碰撞能对K(2S)+HF(X1Σ+)→KF(X1Σ+)+H(2S)反应的影响
2021-07-11陈海亮张志红吕文彩
陈海亮,张志红,吕文彩,3
(1.青岛大学物理科学学院,青岛266000;2.鲁东大学物理与光电工程学院,烟台264025;3.吉林大学理论化学研究所,长春130000)
In recent decades,the reaction K(2S)+HF(X1∑+)→KF(X1∑+)+H(2S)has been extensively studied.It is characterized by the mass Heavy-Light-Heavy(HLH)combination(heavy+light-heavy to form heavy-heavy+light)and possesses a highly endothermic feature.And thus,the study of title reaction can enrich the deep understanding of typical HLH reaction dynamics.Experimentally,the influence of initial conditions on title reaction,such as the alignment between the initial rotational angular momentum of HF(J)and the initial relative velocity(V)[1―3],as well as the initial vibration and rotational excitation of HF[4―6]have been well studied.
Theoretically,several potential energy surfaces(PESs)of KHF system have been constructed by the leading experts in this field.In 2001,ab initiostudy was carried out for this reaction by Sayóset al.[7],which developed the ground state PES1and PES2 for the collinear K+HF configurations using a many body expansion method.Althoughab initiomethod has been performed on analogous simpler systems,such as Li+HF[8―10]and Na+HF[11―13],it was the first time to characterize K+HF system at this level.They also performed the quasiclassical trajectory(QCT)method calculations on PES2.Meanwhile,based on the PES2,Tanget al.[14]not only calculated the total reaction cross section and the temperature dependence of microscopic rate constants employing time-dependent quantum wave packet method,but also compared their results with the experimental results of Heismannet al.[15].Just recently,Mayneriset al.[16]calculated a series of scalar properties of this reaction,such as the reaction cross-sections,the temperature dependence of the rate constant and the rovibrational distributions of product KF molecules,by using time-dependent quantum wave packet and QCT methods,respectively.It should be noted that their results are in good accordance with each other.
As mentioned above,the PES construction of KHF and the study of dynamics properties of K+HF system have made a significant progress.However,the previous investigations only focus on the scalar properties of product[7,14―16].In this work,we will further study the vector properties and obtain the stereodynamics characters of the K(2S)+HF(X1Σ+)→KF(X1Σ+)+H(2S)reaction on the PES2 and comprehensively analyze the influence of collision energy of reactant molecules on the cross section and probability distribution of product molecules.
1 Computational Theory and Details
1.1 QCT Calculations
To shed more light on the vector correlations of the reaction of K(2S)+HF(X1∑+)→KF(X1∑+)+H(2S),we perform QCT calculations at different collision energies(Ec=1.0,1.4 and 1.8 eV)to study the stereodynamics of the title reaction.The QCT calculations have been performed using a code developed in Refs.[17―22].The vibrational and rotational levels of the reactant molecule are taken to bev=0 andj=0,respectively.In our calculation,Batches of 100000 trajectories were run for each reaction with an integration step size of 0.1 fs.The trajectories started at an initial distance of 1.0 nm between the K atom and the center of mass(CM)of the HFmolecule.
The total reaction cross section is defined as

WhereNris the number of reactive trajectories andNis the total number of trajectories.
1.2 Vector Correlations
The CM frame was used as a reference(Fig.1).Thez-axis is parallel to the relative velocity vectork.Thex-zplane is the scattering plane that contains the initial and final relative velocitykandk′.θtis the scattering angle between the reagent relative and product relative velocitykandk′.θrandφrare the polar and azimuthal angles of the final rotational angular momentum j′,respectively.
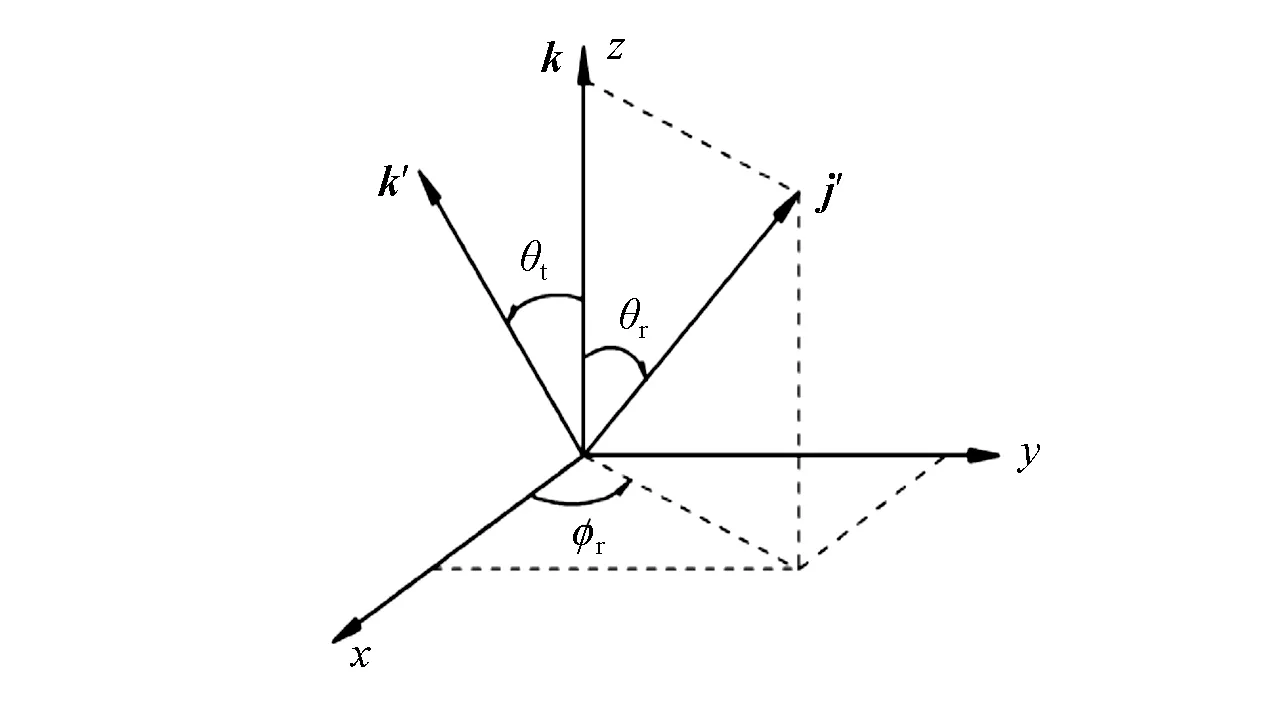
Fig.1 Centre-of-mass coordinate system that describes the correlations k,k′and j′
The differential cross section(DCS)describes the k-k′distribution or the direction of the product,which is given as follows:

Where

The distribution function[P(θr)]describing the k-j′correlation can be expanded in a series of Legendre polynomials as
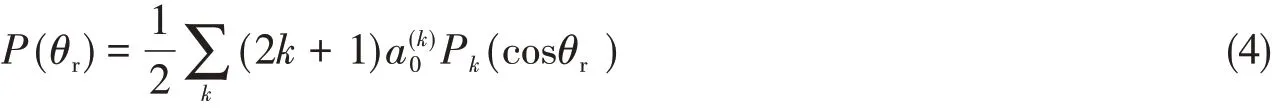
With
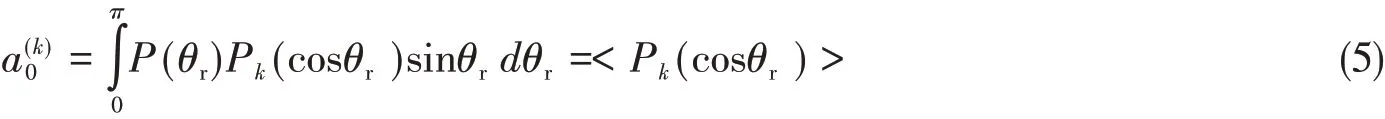
The dihedral angle distribution function[P(φr)]describing k-k′-j′correlation can be expanded in Fourier series as

Where

The vector correlationP(wt,wr)between the reagent and product can be expressed as
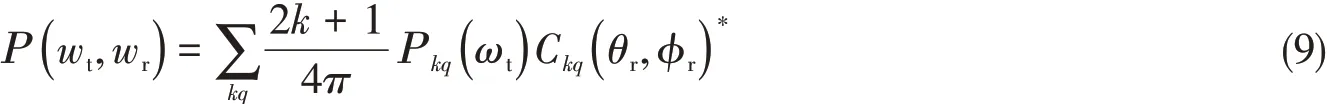

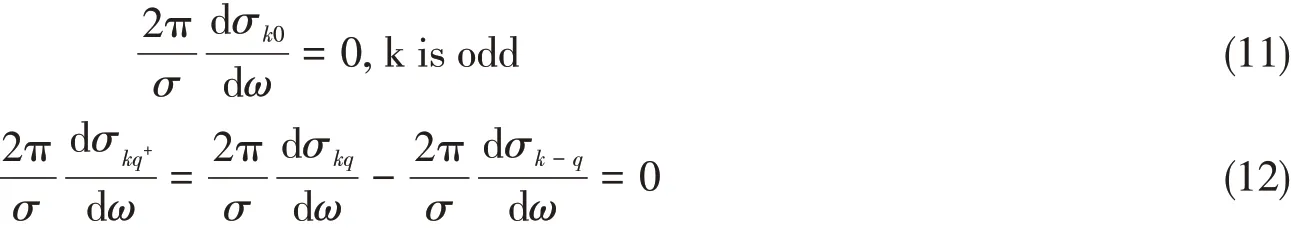
kis even andqis odd orkis odd andqis even.
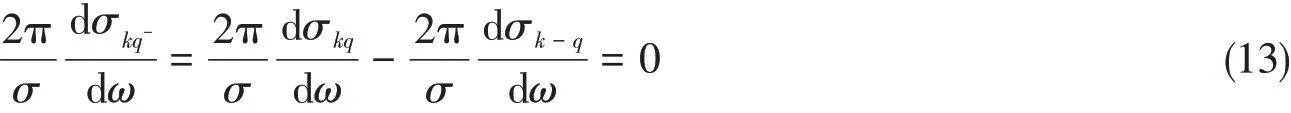
kis odd andqis even orkis odd andqis odd.
PDDCS can then be written as

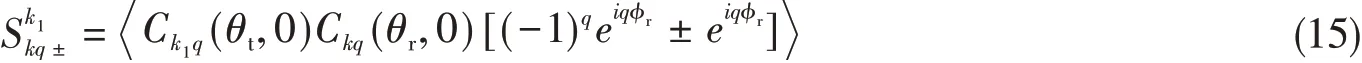
Forq=0,PDDCS is given as

Whereσis the integral cross section and(1/σ)(dσkq/dωt)are the PDDCSs[23,24].(2π/σ)(dσ00/dωt),(2π/σ)·(dσ20/dωt),(2π/σ)(dσ22+/dωt),and(2π/σ)(dσ21-/dωt)were calculated.
2 Results and Discussion
2.1 Correlations of the k-j and k-k′-j′Vectors
Fig.2 shows theP(θr)distributions of the KF product for various collision energies that describek-j′correlations,in which the distributions are maximum forθrclose to 90°and symmetric with respect to 90°.The results indicate thatj′is strongly aligned along the direction at right angles tok.The peak ofP(θr)becomes higher with the collision energy increasing from 1.0 eV to 1.4 eV,elucidating that the effect of the product rotational alignment becomes more prominent.This may result from the expectation values ofP2(j′·k)which are-0.499682,-0.499951 and-0.499971 in Table 1,respectively(the smaller the expectation value ofP2(j′·k),the stronger the rotational alignment).Therefore,the alignment of the K(2S)+HF(X1Σ+)→KF(X1Σ+)+H(2S)reaction is enhanced by increasing collision energy.

Fig.2 Distributions of P(θr),reflecting the k-j′correlation at three collision energies

Table 1 Values of product rotational alignment parameter P2(j′·k)calculated at three collision energies
Fig.3 illustrates the dihedral angle distribution[P(φr)]of the KF product describing thek-k′-j′correlation for three collision energies.Distribution peaks appear at 270°,indicating strong polarization of the angular momentum of the product in the reaction.It is very interesting that the peaks of theP(φr)distributions at three collision energy have the same trend;however,there is a small discrepancy between three collision energies.The peak ofP(φr)of the reaction atEc=1.0 eV is highest and that of the reaction atEc=1.80 eV is lowest atφr=270°.With all three collision energies,the peaks only appear atφr=270°and suggest that the rotational angular momentum vector of product KF is not only aligned but also oriented along the negative direction of theyaxis.The phenomenon in which the title reaction angular momentumj′is symmetric about the relative velocity vector with asymmetricP(φr)distribution can be explained by the impulse model[25,26].For the K(2S)+HF(X1Σ+)→KF(X1Σ+)+H(2S)reaction,the angular momentumj′of the product molecule KF can be written as

Fig.3 P(φr)distribution for j′with respect to the k-k′plane at three collision energies
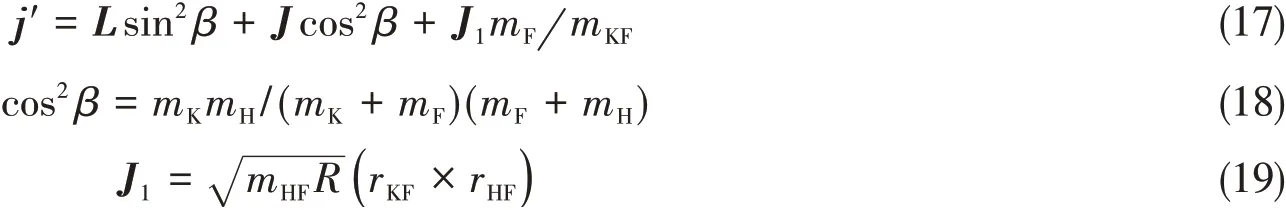
Here,LandJrepresent the reactant orbital momentum and rotational angular momentum,respectively;μHFis the reduced mass of the HF molecule;Ris the repulsive energy;and vectorsrKFandrHFare unit vectors,with F pointing to K and H,respectively.During the chemical bond forming and breaking for the K+HF reaction,the termLsin²β+Jcos²βin the equation is symmetric,while the termJ1mF/mKFshows a preferred direction because of the effect of repulsive energy,which leads to the orientation of the KF product.
In order to get more information of reaction dynamics,the polar distribution function[P(θr,φr)]is also plotted in Fig.4.Each of the threeP(θr,φr)distributions has two distinct peaks located at(90°,90°)and(90°,270°),respectively,which are in good accordance with the distributions ofP(θr)andP(φr),indicating that the products of KF molecules are strongly polarized perpendicular to the scattering plane and rotate in planes parallel to the scattering plane.
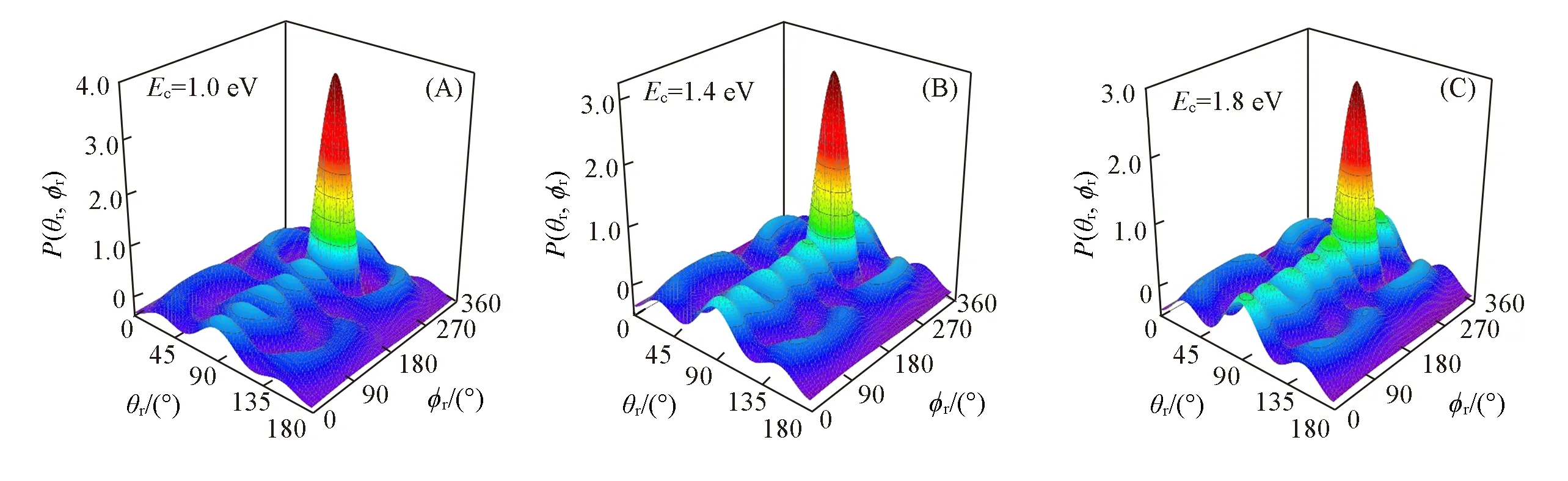
Fig.4 Polar plots of P(θr,φr)distribution averaged over all scattering angles for K+HF(v=0,j=0)→KF+H reaction at the collision energy of 1.0 eV(A),1.4 eV(B)and 1.8 eV(C)
2.2 Generalized PDDCSs
PDDCS describes thek-k′-j′correlation and scattering direction of the product molecule KF,which can be represented by(2π/σ)(dσ00/dωt),(2π/σ)(dσ20/dωt),(2π/σ)(dσ22+/dωt),and(2π/σ)(dσ21-/dωt).The results of the PDDCSs for the title reaction at three collision energies are shown in Fig.5.(2π/σ)(dσ00/dωt),which simply relates to DCS,describes thek-k′correlation[Fig.5(A)].The result shows that the scattering directions of the product molecules are closely related to the collision energy.At the lower collision energy of 1.0 eV,although the KF molecules show an obvious backward scattering,the sideway and forward scattering are weakly visible.With the increase of collision energy,the backward scattering decreases and the scattering feature shift to sideway and forward scattering.
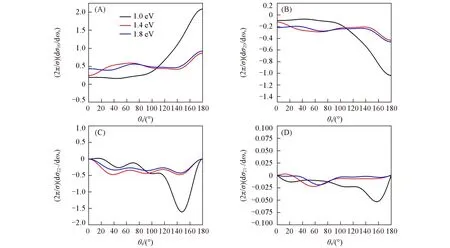
Fig.5 Four PDDCSs for the reaction K+HF(v=0,j=0)→KF+H at three collision energies,plotted as a function of scattering angle(θt)
The PDDCS(2π/σ)(dσ20/dωt),which is related to expectation value of the second Legendre moment and reflects the degree of product alignment alongzaxis,show an opposite variation trend to that of(2π/σ)(dσ00/dωt),as show in Fig.5(B).It is known that the smaller the negative(2π/σ)(dσ20/dωt)is,the stronger alignment of productj′along the direction perpendicular tokis.At all three collision energy,(2π/σ)(dσ20/dωt)are negative in all scattering angle,and thus the productj′align along with the direction perpendicular tokin whole scattering angle.Moreover,the opposite variation trend between(2π/σ)(dσ00/dωt)and(2π/σ)(dσ20/dωt)suggests that the angular distributions in Fig.2―Fig.4 primarily stem from scattering products at scattering angle range with larger DCS.TakingEc=1.0 eV as a typical example,backward scattering dominates the scattering reaction and mainly contribute to the angular polarization distribution.The PDDCS(2π/σ)(dσ22+/dωt)and(2π/σ)(dσ21-/dωt)also describe the alignment of scattering polarized product in other view.The former is related to sin2θrcos 2φr,and the later is related to sin2θrcosφr.When scattering angle is 0°and 180°,thek-k′scattering plane can not be specified and therefore(2π/σ)(dσ22+/dωt)and(2π/σ)(dσ21-/dωt)keep zero in two endpoints,as shown in Figs.5(C)and(D).When(2π/σ)(dσ22+/dωt)is negative,the alignment ofj′is alongy-axis.The smaller(2π/σ)(dσ22+/dωt)suggests the stronger alignment.In the region aroundθt=148°,the product displays a stronger polarization at the lower collisional energyEc=1.0 eV,and apparently weakens with increasingEc.The values of(2π/σ)(dσ21-/dωt)are negative at three collision energies,which indicates thatj′is aligned along the direction of vectorx+zat three collision energies.Especially,The KF product exhibits strong polarization at approximately 160°,which implies that product angular distribution is anisotropic for the reaction.
3 Conclusions
Potential energy surface based on Sayóset al.[7],a series of QCT calculations have been carried out to study the influence of collision energy on the stereodynamics of the title reaction.We have calculated the generalized PDDCSs and plotted the distributions ofP(θr),P(φr)andP(θr,φr).It has been found that the collision energy significantly enhance the forward scattering of product molecules.The product rotational angular momentumj′is not only aligned,but also oriented along the negative direction of theyaxis.Furthermore,the PDDCS(2π/σ)(dσ00/dωt)predicts that the product KF molecules mainly tend to the backward scattering.These characteristics may be ascribed to the HLH mass combination type and the construction of the PES.The QCT results imply that the collision energy play an important role on the stereodynamics of the title reaction.
This work was supported by the National Natural Science Foundation of China(No.21773132).
