Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
2021-07-13YiwenChenLinglingLiQuanlongXuTinarenJiajieFanDekunMa
Yiwen Chen , Lingling Li , Quanlong Xu , Tina Düren , Jiajie Fan 1,,*, Dekun Ma
1 School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450002, China.
2 School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China.
3 Key laboratory of Carbon Materials of Zhejiang Province, College of Chemistry and Materials Engineering, Wenzhou University,Wenzhou 325027, Zhejiang Province, China.
4 Centre for Advanced Separations Engineering, Department of Chemical Engineering, University of Bath, Bath BA2 7AY, UK.
5 Zhejiang Key Laboratory of Alternative Technologies for Fine Chemicals Process, Shaoxing University, Shaoxing 312000,Zhejiang Province, China.
Abstract: The growing frustration from facing energy shortages and unbalanced environmental issues has obstructed the long-term development of human society. Semiconductor-based photocatalysis,such as water splitting, transfers solar energy to storable chemical energy and is widely considered an economic and clean solution.Although regarded as a promising photocatalyst, the low specific surface area of g-C3N4 crucially restrains its photocatalytic performance. The macro-mesoporous architecture provides effective channels for mass transfer and full-light utilization and improved the efficiency of the photocatalytic reaction. Herein, g-C3N4 with an inverse opal (IO) structure was rationally fabricated using a well-packed SiO2 template, which displayed an ultrahigh surface area (450.2 m2·g−1) and exhibited a higher photocatalytic H2 evolution rate (21.22 μmol·h−1), almost six times higher than that of bulk g-C3N4 (3.65 μmol·h−1). The IO g-C3N4 demonstrates better light absorption capacity than bulk g-C3N4, primarily in the visible spectra range, owing to the multiple light scattering effect of the three-dimensional (3D) porous structure. Meanwhile, a lower PL intensity, longer emission lifetime, smaller Nyquist semicircle, and stronger photocurrent response (which synergistically give rise to the suppressed recombination of charge carriers) decrease the interfacial charge transfer resistance and boost the formation of photogenerated electron-hole pairs. Moreover, the existing N vacancies intensify the local electron density,helping increase the number of photoexcitons. The N2 adsorption-desorption test revealed the existence of ample mesopores and macropores and high specific surface area in IO g-C3N4, which exposes more active edges and catalytic sites. Optical behavior, electron paramagnetic resonance, and electrochemical characterization results revealed positive factors, including enhanced light utilization, improved photogenerated charge separation, prolonged lifetime, and fortified IO g-C3N4 with excellent photocatalytic performance. This work provides an important contribution to the structural design and property modulation of photocatalysts.
Key Words: g-C3N4; Inverse opal (IO); Photocatalysis; H2 evolution
1 Introduction
Photocatalytic technology, using inexhaustible sunlight and semiconductor, has gained broad research interest because it is a sustainable approach in tackling the environmental pollution and energy crisis facing human beings1–3. Especially, as a promising photocatalyst, polymeric g-C3N4could harvest visible light because of its intrinsic bandgap (ca.2.7 eV). Furthermore, g-C3N4is of high physicochemical stability, which stems from the conjugated graphitic plane consisting ofsp2-hybridized C and N atoms4−7.
Heterogeneous g-C3N4is the focus of abundant multidisciplinary research in energy and environmental applications, including photolysis of water to generate hydrogen or/and oxygen, photolysis of CO2to produce clean hydrocarbons, and pollutant removal8–11. Despite the remarkable achievements in g-C3N4-based photocatalysts, it is still unable to meet the requirements for practical application.Especially, the low specific surface area (SBET), poor charge separation efficiency and marginal visible light response range of g-C3N4bring about poor photocatalytic performance12. Faced with these problems, extrinsic and intrinsic strategies on g-C3N4have been carried out, respectively.
An example of an extrinsic strategy is the introduction of metal/non-metal atoms or defects into g-C3N4framework13–15.The atoms or defects can work as active sites, on the other hand,can extend the light harvesting range and to adjust its electronic band16–19. Modification with noble metal nanoparticle(cocatalyst or surface plasmonic properties) and carbonaceous materials are used to improve charge separation efficiency,which may also reduce the activation barrier20–22. Besides,integration with other suitable semiconductors also benefits the charge carrier separation process23–26. Intrinsic self-structural manipulations, which involves shape, size and crystallinity modification, can improve light harvesting efficiency, exploit high reactive planes, and reduce charge carrier recombination behavior27–30.
Recently, a kind of porous structure photocatalyst,i.e., inverse opal (IO) structure photocatalyst was designed for photocatalysis applications31–33. Generally, IO structure was inspired from opal photonic crystal template (PCT). The prepared IO structure photocatalyst has ample active sites and display strong reactant adsorption ability because of its highSBET. Meanwhile, The IO photocatalyst exhibits superb optical properties in an optical path lengthening way, caused by its unique slow photon effect of the photonic crystal34,35. Additionally, the multiple scattering behavior of incident photons displays enhanced light-matter interaction, which can increase the light utilization efficiency36.However, the synthesis process of IO structure is challenging because of complex operational steps37–39. Furthermore, the low yield is also a critical issue32,40,41.
Herein, for designing IO g-C3N4structure with high yield,close-packed configuration SiO2were used as templates(Scheme 1). In the first step, the pre-synthesized SiO2spheres were assembled into a close-packed configuration through a facial centrifugation treatment, which can provide sufficient SiO2PCT (several grams in each batch). Then, infiltration and formation of g-C3N4in the interparticle voids of SiO2PCT was achieved by heat treatment of melamine in a chemical vapor deposition (CVD) process. After etching the SiO2PCT with NaOH, g-C3N4with inverse opal structure was collected.Benefited from the porous structure, IO g-C3N4displayed superior photocatalytic performance toward water splitting under visible light irradiation.
2 Experimental
2.1 Sample preparation
Firstly, dispersed silica spheres with diameter of 250 nm were prepared similar to the previous work4. After obtaining the uniform SiO2spheres, the silica PCT was obtainedviaa centrifugation treatment (1500 r·min−1, 90 min), then followed with drying at 60 °C for 12 h. Finally, IO g-C3N4was prepared through a CVD method using close-packed configuration SiO2as template.
To be specific, 5 g of melamine was laid into a crucible firstly.Subsequently, 2.4 g of silica photonic crystal template was uniformly placed on the surface of melamine, and held at 550 °C in muffle furnace for 2 h. The melamine underwent pyrolysis and thermalpolymerized to produce g-C3N4under the heating process, while the product filled into the voids of SiO2PCT.After that, the obtained g-C3N4/SiO2was treated with 100 mL of 2 mol·L−1NaOH for 12 h for etching SiO2, followed with washing by using water and ethanol. Finally, g-C3N4with IO structure was collected after drying for 12 h at 60 °C, denoted as IO g-C3N4. For comparison, bulk g-C3N4was synthesized under the same procedure without using SiO2PCT.
The reagents used in the experiment are analytically pure without further treatment.
2.2 Characterization
X-ray diffraction (XRD) was used to examine the crystal structure of the prepared samples and investigated by a D/MAXRB diffractometer (Rigaku, Japan) with CuKαradiation.Scanning electron microscope (SEM) images were obtained by using a FEI NOVA NANOSEM 200 (USA) with accelerating voltage of 10 kV, and Transmission electron microscope (TEM)images were recorded through a JEOL JEM-1200EX instrument(Japan). N2adsorption-desorption isothermals were performed on a Micromeritics nitrogen adsorption apparatus (ASAP 2020,USA). X-ray photoelectron spectroscopy (XPS) was measured on Kratos AXIS Supra spectrometer (Japan) with Monochromated AlKαradiation serving as the photon source.Room temperature electron paramagnetic resonance (EPR) was measured on a Bruker MEX-nano instrument (Germany) in the dark. Fourier transform infrared spectroscopy (FT-IR) was conducted on a Nicolet iS50 spectrometer (Thermo Scientific,USA). Diffuse reflection spectrum (DRS) was measured a UV 2550 Shimadzu spectroscopy (Japan) using BaSO4as the reference. Room temperature photoluminescence (PL) was conducted on F-7000 Hitachi (excitation wavelength: 365 nm;Japan). Time-resolved photoluminescence (TRPL) measurement was performed on an Edinburgh FLS1000 (UK) under 375 nm excitation. The fitting curves and average emission lifetimes were achievedviaEqs. (1) and (2), respectively.

2.3 Photoelectrochemical characterizations
The photoelectrochemical characterizations,i.e.,electrochemical impedance spectroscopy (EIS) (from 1 Hz to 100 kHz), photocurrent densities (under 300 W Xenon lamp),and Mott-schottky plots (at the AC frequency of 1 and 2 kHz,respectively), were conducted on Shanghai Chenhua CHI-660C workstation, with a standard three-electrode cell as described in our previous publication42,43. The Mott-Schottky equation44:

2.4 Photocatalytic H2 evolution
The photocatalytic test was evaluated by water splitting to generate H2under visible light irradiation. 350 W xenon lamp equipped with 420 nm cut-off filter was applied as light source.Triethanolamine (TEOA, 15%, volume fraction) was used as sacrifice reagent to consume photogenerated holes. Specifically,50 mg of samples was suspended into 100 mL of deionized water containing sacrifice reagent for photocatalytic H2evolution. 3%(mass fraction) Pt nanoparticles were introduced as cocatalystviaanin situphoto-induced reduction process. To remove the air firstly, the closed system was vacuumed before being exposed to light. The yield of H2was monitored by using on-line gas
Scheme 1 Illustration of the fabrication of inverse opal g-C3N4.chromatography equipped with thermal conductivity detector.The apparent quantum efficiency (AQE) of IO g-C3N4under the irradiation of 420 nm light was studied using similar above operation except for the replacing of 420 cut-off filter by 420 band-pass filter.
3 Results and discussion
IO g-C3N4was prepared by using a hard template approach,as illustrated in Scheme 1. According to the SEM and TEM images displayed in Fig. 1a–d, IO g-C3N4displays typical inverse opal structure consisting of a three dimensional (3D)porous structure. The pores, surrounded by thin layer of g-C3N4,are interconnected forming a 3D network. Such unique macromesoporous structure ensures superior mass transfer capacity.Furthermore, IO g-C3N4forms an imperfect ordered arrangement. It is believed that the partial periodic pore structure significantly improves the light harvest efficiency through multiple scatter effect and the order-disorder interfaces can tailor the charge dynamics effectively45,46. Note that conventional fabrication methods using hard templates always result in low yield of samples. In contrast, although hard template was used during the synthesis process of IO g-C3N4, it still displays a great sample yield, which is beneficial for the practical application.
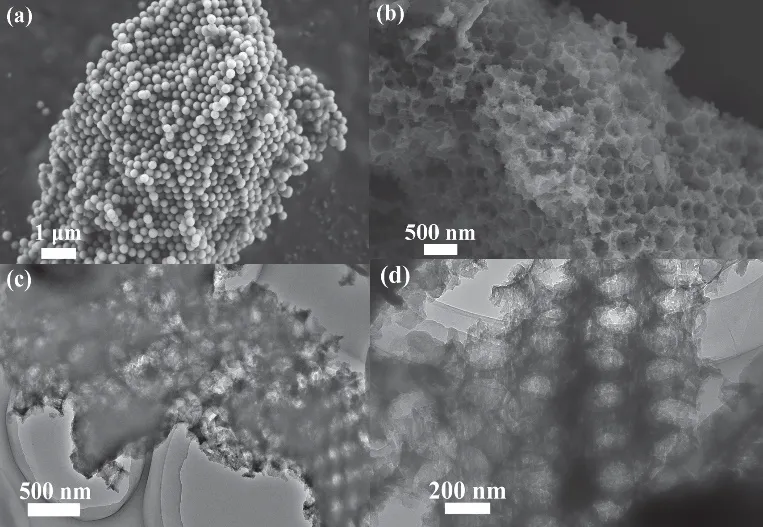
Fig. 1 SEM images of SiO2 PCT (a) and IO g-C3N4 (b),(c, d) TEM images of IO g-C3N4.
More detailed information about textural properties of the photocatalysts were examined by N2adsorption-desorption isotherms (Fig. 2a,b), confirming that 3D porous structure results in significantly increasedSBET. Accordingly, the specific surface area of IO g-C3N4is 450.2 m2·g−1, which is approximately 33-fold larger than that of bulk g-C3N4(13.8 m2·g−1). In addition,the hysteresis loop atP/P0> 0.8 reveals the existence of ample mesopores and macropores.
Fig. 2c shows crystal structure of IO and bulk g-C3N4. Both samples exhibit two diffraction peaks, which can be assigned to the (100) and (002) diffraction peaks of g-C3N4, respectively46,47.For the bulk g-C3N4, the two peaks lay at 13.1° and 27.3°,respectively. Differently, the (002) peak of the IO sample displays a slight shift towards high angles, revealing the interlayer distance reduction, which benefits the charge transfer between interlayers. This reduction can be rationalised by the polymerization process occurred in the confined voids of SiO2PCT during the IO g-C3N4fabrication procedure, which may induce the interlayer compression of g-C3N4. FT-IR characterization was conducted to examine the basic framework of the IO and bulk g-C3N4(Fig. 2d). Both FT-IR spectra show similar features. The assignments of peaks are listed in Table 14.In addition, IO g-C3N4displays intensified absorption compared with bulk g-C3N4, which is ascribed to the enlarged surface area and more exposed surface CN heterocycles.

Fig. 2 N2 adsorption-desorption isothermals and pore size distribution of bulk g-C3N4 (a) and IO g-C3N4 (b), XRD patterns (c) and FT-IR spectra (d) of bulk g-C3N4 and IO g-C3N4.

Table 1 FT-IR spectra modeling parameters for the IO and bulk g-C3N4.
The chemical compositions and states of the synthesized samples were studied by XPS. The peaks, including C 1s, N 1sand O 1s, can be clearly observed (Fig. 3a). For the deconvoluted high-resolution XPS spectrum of C 1s(Fig. 3b), the peak of 284.6 eV is assigned to the residual carbonaceous, which is used as the reference. The other two peaks are ascribed tosp2-bonded carbon (N―C=N) and C―O bond, respectively. The highresolution XPS spectrum of N 1s(Fig. 3c) could be deconvoluted into four distinct peaks, which are corresponded tosp2-bonded nitrogen (C―N=C), tertiary nitrogen, C―NH2species, and charge effects, respectively. Interestingly, the C 1sand N 1sXPS spectra of IO g-C3N4display negative shift relative to bulk g-C3N4, indicating the surface electron rich properties. Thus, it can be deduced that N vacancies were formed during the synthesis process48. In addition, the existence of C―O bond (Fig. 3d)indicates the presence oxygen-containing groups, which results from the incomplete condensation of melamine43. Although residual sodium ions are present in IO g-C3N4, they make no contribution to improving photocatalytic performance49.
The photoactivity of the prepared samples was measured using 15% (volume fraction) TEOA aqueous solution under visible light irradiation, and Pt nanoparticles werein situloaded on the surface of given photocatalyst serving as cocatalysts. IO g-C3N4exhibited greater photocatalytic H2evolution rate (21.22 μmol·h-1), which is almost six times superior than bulk g-C3N4(3.65 μmol·h-1) (Fig. 4a). Especially, as a night passed between the first test and the second, the photocatalytic activity is still very high after three cycles (Fig. 4b), indicating that IO g-C3N4has good stability. The AQE of IO g-C3N4under light irradiation with wavelength of 420 nm is calculated to be 0.8%.
With respect to the optical behaviour of samples in the range of 440–800 nm and belowca.390 nm, IO g-C3N4demonstrates better absorption capacity than bulk g-C3N4, mainly owing to the 3D porous structure. Moreover, the intrinsic absorption edge of IO g-C3N4is blue-shifted (Fig. 5a) relative to the bulk sample,caused by the quantum confinement effects within the layered g-C3N4. According to the DRS spectra, theEgof IO g-C3N4and bulk g-C3N4are 2.89 and 2.79 eV, respectively. Furthermore,charge carrier separation properties were studied by using PL spectroscopy (Fig. 5b). The intense emission peak locatedca.466 nm is caused by the recombination of the photoinduced electron-hole pairs of bulk g-C3N4. The quenched PL of IO g-C3N4indicates more effective interfacial charge transfer emerging from the porous interconnected architecture.

Fig. 3 XPS spectra of bulk g-C3N4 and IO g-C3N4: (a) survey, (b) C 1s, (c) N 1s, and (d) O 1s.
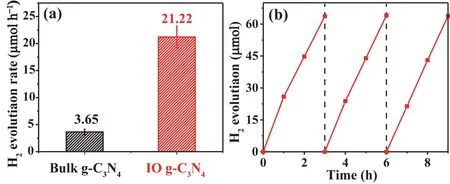
Fig. 4 (a) The photoactivity of the two materials, (b) the stability of IO g-C3N4.

Fig. 5 (a) UV-vis diffuse spectroscopy, (b) steady-state PL spectra, (c) time-resolved transient PL decay, and(d) EPR spectra of bulk g-C3N4 and IO g-C3N4.
To further understand the charge separation behaviour, timeresolved photoluminescence (TRPL) spectroscopy was carried out at 375 nm emission wavelength. The acquired TRPL decay curves were fitted by biexponential function (Fig. 5c), indicating that there exist two pathways on charge relaxation, one of which is faster than the other50. Specifically, the fast decay process is ascribed to nonradiative recombination because of the existed defects, while the slow decay behaviour is stemmed from the radiative process of the free exciton recombination51,52. The PL lifetimes and the corresponded fractional intensities are summarized in Fig. 5c. Apparently, IO g-C3N4displays longer amplitude weighted average exciton lifetime (τave,) than that of bulk g-C3N4, resulting from the enhanced charge separation efficiency because of the ultrafast charge transfer. The decrease in the PL lifetime is ascribed to the more dominant fast component (τ1).τ2is attributed to the indirect recombination of photoinduced charge carriers. This indicates that IO g-C3N4contains sufficient defect to induce self-trapping of charge carriers, thus hindering electron-hole recombination.
The defects or paramagnetic information of the samples was identified by EPR (Fig. 5d). Both IO g-C3N4and bulk g-C3N4possess single Lorentzian line with agvalue of 2.012, indicating that only one kind of paramagnetic specie existed which comes from the unpaired electrons of the carbon atoms within theπconjugated heptazine rings53,54. Besides, the intensity of IO g-C3N4EPR signal is enhanced compared to bulk g-C3N4, resulting from the generation of two-coordinated nitrogen vacancies in the IO g-C3N455,56. This result is further confirmed by the Mott-Schottky test. More delocalized electrons favour the photocatalytic reaction.
Electrochemical measurements were carried out on the different g-C3N4materials to evaluate the photoelectron transfer behaviour (Fig. 6). The transient photocurrent response evoked by the absorbed light can reflect the separation efficiency of charge carriers of photocatalysts. Photoanode deposited with IO g-C3N4display significant improvement in photocurrent intensity relative to bulk g-C3N4, indicating that more photoinduced electrons transfer to the collecting electrode. EIS was tested to examine the electrical conductivity of the IO and bulk g-C3N4. As displayed in Fig. 6b, the reduced arc radius of the Nyquist plot for IO g-C3N4implies lower charge transfer resistance than bulk g-C3N441. Additionally, according to the Mott-Schottky plots, the electron density is estimated as 1.6-fold higher than bulk g-C3N4taking 1 kHz (shown in the Fig. 7)57.The remarkable photoelectric performance of IO g-C3N4can be traced back to its unique 3D porous structure, which improves the light utilization immensely and facilitates the photogenerated charge transfer. Besides, it can be acknowledged that the IO g-C3N4isn-type semiconductor with CB potential of −1.7 Vvs.Ag/AgCl at pH 7. Considering that the band gap of IO g-C3N4is 2.89 eV, the VB potential of IO g-C3N4is 1.19 V (vs.Ag/AgCl at pH 7).

Fig. 6 (a) Transient photocurrent responses and (b) Nyquist plotsof bulk g-C3N4 and IO g-C3N4.
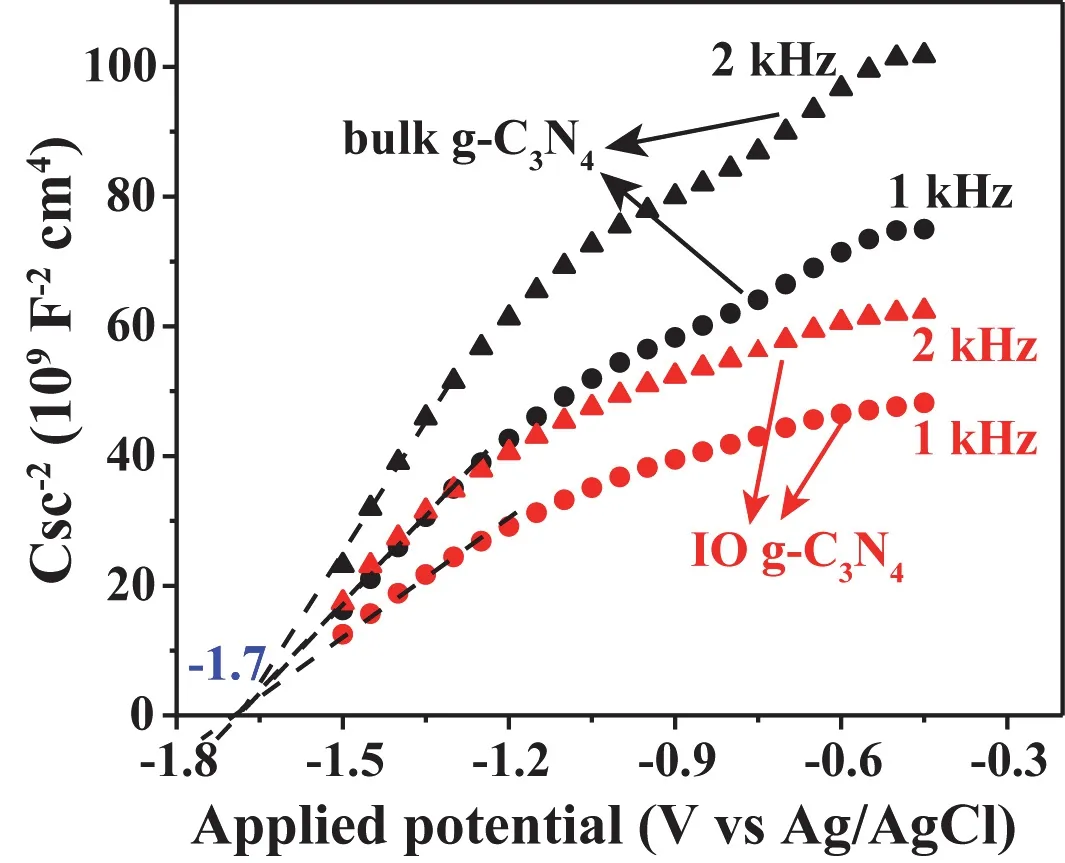
Fig. 7 Mott-Schottky curves of bulk g-C3N4 and IO g-C3N4 measured in 1 and 2 kHz, respectively.

Fig. 8 Schematic illustration of the mechanism for hydrogen evolution over IO g-C3N4.
As IO g-C3N4demonstrates better light harvesting, lower PL intensity, longer emission lifetime and smaller Nyquist semicircle and stronger photocurrent response, which synergistically give rise to the supressed recombination of charge carriers, dropped resistance of interfacial charge transfer as well as boosted formation photogenerated electron-hole pairs.Besides, the N vacancy served as photoinduced electron trap center to promote the charge separation. The proposed mechanism is illustrated in Fig. 8.
4 Conclusions
In summary, we presented a new fabrication route to synthesize IO g-C3N4for photocatalytichydrogen evolution. The significant improvement of H2generation over IO g-C3N4can be rationalised by the listed below reasons. First, the thermal polymerization of melamine to form g-C3N4was confined into SiO2PCT voids, endowing decreased interlayer distance and improved charge diffusion. Second, the holey g-C3N4thin layer attached to the voids can expose more active edges and catalytic sites. Moreover, the interconnected voids allow for better mass infiltration and light utilization thanks to the multiple light scattering effect. Fourth, the order-disorder interface formed within IO g-C3N4layer can promote the separation of charge carriers. Last, the existing N vacancies intensify local electron density, which helps increasing photoexcitons. Overall, this work offers a novel effective way to the synthesis of g-C3N4with inverse opal structure and achieve enhanced photocatalytic conversion and high stability.
杂志排行
物理化学学报的其它文章
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst
- 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S 型异质结及其光催化制氢性能
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- S-Scheme Heterojunction Photocatalyst for CO2 Photoreduction
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion
