practical photocatalytic hydrogen evolution. Recently, 1D MnxCd1−xS nanostructures have shown superior light absorption, charge separation, and H2-evolution activity owing to their shortened diffusion pathway of carriers and high length-to-diameter ratios. Thus, 1D MnxCd1−xS nanostructures have been applied in photocatalytic H2 evolution. However, a single MnxCd1−xS photocatalyst still has some disadvantages for photocatalytic H2 evolution, such as the rapid recombination of photogenerated electron-hole pairs and low quantum efficiency. Herein, to further boost the separation of photogenerated charge carriers and H2-evolution kinetics, an in situ solvothermal method was used to synthesize the 1D/2D Schottkybased heterojunctions between the Mn0.2Cd0.8S nanorods (MCS NRs) and Ti3C2 MXene nanosheets (NSs). Furthermore,various characterization methods have been used to investigate the crucial roles and underlying mechanisms of metallic Ti3C2 MXene NSs in boosting the photocatalytic H2 evolution over the Mn0.2Cd0.8S nanorods. X-ray Diffraction (XRD),Transmission Electron Microscope (TEM), High Resolution Transmission Electron Microscopy (HRTEM), element mapping images, and X-ray Photoelectron Spectroscopy (XPS) results clearly demonstrate that hybrid low-cost Schottky-based heterojunctions have been successfully constructed for practical applications in photocatalytic H2 evolution. Additionally,the photocatalytic hydrogen evolution reaction (HER) was also carried out in a mixed solution of Na2SO3 and Na2S using as the sacrificial agents. The highest hydrogen evolution rate of the optimized 1D/2D Schottky-based heterojunction is 15.73 mmol·g−1·h−1, which is 6.72 times higher than that of pure MCS NRs (2.34 mmol·g−1·h−1). An apparent quantum efficiency of 19.6% was achieved at 420 nm. The stability measurements of the binary photocatalysts confirmed their excellent photocatalytic stability for practical applications. More interestingly, the UV-Vis diffuse reflection spectra,photoluminescence (PL) spectrum, transient photocurrent responses, and Electrochemical Impedance Spectroscopy (EIS)Nyquist plots clearly confirmed the promoted charge separation between the MCS NRs and Ti3C2 MXene NSs. The linear sweep voltammetry also showed that the loading of MXene cocatalysts could greatly decrease the overpotential of pure MCS NRs, suggesting that the 2D Ti3C2 NSs could act as an electronic conductive bridge to improve the H2-evolution kinetics. In summary, these results show that the 2D/1D hybrid Schottky-based heterojunctions between metallic Ti3C2 MXene NSs and MCS NRs can not only improve the separation of photogenerated electrons and holes but also decrease the H2-evolution overpotential, thus resulting in significantly enhanced photocatalytic H2 generation. We believe that this study will inspire new ideas for constructing low-cost Schottky-based heterojunctions for practical applications in photocatalytic H2 evolution.
Key Words: Photocatalytic hydrogen evolution; 1D Mn0.2Cd0.8S nanorods; Ti3C2 MXene NSs;2D/1D Schottky-based heterojunctions; Solar fuel
1 Introduction
Energy shortage and environmental pollution are becoming more and more severe in today’s world1,2. Exploring the environmentally friendly clean energy is urgent for the sustainable development3. Making full use of clean energy sources in nature, such as solar energy and wind energy, to produce the sustainable fuels and chemicals has been attracting more and more interest in recent years2–5. Especially, H2is an ideal fuel due to high combustion value as well as the only combustion product, H2O, without any other pollution6. Thus,since Honda and Fujishima first found photoelectrochemical H2production over Pt attached to a photoanode ofn-type semiconductor titanium dioxide (TiO2) under ultraviolet light in 19727, many semiconductors, such as, TiO28, CdS9–14, g-C3N415–19and have been discovered and applied in photocatalytic H2evolution20,21. Recently, metal sulfides have been received increasing attention because they have admirable light harvesting property, narrow enough band gap and suitable redox potentials of conduction and valance bands.However, single photocatalyst still has limited applications in photocatalysis due to its disadvantages of the rapid recombination of photogenerated electron-hole pairs and low quantum efficiency13,14.
Recently, ternary sulfide solid solutions have been drawn extensive attention22, owing to their tunable electronic structure and superior light absorption7,23. ZnxCd1−xS24–27, MnxCd1−xS7,28and ZnIn2S429have been synthesized for photocatalytic HER.Among these solid solutions, MnxCd1−xS solid solution has been seen as one of promising semiconductor photocatalysts for producing H2from water under visible light30. Especially, 1D MnxCd1−xS nanostructures contribute to the improved separation of photogenerated electrons and holes and the light absorption because of their characteristics of shortening the diffusion pathway of carriers, and high length-to-diameter ratios31. Even so, single ternary solid solution is failure to satisfy all the needs in photocatalysis. Thus, in order to further improve hydrogen production efficiency and the apparent quantum efficiency(AQE) of metal sulfides, many approaches have been employed,for example, the design of Z-scheme (or S-scheme) system32–35,p-njunction36, loading of conductive nanomaterials and cocatalysts6,21,37–39. Among them, the strategy of coupling conductive nanomaterials with semiconductors has been confirmed as one of the efficient and facile method for enhancing charge separation and photocatalytic efficiency. Meanwhile,metallic MXenes have aroused growing concerns due to their amazing two-dimensional (2D) structure, high conductivity and superior electrocatalytic activity40. Among of various kinds of transition metal carbide/nitride MXenes with graphene-like materials, metallic Ti3C2has been extensively researched41,42.2D Ti3C2NSs possessed superior visible light absorption, good structural stability as well as excellent electrical conductivity43.Notably, metallic Ti3C2NSs have been used as cocatalysts for boosting photocatalytic H2evolution or CO2reduction41,42,44–46.Impressively, various nanostrcutured Schottky-heterojunctions between different dimension semiconductors and Ti3C2MXene,such as Ti3C2/g-C3N443,47, Ti3C2/ZnIn2S448, ZnS/Ti3C249,CdLa2S4/MXene50, ZnxCd1−xS/MXene49, CdS/MXene51,52and TiO2/MXene Ti3C253, have been widely constructed and applied in efficient photocatalytic H2evolution. More interestingly,MXene-based dual cocatalysts, such as Mxene/MoS254and Mxene/WS255, and MXene NS-confined cocatalysts56have been widely demonstrated to be excellent strategies for further boosting their photocatalytic H2generation. However, to date, to our best knowledge, MnxCd1−xS/Ti3C2has been not reported for photocatalytic H2evolution.
Herein, we successfully constructed the 2D/1D hybrid Schottky-based heterojunctions between metallic Ti3C2MXene NSs and Mn0.2Cd0.8S nanorods, and deeply investigated the key roles and underlying mechanisms of metallic Ti3C2MXene NSs in boosting the photocatlytic H2evolution over Mn0.2Cd0.8S nanorods. It is expected that this study could render a promising strategy to design low-cost sulfide-based hybrid photocatalysts for markedly enhanced visible-light photocatalytic H2generation.
2 Experimental and computational sections
2.1 Preparation of photocatalyst
2.1.1 Raw materials
All chemical reagents are of analytic grade and used as received without further purification. Mn(CH3COO)2·4H2O (≥99.0%), Cd(CH3COO)2·2H2O (≥ 99.5%), Ti3AlC2(98%),CH4N2S (≥ 99.0%), (AR, 40.0%), C2H8N2(≥ 99.0%), H2SO4(95.0%–98%), Na2SO4(99.0%), Na2S·9 H2O (≥ 99.0%), Na2SO3(≥ 97.0%).
2.1.2 Synthesis of 2D Ti3C2MXene NSs
Ti3C2NSs were fabricated through following the previously reported processes43. In detail, 1 g Ti3AlC2was slowly put into 20 mL HF (AR, 40.0%) and stirred for 3 days at room temperature. Then, the black suspension was washed by purified water and ethyl alcohol until the pH becoming neutral. At last,the prepared Ti3C2MXene NSs was dried at 60 °C.
2.1.3 Synthesis of Mn0.2Cd0.8S/xTi3C21D/2D heterojunctions
The Mn0.2Cd0.8S/xMXene heterojunctions were synthesized by a simplein situsolvothermal method. In a typical process,0.6 mmol of Mn (CH3COO)2·4H2O, 2.4 mmol of Cd (CH3COO)2·2H2O and excess of thioacetamide were dissolved in 60 mL of ethane diamine. Subsequently, the Ti3C2NSs (0, 0.3, 0.5, 1, 2, 5 mg) were added into the suspension with an ultrasound for about 30 min. Then the prepared suspension was transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 160 °C for 24 h. Finally, the as-obtained product was washed with DI water and ethanol three times, respectively, which were denoted as MCS, MCS/0.3 MXene, MCS/0.5 MXene, MCS/1 MXene,MCS/2 MXene and MCS/5 MXene, respectively.
2.2 Characterization
X-ray diffraction (XRD) was employed to identify the chemical compositions and crystal structures of 1D Mn0.2Cd0.8S nanorods as well as the heterojunctions. Shimadzu UV-2600 PC diffuse reflectance spectroscope (UV-Vis DRS, Kyoto, Japan)was performed to analyze the light absorption ability of the photocatalysts. By using transmission electron microscopy(TEM) and high resolution TEM (HRTEM; JEM-2100HR 200 kV, Japan), the structures and morphology of the photocatalysts were studied. X-ray photoelectron spectroscopy (XPS, a VG ESCALAB250 surface analysis) data was performed to obtain the chemical composition and surface states. Photoluminescence(PL) spectra were performed on a LS 50B PerkinElmer with an excitation wavelength of 331 nm. Brunauer-Emmett-Teller(BET) method was used to analyze the specific surface area.
2.3 Photocatalytic HER tests
Photocatalytic HER experiment was taken in a 100 mL threeneck round-bottomed Pyrex flask under illumination of a 300 W Xe lamp (PLS-SXE300, Beijing Perfect Light Technology Co.,Ltd., with intensityca.160 mV·cm−2) with a light filter (λ≥ 420 nm) at room temperature. In a typical experiment, 5 mg of photocatalyst was put into 80 mL of aqueous solution,containing 0.25 mol·L−1Na2SO3and 0.35 mol·L−1Na2S·9H2O.The suspension was sonicated for 15 min and then evacuated with N2for 15 min to remove the gas of the flask. The generated hydrogen was detected by GC-9500 online chromatograph. The formula for calculating the AQE was similar to our previous works11,57,58.
You see, I had already learned that one can derive instructive benefit from bad examples -- by avoiding that behavior. Alcoholism was a serious problem in my mother’s family. As a result of having seen enough examples of alcoholic9 excess in my childhood, I have never had any interest in drinking. The same applies to smoking. My poor mother was a two-pack a day victim of nicotine10 addiction11, and because of the endless clouds of smoke, the coughing, the overfilled ashtrays12, and the ugly smell of cigarette smoke in the house and in my clothing, I have never smoked a cigarette in my life.
2.4 Photoelectrochemical measurements
The working electrodes were prepared as follows: 5 mg of asprepared photocatalyst was added into the solution containing ethanol (2 mL) and Nafion solution (20 μL 0.25%). After sonicating for 2 h, 50 μL of the solution was injected onto a 2 ×3.5 cm2fluorine-doped tin oxide (FTO) glass substrate and dried under an infrared lamp (repeating the process 10 times). The resulting electrodes were then dried in an oven and calcined at 150 °C for 1 h in a N2gas flow.
2.4.1 Transient photocurrent tests
The transient photocurrent test was measured with an electrochemical analyzer CHI 660 (CH Instruments, Shanghai,China) in a three-electrode system. The as-prepared electrode,Ag/AgCl, and Pt plate were used as the working electrode,reference electrode, and counter electrode, respectively. 0.1 mol·L−1Na2SO4solution was used as the electrolyte. A Xe lamp(300 W) with a UV cut off filter (λ≥ 420 nm) was used as a light source.
2.4.2 Electrochemical impedance spectra (EIS) tests
The electrochemical impedance spectra (EIS) were also tested in a three-electrode system in a frequency range of 0.01–105Hz with an ac amplitude of 2 mV in the dark. The 0.1 mol·L−1Na2SO4aqueous solution was used as the electrolyte.
2.4.3 Mott-Schottky (MS) tests
2.4.4 Electrocatalytic hydrogen evolution
The electrocatalytic hydrogen evolution was tested in a threeelectrode cell, using Ag/AgCl as a reference electrode and Pt plate as the counter electrode. The test was performed in 0.5 mol·L−1H2SO4electrolyte solution with a 5 mV−1scan rate. The working electrodes were prepared as follows: 6 mg of photocatalyst powder were added into 2 mL of deionized water and sonicating for 2 h. The resulting samples were deposited on a glassy carbon electrode with 3 μL of prepared solution. After drying under the infrared lamp, 3 μL of Nafion solution (0.5%)was added on the catalyst layer and dried under the infrared lamp.
3 Results and discussion
3.1 The structure and morphology
The phase structure of the photocatalysts was tested by XRD.As shown in Fig. 1a, none of new peaks could be found after loading MXene on the MCS due to its low contents. Meanwhile,the crystallinity has obvious change after coupling with Mxene.With increasing the Ti3C2MXene content, the crystallinity obviously increased at first. The MCS/0.5 MXene heterojunction has the best crystallinity. On the contrary, when Ti3C2MXene content continuous to increase, the crystallinity of the heterojunctions became decreased. However, the crystallinity of MCS/5 MXene and MCS/2 MXene were still better than pure MCS NRs. Based on the previous reports, the crystallinity of the photocatalysts would significantly affect the photocatalytic HER. To some extent, the high crystallinity layer would result in better photocatalytic HER59,60. Additionally, the peaks of MCS show a little shift after loading MXene, especially for the angles of MCS/0.3 MXene and MCS/0.5 MXene. As shown in Fig. 1b,the peak of Ti3AlC2MXene at 19.17° belongs to (004) crystal facet, which was broadened and shifted to lower angle compared with the Ti3AlC2MXene after etching61. In addition, the peak at 39.0° absolutely disappeared after HF etching for 3 days, which indicated that the Al element in Ti3AlC2was thoroughly removed. The peak at 60.8° belongs to (110) crystal face of Ti3C2. Meanwhile, the peaks of Ti3AlC2after etching turned into weaker and broader. These results indicated that Ti3C2MXene was successfully synthesized.
We used the TEM and HRTEM to study the morphology and size of Mn0.2Cd0.8S solid solution. From the Fig. 2a, we can clearly see the nanostructure of the Mn0.2Cd0.8S solid solution.According to the previous report31, the 1D Mn0.2Cd0.8S nanorods with smooth surface were successfully synthesized. The Fig. 2b–d displayed the structure and morphology after loading MXenes.These images show that the MCS nanorods were successfully deposited on 2D Ti3C2MXene NSs with width of around 32–65 nm and length of around 0.5 to 3 μm. Although the new peaks of Ti3C2MXene NSs were not detected in the XRD results, we can readily find the existence of Ti3C2in the TEM results.Meanwhile, we could also find that the 2D layer structure of Ti3C2MXene clearly in the Fig. 2b–e, which suggest that the ultrathin Ti3C2NSs had been successfully synthesized. Some Ti3C2NSs were dark in color, which can be attributed to the bending of highly flexible Ti3C2NSs43. As shown in Fig. 2f, the lattice fringe of 0.240 nm was attributed to the (102) planes of Mn0.2Cd0.8S solid solution62, while the lattice fringe of 0.264 nm can be ascribed to Ti3C2NSs.
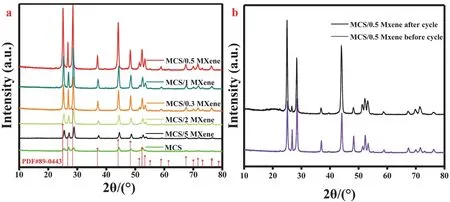
Fig. 1 (a) XRD patterns of MCS NRs, MCS/0.3 MXene, MCS/0.5 MXene, MCS/1 MXene, MCS/2 MXene and MCS/5 MXene.(b) Ti3AlC2 before and Ti3AlC2 after etching.

Fig. 2 (a) The morphology of Mn0.2Cd0.8S; (b), (c) and (e) the TEM of the composites of MCS/0.5 MXene;(d) the 2D Ti3C2 MXene NSs; (f) the lattice fringe of MCS and Ti3C2.

Fig. 3 (a) Field-emission scanning electron microscopy (FESEM) of MCS/0.5 MXene heterojunctions and corresponding EDS element mapping images of (b) Mn, (c) Cd, (d) S, (e) Ti and (f) C.
Fig. 3a–f show energy-dispersive X-ray spectroscopy (EDS)mapping of MCS/0.5 MXene heterojunctions. The Fig. 3a was the general view of the other pictures of Fig. 3. The EDS measurements of MCS/0.5 MXene composites confirmed the existence of Mn, Cd, S, Ti and C elements. This result was consistent with those of XRD measurements. Through further observation, we found that the Ti and C elements distribution areas were larger than the other three elements, which further verified the Mn0.2Cd0.8S nanorods were deposited on Ti3C2NSs.

Fig. 4 The XPS spectra of Mn0.2Cd0.8S/0.5 MXene composites and pure MCS: (a) the wide scan XPS spectrum of Mn0.2Cd0.8S/0.5 MXene,(b) Cd 3d, (c) Mn 2p, (d) Mn 3s, (e) S 2p, (f) Ti 2p, (g) C 1s.

Fig. 5 (a) UV-Vis absorption spectra of all photocatalysts; (b) Tauc plots of the UV-Vis spectra.
In order to analyze the chemical element compositions as well as the surface oxidation states of MCS/0.5 MXene composite,the X-ray photoelectron spectroscopy (XPS) was measured.From the Fig. 4a, we can clearly see the existence of Mn, Cd, S and C elements, which was consistent with EDS results. Due to the low content, Ti element was not appeared in XPS survey. In addition, from the Fig. 4f, the broad peak area of Ti element was rather low, indicating the successful etching of Ti3AlC2. As shown in Fig. 4b, Cd 3dspectra has two evident strong peaks located at 411.52 and 404.73 eV, corresponding to Cd2+3d3/2and Cd2+3d5/2, respectively. Two peaks in Fig. 4c at 651.91 and 640.98 eV correspond to Mn2+2p1/2and Mn2+2p3/2, respectively.In Fig. 4d, the Mn 3sorbits are originated from MnO whose binging energies are 87.79 and 82.57 eV. The presence of Mn2+oxidation state may be due to O2−in H2O. The peaks of S 2p3/2and S 2p1/2can be attributed to 161.04 and 162.43 eV in Fig. 4e respectively. The XPS results demonstrated the successful fabrication of MCS/MXene. In order to compare with oxidation states of pure MCS, the XPS spectra of pure MCS were measured. As observed from Fig. 4, all XPS peaks for pure MCS were similar to those of MCS/0.5 MXene composite, indicating there is no obvious change for MCS in binary composite. All the results clearly confirmed that the MCS/0.5 MXene composite has been successfully constructed.
3.2 Optical performance
In order to study the light absorption performance of all the prepared photocatalysts, the UV-Vis diffuse reflection spectra(UV-Vis DRS) was presented in Fig. 5a. The pure 1D Mn0.2Cd0.8S nanorods exhibit the evident absorption edges at 530 nm, while pure 2D Ti3C2MXene NSs exhibit the strong absorption in the spectrum of 400–700 nm, which may be originated from its narrow band gap and black color63. The UVVis diffuse reflection spectra of MCS/xMXene composites show a similar absorption edge with a blue shift, as compared with that of pure MCS. The band gaps of MCS and MCS/0.5 MXene composites could be calculated from the Tauc plots as shown in Fig. 5b, which are 2.24 and 2.27 eV for MCS and MCS/0.5 MXene, respectively.
The separation and transfer of photogenerated charge carriers are important for photocatalytic water splitting, so the photoluminescence (PL) spectrum was performed at the excitation wavelength of 331 nm. Fig. 6a, the MCS showed much higher PL peak intensity than MCS/0.5 MXene, which demonstrated that the binary composite had the better e−-h+separation and transfer11. The MCS/0.5 MXene and MCS were tested by N2adsorption-desorption isotherms. The adsorption/desorption isotherms of MCS/0.5 MXene and MCS belong to the classic type-IV curve, indicating the mesoporous structures. The BET surface areas of MCS/0.5 MXene composite and the MCS were 16.08 and 31.39 m2·g−1, respectively, which may be attributed to the aggregation of MCS nanorods on both sides of Ti3C2.

Fig. 6 (a) PL spectra; (b) N2 adsorption/desorption isotherms of MCS/0.5 MXene and MCS.

Fig. 7 (a) the H2 evolution rate of MCS and the composites of MCS/xMxene; (b) the apparent quantum efficiency;(c) the overall photocatalytic water splitting without sacrificial agent and (d) the cycling stability of MCS/0.5 MXene photocatalysts.
3.3 Photocatalytic activity and stability
Fig. 7a showed the H2evolution rates of MCS samples with different molar ratio of Mn with Cd. From the picture, it was clearly demonstrated that the sample with the Mn and Cd molar ratio of 2:8 showed the highest H2evolution rate of 2.34 mmol·g−1·h−1. Single 1D Mn0.2Cd0.8S NRs exhibited a relative low hydrogen evolution reaction activity due to the rapid recombination of photogenerated electron-hole pairs and relatively low content of active sites. Fig. 7b, after loading 2D Ti3C2MXene NSs, the hydrogen evolution rate was significantly enhanced. The mass of Ti3C2MXene increased to 0.5 mg, the H2evolution rate of the binary photocatalyst reached the highest value of 15.73 mmol·g−1·h−1. On the contrary, the photocatalytic performance of the binary photocatalysts gradually decreased with further increasing the mass of Ti3C2MXene. This result may be caused by the hindered light absorption of 1D Mn0.2Cd0.8S nanorods as well as the decreased active sites due to the excessive loading64. Even so, the HER rate of MCS/5 Mxene composite was still 2 times higher than that of pure MCS,indicating that the use of 2D Ti3C2MXene as the electronic conductive cocatalysts and the formation of interface Schottky junction could effectively accelerate the transfer and separation of photogenerated electrons. In Fig. 7c, the AQE values of MCS/0.5 Xene were 19.6%, 12.3%, 16.0%, 14.6 and 4.3% at 420, 435, 450, 475, and 500 nm, respectively. The MCS/0.5 MXene also exhibits the potential application for overall photocatalytic water splitting without using sacrificial agent. In the first hour, HER rate reached 338.57 μmol·g−1. However,unfortunately, in the second hour, the MCS/0.5 MXene composite only exhibits a small increasement of 135.58 μmol·g−1, due to the unexpected photocorrosion (Fig. 7d).
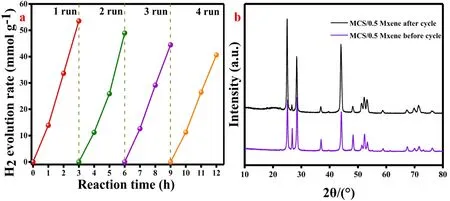
Fig. 8 (a) The cycling stability of MCS/0.5 MXene photocatalysts; (b) The XRD of binary composite before and after cycle.
Fig. 8a, the cycling stability of MCS/0.5 MXene compounds was conducted under the same condition. The composite maintained a good stability, with 75% performance retention after the fourth cycle. The reason may be ascribed to the photocorrosion of Mn0.2Cd0.8S nanorods and depletion of sacrificial agent. Although the HER rate of binary photocatalyst decreased by about a quarter, its excellent photocatalytic performance still had a great application prospect in the field of photocatalytic HER. Fig. 8b, there were not any other peaks emerged after photocatalytic reactions, it was suggested that the MCS/0.5 MXene remained good stability after 12 h illumination.The Table 1 was the HER and AQE comparation of MnxCd1-xS composites.

Table 1 Comparison of the photocatalytic HER performance of MnxCd1−xS based photocatalysts.

Fig. 9 (a) Transient photocurrent responses (i–t curves); (b) LSV curves; (c) Mott-Schottky plots; (d) EIS Nyquist plots of MCS, MCS/0.5 MXene.

Fig. 10 The photocatalytic mechanism of the MCS/Ti3C2 MXene system.
3.4 Photocatalytic mechanism analysis
To understand the separation efficiency of photogenerated electron-hole pairs, transient photocurrent responses (i–tcurves), EIS Nyquist plots, Mott-Schottky (MS) plots and the HER polarization curves were measured. Fig. 9a shows the photocurrent densities of the as prepared photocatalysts. The photocurrent density of MCS/0.5 MXene is much higher than that of pure MCS photocatalyst, suggesting that 2D Ti3C2MXene NSs as a conductive cocatalyst can efficiently accelerate the transfer and separation of photogenerated electrons.Furthermore, we tested the linear sweep voltammetry (LSV). As depicted in the Fig. 9b, pure Mn0.2Cd0.8S shows much higher overpotential than binary hybrid photocatalysts with Mxene cocatalysts, suggesting that the 2D Ti3C2NSs could act as electronic conductive bridge to improve the H2-evolution kinetics. In general, it is known that a low overpotential is more benifical for surface electrocatalytic HER due to the accelerated reaction kinetics57,67. The results of LSV curve are consistent with the above analysis results. Mott-Schottky (MS) was also performed to study the flat bands of MCS and MCS/0.5 MXene.From the Fig. 9c, compared with that of pure Mn0.2Cd0.8S (−1.25 eV), the flat band potential of MCS/0.5 MXene is more negative(−1.62 eV), indicating that the composite of MCS/0.5 MXene has much stronger reduction capacity. Forp-type semiconductors, the flat band is close to valence band (VB),which can be deemed as the VB position. Forn-type semiconductors, the flat band site is located near the conduction band (CB), which can be considered as the CB position. As seen from the slope of the tangent line of the curve, the photocatalyst of Mn0.2Cd0.8S isn-type semiconductor, and the VB of Mn0.2Cd0.8S was calculated to be about 0.99 eV. After loading the Ti3C2cocatalysts, the band gap of the binary photocatalyst is 2.37 eV, while the CB and VB of MCS/0.5 MXene is −1.62 eV and 0.75 eV, respectively. Comparing with binary photocatalyst MCS/0.5 MXene, the MCS has a little more positive VB,indicating the pure MCS had a stronger oxidizing ability for the Na2S-Na2SO3sacrificial agent. Through the comparison of the EIS Nyquist plots of the photocatalysts (Fig. 9d), we can see the MCS/0.5 MXene has the lowest radius of circular arc, which clearly demonstrates that after loading 2D Ti3C2NSs on the 1D Mn0.2Cd0.8S NRs, the separation of photogenerated carriers in Mn0.2Cd0.8S were greatly improved. All these results are in good accordance with the H2evolution rate and photocurrent density.
As observed from the above analysis, the photocatalytic mechanism of the MCS/Ti3C2MXene system was shown in Fig.10. The binary photocatalysts synthesized by solvothermal method successfully built an interfacial Schottky junction between MCS and 2D Ti3C2MXene NSs. Upon this tight contact, the photogenerated electrons tended to shift spontaneously from MCS to Ti3C2until the both Fermi levels reached equilibrium. The charge migration led to the bending of the band edge as well as the formation of an internal electric field between the interfaces of MCS and 2D Ti3C26. Under visible light irradiation, the electrons were excited from the VB to the CB of the MCS. While, due to the poor separation-efficiency of photogenerated-hole pairs, the photogenerated electron-hole pairs would recombine immediately. Thus, the pure MCS shows poor photocatalytic performance. Fortunately, when coupling MCS with 2D Ti3C2MXene NSs, an interfacial Schottky junction was successfully constructed between MCS and 2D Ti3C2MXene NSs. Therefore, the photogenerated electrons on the surface of MCS could easily migrate to the surface of 2D Ti3C2MXene NSs through the Schottky junctions and thus trigger hydrogen evolution reaction. Meanwhile, the holes in the VB of MCS are consumed by Na2SO3. As a result, charge recombination is diminished in the composite of MCS/Ti3C2,thus achieving a greatly enhanced photocatalytic H2generation.
4 Conclusions
In this article, we successfully synthesized the binary photocatalysts between 1D Mn0.2Cd0.8S nanorods and 2D Ti3C2MXene NSs by a facilein situsolvothermal method. The asconstructed interfacial Schottky junction could facilitate the transfer and separation of photogenerated carriers, thus resulting in the enhanced photocatalytic H2evolution. The HER activity of binary photocatalysts was about 6.72 times higher than that of pure Mn0.2Cd0.8S nanorods. The apparent quantum efficiency of 19.6% was achieved. According to the above analysis, metallic Ti3C2MXene was found to be an excellent cocatalyst for promoting separation of photogenerated electrons and holes and improving the H2-evolution kinetics. This work proved that metallic Ti3C2MXene hold a great potential in enhancing photocatalytic water splitting.
杂志排行
物理化学学报的其它文章
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S 型异质结及其光催化制氢性能
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion
