利用介孔分子筛中高度分散的Cu-Co氧化物的高效协同作用提高VOCs的催化消除效率
2021-07-10刘梦婷陆雨桐王旭裕袁爱华李露露
欧 锐 刘梦婷 陆雨桐 王旭裕 袁爱华 李露露 杨 福
(江苏科技大学环境与化学工程学院,镇江 212003)
0 Introduction
For the past few years, the efficient remediation of toxic volatile organic compounds (VOCs) has come into view and has become increasingly focus on air pollution control with the development of petrochemical engineering[1-4]because of the brought following serious threat to the health of humans. For now, various VOCs abatement end-processing technologies have emerged,among which, catalytic oxidation degradation has been regarded as the most promising VOCs abatement technology[5-13]. VOCs are supposed to be degraded into harmless carbon dioxide and water at relatively lower temperatures using the promising regenerative catalytic oxidation (RCO) compared to regenerative thermal oxidizer (RTO), which is more eco-friendly and efficient.Therefore, the development of highly-efficient and lowcost catalysts used for the catalytic degradation of VOCs is fairly desirable and also is the center key in heterogeneous environmental catalysis field.
Great efforts have been made to obtain valid functional catalytic materials for VOC oxidation so far. Several developed catalysts supported by noble metals(mainly Pt and Pd)have already exhibited high catalytic activity in the previous studies. Xu et al.[14]prepared a Pd-based catalyst supported by mesoporous alumina and TiO2nanosheet and found that the most active Pd/ba-ma catalyst reached the complete transformation of toluene at 187 ℃. Though very active for the elimination of VOCs, the corresponding shortcomings of noble metal-based catalysts are still obvious including highcost, low thermal-stability, and an easy poisoning tendency which limits its further wide applications in the practical process. By contrast, catalysts supported by transition metals (i.e., V, Ce, Mn, Cr, Cu, Co, and Ni)have been considered promising candidates to replace the noble metal counterparts due to their high thermal stability and low-cost[15-19].Particularly,it is worth noting that Cu and Co as active species have been proved to be extraordinarily active for the catalytic oxidation of VOCs compared with some related reports mentioned[20-24].Nonetheless, the main disadvantage of transition metals is the limited intrinsic activity compared to the more active noble metal, but which can be solved by improving the dispersion of active species, increasing the active sites, regulating the metal valence state, and so on. Sun et al.[25]reported Co3O4with different spatial structures and discovered that the two-dimensional Co3O4nanosheet materials showed the optimized catalytic performance for methane oxidation. Heynderickx et al.[26]fabricated Cu-based series oxide catalysts by different synthetic methods and determined that the oxidation state of copper plays a key role in the catalytic reaction.Yet the structure space of the catalyst available for the reaction region is small and also limited to a single metal. More strikingly, composite catalysts decorated by bimetals or their oxides tend to be positive and meaningful for the catalytic process due to the visible synergetic effects.Clarke et al.[27]developed Cu-Mn mixed oxide catalysts at different calcining temperatures and concluded that the catalysts calcined at 400 ℃revealed the best catalytic activity for naphthalene (T50=229 ℃,T90=238 ℃, whereT50is the reaction temperature when the naphthalene conversion is 50%,andT90is the reaction temperature when the naphthalene conversion is 90%). Similar works inspire us to focus on the bimetal composite catalysts by constructing the structural engineering and valence engineering of active species in heterogeneous catalysts. The main difficulty in obtaining a highly-efficient bimetallic oxide catalyst lies in how to construct the reactionpreferred catalyst micro-scale structure and increase the density of active metal oxides to induce the applicable reaction contact between VOCs molecules and active substances. Whereas, the desirable results are often difficult to accurately control and repetitive.What′s more, the low atom-efficiency of aggregated reaction active species on the catalytic supports prevented the further promotion of catalytic activity of the material.
Additionally, the carrier of catalysts is another key factor for obtaining excellent catalytic performance. Mesoporous silica has emerged as a promising carrier or reactor of the catalyst due to its high specific surface area, oriented channels, and appropriate pore volume, which are ideal characteristics as molecules reaction zone. To prevent the migration and accumulation of metal species on the carrier or their further escape from mesopores during the calcination, a strategy that can comprehensively take advantage of the constraint effect and metal-carrier interaction to obtain a more active metal phase is deserved to be developed.
Inspired by the above-mentioned thoughts, we attempted to prepare series of mesoporous silica-based catalystsin-situdecorated with close-contact dual metal species by introducing synergetic Cu-Co oxides into hexagonal mesoporous silica (HMS) based on an improved micelle template method[28-30]. As was illustrated in Scheme 1a, the controlled ratios of Cu2+and Co2+were embedded into the micelle template (DDA) and the silica wall, in which metal cations play an important intermediary role through the physical-chemical interaction between surfactants and silicate substances(metal cation could coordinate with the surface amino group to form metallomicelle which matched with the negatively-charged siliceous oligomers). After that, Cu-Co oxides werein-situformed on the mesoporous surface by calcination at high temperatures. This method bears the advantage of high dispersion of active catalytic substances by confinement and guest-host effect in mesoporous silica, also, the creating synergistic effect of bimetals can further promote the catalytic process of VOCs. By contrast, the post-impregnation method(Scheme 1b) in some reported works is regarded as a long way from the method mentioned above. Obviously,it′s easy to cause the block of the diffusion channel,which will seriously hinder the catalytic efficiency of samples.The received Cu or/and Co-modified HMS catalyst with typical spherical morphology and the size of~200 nm was used for catalytic oxidation of toluene.The effect of different ratios of metal addition dosage on the textural property and catalytic performance of toluene was further ascertained. Valid characterizations including scanning electron microscopy(SEM),Xray diffraction (XRD), nitrogen adsorption-desorption test were used to study the related physicochemical properties change of the materials. Finally, some cores related to catalyst active sites, bimetal synergistic effect, and atomic efficiency were also roughly discussed.
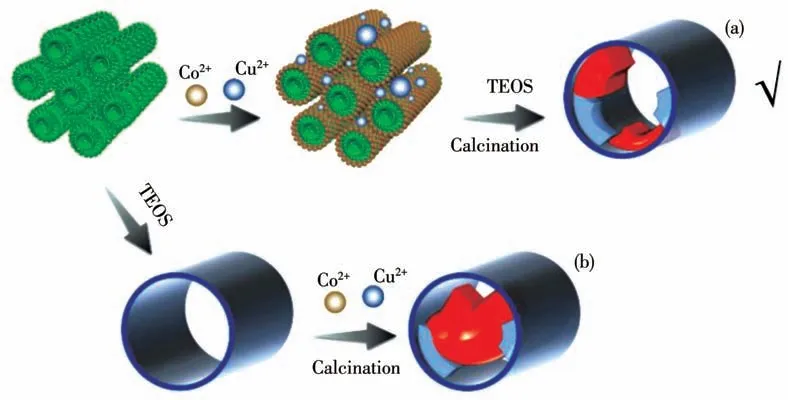
Scheme 1 Schematic description for synthesis of confined dual Cu-Co oxides-bearing mesoporous silica:(a)micelle-assisted approach,(b)post-impregnated approach
1 Experimental
1.1 Chemicals and materials
Dodecyl amine (DDA,≥98%) was purchased from Aladdin. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O,≥99%), copper nitrate trihydrate (Cu(NO3)2·3H2O,≥99%), tetraethyl-orthosilicate (TEOS, AR), absolute ethyl alcohol (C2H5OH, AR) and toluene (C7H8, AR)were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were commercially available and used directly without any purification.
1.2 Synthesis of Cu-Co co-modified nanospherical mesoporous silica
The typical synthesis details of nano-spherical mesoporous silica (NS-MS) modified by bimetallic Cu-Co oxides are described as follows:1.85 g neutral DDA surfactant (0.01 mol) was dissolved in a mixed solution containing 25 mL ethanol and 62.5 mL deionized water. The addition of different ratios of bimetal Co(NO3)2·6H2O and Cu(NO3)2·3H2O (nCo∶nCu=1∶1,2∶1,3∶1, 4∶1) resulted in that the color of the solution immediately turned blue. After cooling to room temperature, 10.7 mL TEOS was added to the mixed solution for 4 h under intense stirring,and then the mixture was heated at 45 ℃for 18 h. Finally, the collected samples were further centrifuged and washed with water and ethanol. The dried sample was calcined under air purge for 6 h at 550 ℃with a heating rate of 1 ℃·min-1, and then naturally cooled to room temperature.The final samples with different ratios of cobalt to copper before calcination were labeled as 1Cu/NS-MS,3Co/NS-MS, and 1CuyCo/NS-MS (y=1, 2, 3, 4) and the calcined samples were labeled as 1Cu/NS-MS-s, 3Co/NS-MS-s,and 1CuyCo/NS-MS-s(y=1,2,3,4).
1.3 Characterization
SEM images were obtained from a Merlin Compact Field Emission Scanning Electron Microscopy(Zeiss,Jena,Germany,0.02~30 kV).
High-resolution transmission electron microscope(HRTEM) images were obtained from a JEM-2010 EX microscope(JEOL,Tokyo,Japan)operated at the accelerated voltage of 200 kV, equipped with energy dispersive X-ray detectors and element mapping analysis.
The XRD patterns of all of the samples were collected using XRD-6000X (Shimadzu Corporation,Tokyo, Japan) equipped with a rotating anode and CuKαradiation(λ=0.154 178 nm).The voltage was 40 kV,the current was 35 mA,the test angle(2θ)was 10°~80°,and the scanning rate was 5(°)·min-1.
FT-IR spectra of the samples were collected by Fourier transform infrared spectroscopy (Agilent,USA), and the samples were prepared by the potassium bromide tableting method,and the wavenumber was recorded from 400 to 4 000 cm-1.
UV-Vis diffused reflection spectra (UV-Vis DRS)of the samples were obtained in a range of 200~800 nm by using a Lambda 950 spectrophotometer (PerkinElmer,Waltham,MA,USA).
X-ray photoelectron spectroscopy (XPS) was recorded on a PHI 5000 Versa probe X-ray photoelectron spectrometer(ULVAC-PHI,Kanagawa,Japan)equipped with AlKαradiation (1 486.6 eV). The reference C1speak of 284.6 eV was used as the standard.
Hydrogen temperature-programmed reduction (H2-TPR) measurements were carried out in a fixed bed reactor from room temperature to 800 ℃with 10% H2/Ar (volume fraction) atmosphere flow and a heating rate of 10 ℃·min-1. Before analysis, the samples were purified by flowing 30 mL·min-1argon at 300 ℃for 1 h and then cooled to room temperature. During the reduction, the consumption of H2was continuously recorded using the thermal conductivity detector(TCD).
The N2adsorption-desorption tests were carried out using BELsorp-MAX volumetric adsorption analyzer(BEL Japan, Osaka, Japan). The samples were outgassed in a vacuum at 180 ℃for 12 h before measurement.The specific surface areas and pore size distribution were calculated through Brunauer-Emmet-Teller(BET) and non-local density function theory (NLDFT)methods,respectively.
The toluene temperature-programmed desorption(Toluene-TPD) measurement was performed in a fixed bed microreactor. The sample (100 mg) was first pretreated in a He flow at 300 ℃for 30 min and then cooled down to 30 ℃, prior to the experiment. Then,toluene gas was fed into the catalyst until adsorption saturation,which was followed by purging for 1 h under a He atmosphere to remove weakly adsorbed toluene.Finally, temperature programming was initiated with a heating rate of 10 ℃·min-1from room temperature up to 500 ℃. The concentration signals of the desorbed toluene were detected.
1.4 Catalysis assessment for toluene degradation
The catalytic activity test was carried out in a tubular fixed-bed reactor system connected to the online gas chromatograph(GC)equipped with a flame ionization detector (FID) and KB-1 column. Toluene was selected as the representative of VOCs to evaluate the catalytic activity ofxCuyCo/NS-MS-s series catalysts.The gas resources mixture was composed of 4.115 g·m-3toluene, and the volume ratio of compressed air (as equilibrium gas) to toluene vapor was 91∶9. The total reaction gas was mixed in a tubular fixed bed with a flow rate of 100 mL·min-1. The space velocity (GHSV)was 60 000 mL·h-1·g-1under 0.100 g catalyst (40~60 mesh).
The toluene conversion rate (Xtoluene) at different temperatures was calculated according to the following calculated formula:Xtoluene=[(ctoluene,in-ctoluene,out)/ctoluene,in]×100%, wherectoluene,inis the inlet toluene concentration andctoluene,outis the outlet toluene concentration.
For a better understanding of catalytic thermal removal of toluene, we studied the kinetic law of the composite catalyst. Generally speaking, the catalytic oxidation reaction process conforms to the first-order reaction kinetics. The following formula can be used to calculate the activation energy (Ea) of different catalysts to evaluate the activity:
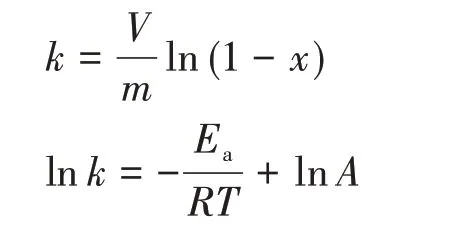
whereXis the conversion rate of toluene,kis the reaction rate constant at a certain temperature;Vandmare the volume flow rate of gas and the feed amount of catalyst, respectively. When the gas volume flow rate was 9 mL·min-1and the catalyst feeding amount was 0.1 g,the conversion rateXat different temperatures can be measured andkcan be further calculated. Then,according to Arrhenius formula, the relation graph of lnkvsT-1was obtained, and theEaof different catalysts could be calculated by obtaining different slops(-Ea/R)through linear fitting.
2 Results and discussion
The designed synthesis of Cu-Co co-modified mesoporous silica by the micelle-assisted approach could exploit the coordinated-oriented function by the oriented self-assemble route, DDA-Co2+/Cu2+—Si—O—,where the metal ions would act as the media to interact with DDA micelle and negatively-charged siliceous oligomers. The metal ions firstly coordinated with DDA molecule as cationic metallomicelle, and then selfassembled with the siliceous oligomers via counterions media. Such a tactic could orientedly insert the metal ions into the interface of the micelle and silica wall, which is beneficial to make the metal oxides form in the mesopore of silica. This process could avoid the random distribution of introduced metal species inside or outside the mesopore, and trigger the strong metalsupport interaction to enhance the dispersion of active metals species when compared to the post-impregnation approach.
2.1 Morphology and structural characteristics

Fig.1 SEM images of the samples:(a)1Cu1Co/NS-MS-s,(b)1Cu2Co/NS-MS-s,(c)1Cu3Co/NS-MS-s,(d)1Cu4Co/NS-MS-s,(e)1Cu/NS-MS-s,and(f)3Co/NS-MS-s;Typical TEM images(g,h),HRTEM images(i),dark-field SEM images(j)and elemental mapping analysis for 1Cu3Co/NS-MS-s
Morphology character would determine the external diffusion pathways of reactant molecule, and small particle size could benefit to eliminate external diffusion effect of reactant molecules.Herein,the SEM technique is employed as an effective means of observation for the microscopic surface morphology characteristics of the catalysts. Fig.1a~1f display the representative SEM images for the calcined series Cu-Co modified NS-MS at the same magnification, which revealed the dominant spherical morphology feature and relativelyuniform particle size distribution. In addition, among the samples loaded with different cobalt content,1Cu3Co/NS-MS-s afforded the smallest particle size and optimized morphology, which possibly could promote the reaction process by reducing the external diffusion effect. Also, judging from 1Cu1Co/NS-MS-s to 1Cu4Co/NS-MS-s, the particle size showed a tendency of decreasing first and then increasing with the increment of cobalt loading, which indicated that the loading amount of cobalt may affect the particle size of the sample due to the possible participation self-assemble process of Co and Cu ions when coordinating with the DDA micelle. Compared with the bimetal optimized catalysts, the spherical morphology characteristic of the single metal modified sample was clearer. At the same time,the latter presented a large range of obvious agglomeration of nanoparticles, and the particle size of 1Cu3Co/NS-MS-s was much smaller. The morphology of 1Cu3Co/NS-MS-s was further confirmed by TEM techniques. Specifically, Fig.1 also gives typical TEM(g, h) and HRTEM (i) images of 1Cu3Co/NS-MS-s and demonstrates the spherical status of the sample and wormhole-like mesoporous character of pore structure according with the reported result[31]. What′s more, the complete absence of visible lattice fringes of the metal oxides sintered particles in the high-resolution images(Fig. 1i) indicated that metal oxides were highlydispersed in mesopores. Beyond that, the elemental mapping patterns in Fig.1j could further verify the coexistence of Cu-Co bimetals in the obtained catalysts and the above-claimed conclusion. Taken together, the above observation further demonstrates that the 1Cu3Co/NS-MS-s affords the reaction-preferred structural characters including small particle size and refined active species dispersed on the large surface area carrier.
To further determine the detailed structural properties and parameters,the obtained materials were evaluated with N2adsorption-desorption measurement.
Fig.2 shows N2adsorption-desorption isotherms of the as-preparedxCuyCo/NS-MS catalyst before (a) and after (b) calcination. Specifically, the specific surface areas of the calcined samples were generally much higher than those of the samples before calcination owing to the removal of the template. As shown in Table S1(Supporting information),SBETof allxCuyCo/NS-MSs samples (except for 1Cu4Co/NS-MS-s) presented a growing trend after calcination, which is attributed to the fact that high-temperature calcination is conducive to the improvement of the porous structure. While no obvious increase in specific surface area of 1Cu4Co/NS-MS-s catalyst was observed, which indicates the negative impact on various structural parameters in the case of excessive metal species introduction in the catalyst.The feeble enhancement of porous property for 1Cu4Co/NS-MS-s should be explained by the abnormal pore-making process via the micelle liquid-crystal template. In addition, series of uncalcined and calcined catalysts revealed typical type Ⅳisotherms with type H3 hysteresis loops, which is consistent with the characteristic results of typical mesoporous structures of HMS due to the removal of surfactant template. What′s more, the corresponding pore size distribution curve(Fig.S1) was given on the basis that there were abundant mesopores in the material,which would contribute to improving the catalytic property of the prepared catalyst by providing enough reactive active centers on the accessible mesoporous surface. Besides, we noticed that the cobalt oxides encapsulated into mesopore resulted in the decrement of pore size compared to the only Cu-bearing counterpart, which approves the successful incorporation of cobalt species into silica nanopore. More importantly, 1Cu4Co/NS-MS-s almost forfeited the great mesoporous structure compared to the lower Co loading one,in turn,1Cu3Co/NS-MS-s still afforded the appreciable porous properties at relatively high metal loading,and had a strong implication for the activity-preferred reaction process.This also might provide a hint on the possible poor catalytic activity for 1Cu4Co/NS-MS-s owing to the weakening structural properties.
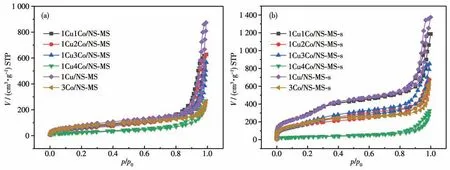
Fig.2 N2 adsorption-desorption isotherms of uncalcined(a)and calcined(b)xCuyCo/NS-MS catalysts
2.2 Characterizations of bimetallic Cu-Co oxide status
Fig.3 shows the wide-angle XRD patterns of Cu-Co modified NS-MS catalysts. As observed, all curves of different samples showed a broad diffraction peak from 20° to 30°, which corresponds to the characteristic diffraction peak of amorphous SiO2[32]. More importantly, no other obvious diffraction peak ascribed to aggregated metals oxide nanocrystals was observed in the XRD patterns, indicating that the small-enough size and high dispersion of the bimetallic oxides active species in the internal pore should thank to the confinement and host-guest effect of mesoporous silica inhibiting the aggregation of active metals species on the silica. Such an observation strongly suggests the high atom-exposure degree of active metal species on the mesoporous surface, which could provide enough active centers for reaction owing to thein-situimplant strategy with the assistance of micelle.
Infrared spectroscopy can effectively further show the characters of functional groups contained in the molecular sieve, which plays an important role in the study of the composition of the catalyst. FT-IR spectra of the uncalcined (a) and calcined (b)xCuyCo/NS-MS are exhibited in Fig.4. In addition, the curves of the series samples were approximately consistent with silica materials in previous reports. The absorption band at 3 397 cm-1is attributed to the characteristic peak of absorbed water of the material exposed to the pores in the air[33].The peak value ofca.2 932 cm-1is obviously ascribed to the absorption band of surfactant DDA assembled in the as-synthesized uncalcined sample[34],which completely disappeared after calcination indicating the successful formation of mesopore. The bands in the 1 600~1 300 cm-1region are assigned to surface carbonate species[35]. The absorption bands at around 1 091, 800, and 466 cm-1are due to the Si—O—Si stretching vibration[36]. The peak located atca. 620 cm-1observed for the sample modified by bimetal species may be due to Cu/Co—O vibrations in metal oxide lattices[37-38], confirming the successful loading of metal oxides.In addition,the absorption peak atca.964 cm-1is derived from the stretching vibration of the surface groups of Si—O—H[39]. Nevertheless, the absorption intensity of the calcined sample weakened slightly with the increment of cobalt content, which could be mainly ascribed to the augment of the coverage of cobalt oxide on the surface groups of Si—O—H by the chemical interaction method with silica wall. Such observation further approves the efficient surface modification and high-exposure of metal species on the mesoporous surface through the generated strong metal-support interplay based on the proposedin-situsynthesized tactics.While the exposed active metals centers could provide more efficient reactive sites for contacting the diffused reactant molecules.

Fig.3 Wide-angle XRD patterns of uncalcined(a)and calcined(b)xCuyCo/NS-MS catalysts
UV-Vis spectroscopy was further employed for the study of local coordination environment of the isolated metal species and the electronic states of aggregating metal oxides. The UV-Vis DRS was recorded and converted to absorption spectra to analyze and infer the composition and content of metal, and the structure of the various samples. Fig.5a and 5b illustrate the UVVis DRS spectra of uncalcined and calcined 1CuyCo/NS-MS catalysts, respectively. The thought that the characteristics of the spectra were closely related to the crystalline state of the loaded metal oxide species has been reported before[40-41]. From the observation, the absorption peak around 220 nm is attributed to the silica matrix,and that at 262 nm corresponds to the isolated Cu2+species in the catalyst given in the previous study[39]. Besides, we noticed that the absorbance band at 300~400 nm can be identified and indexed to the cluster-like CuO species on the silica support, and this absorbance ascribed to the CuO cluster was relatively weak in 1Cu1Co/NS-MS-s, 1Cu2Co/NS-MS-s, and 1Cu3Co/NS-MS-s, but 1Cu4Co/NS-MS-s exhibited the obvious enhancement compared to above other counterparts. This variation might associate with the certain aggregation of CuO from original isolated Cu in the mesopore owing to the excessive introduction of CoOxspecies thereby inducing the weakening interaction of Cu—O—Si neighbored by Co.All the 1CuyCo/NS-MS-s samples displayed three different absorption bands in the 450~700 nm range, centered at 527, 594, and 645 nm, respectively. This could be typically attributed to the coordination fieldd-delectron transitions of octahedral Coギ[42]. According to the references[43-44], they can be defined as highly-dispersed oxidative cobalt species that strongly interact with the surface of silica carriers.Additionally,the intensity of the absorption band corresponding to the copperギweakened a little while that of the cobaltギbecame stronger after calcination, suggesting the stronger electron transfer from oxygen to cobalt after calcination because of the Co—O bond formation from ionic Co2+. Besides, we observed the certain shift of Co absorbance bands in the local region before and after calcination, further confirming the surface state of CoOxand the removal of micelles interacted with ionic Co species after calcination. The above results indicate the certain interaction between CuO and CoOxin the neighboring state which could trigger the possible synergy in the catalysis process.
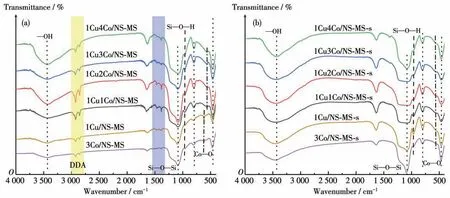
Fig.4 FT-IR spectra of uncalcined(a)and calcined(b)xCuyCo/NS-MS catalysts
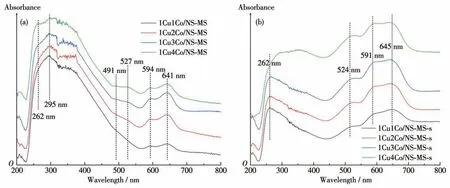
Fig.5 UV-Vis DRS spectra of uncalcined(a)and calcined(b)1CuyCo/NS-MS catalyst
The nature such as the redox ability of CuO and Co3O4within the channel of silica also can be identified from H2-TPR measurements. Fig.6 shows the H2-TPR profiles of calcinedxCuyCo/NS-MS-s catalysts.The observed reduction peaks of those catalysts centered at lower temperature region (276~340 ℃) are primarily attributed to the integrated reduction of welldispersed CuO species on the silica support[45], which are relatively stable in peak proportion. The reduction peak of copper oxides gradually shifted to higher temperature regions owing to the higher cobalt loading,which could be ascribed to the strong interactions between surrounding more cobalt species and silica support and copper oxide species[37]. Additionally, all the Co-contained bimetallic catalysts presented two main reduction peaks atTmaxof ~260 and ~700 ℃,respectively. The first peak at low reduction temperature could be assigned to the reduction of Co バto Coギspecies (Eq.1), and gradually weakened with the increased Co content in the catalyst.This could suggest that more loaded cobalt oxides are formed as Co ギstate at higher concentration, while the little cobalt oxides partially interacted with copper species are easily converted as higher valence Co バunder the effect of Cu when the cobalt loading was low. The second higher reduction temperature accounts for the reduction of Coギto Co0[46](Eq.2),which was increased to higher reduction temperature owing to the existence of the surrounding copper oxides. The intensity of the second reduction peak at 700 ℃increased with more added cobalt content in the catalyst[47], suggesting more cobalt oxides species or oligomeric clusters anchor on the silica surface and strongly interact with CuO species in the neighboring state by bonding interaction which are more difficult to be reduced.

Fig.6 H2-TPR profiles of xCuyCo/NS-MS-s catalysts
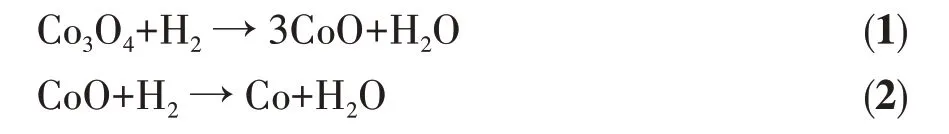
In summary, the observed reducibility behavior may be explained on the basis of the strong intermetallic interaction with each other and the support owing to their close-contact in mesopore, which might act as the possible evidence for the constructed synergy.
Fig.7 XPS spectra:O1s(a)and Cu2p(b)of 1Cu3Co/NS-MS-s catalyst,Co2p core levels of 1Cu3Co/NS-MS-s and 3Co/NS-MS-s(c)
XPS was used for further studying the elemental valence and interaction of the surface metal oxide based on the variation of binding energy. Herein, highresolution XPS spectra of O1s, Cu2p, and Co2pcore levels of 1Cu3Co/NS-MS-s catalyst and Co2pcore level of 3Co/NS-MS-s catalyst are displayed in Fig.7a~7c.The Cu2pXPS results (Fig.7b) display two main peaks centering around 933.5 and 953.9 eV,corresponding to Cu2p3/2and Cu2p1/2orbit splitting, respectively, which affords a certain shift towards lower binding energy compared to the reported monometal Cu catalyst[39].This associate with the increased electron cloud density of Cu atom when neighboring the coexisted Co species,indicating the electron transfer and interaction between CuO and CoOxspecies in the confined mesopore[48-50]. Besides, the binding energy peaks of Co atom located at 782.5 and 797.9 eV in the Co2pelectron region are assigned to Co2p3/2and Co2p1/2core levels,respectively. Compared with the Co2pcore level of Cu-free 3Co/NS-MS-s, the interaction of bimetals decreased the electron cloud density of Co atom, which induced an increment in the binding energy of Co core level and coincided with the variation of Cu binding energy. In the conjunction with the variation of Cu and Co core levels, it is inclined to reach the conclusion that the co-existed Cu-Co affords the strong interaction and neighboring position state in the mesoporous surface. This could provide a possible precondition for the catalytic synergetic effect. Additionally, the deconvolution of O1score levels displayed two binding energies at 529.1 and 530.6 eV indexed to two independent chemical statuses in the surface compositions. It could be indicated that the surface oxygen existed as Si—O—Si and M—O—M species (M represents metal including Co or Cu), and the state of existed interaction was relatively uniform and stable.Besides,it should be noted that the orbital splitting between 2p1/2and 2p3/2peaks of Co2pwas 16.2 eV for 3Co/NS-MS-s, which is in accordance with that of Coギ(16.0 eV) rather than Coバcompounds (15.0 eV)[51]. In contrast, the Cu-Co coexisted 1Cu3Co/NS-MS-s afforded 15.4 eV of orbital splitting energy difference between 2p1/2and 2p3/2peaks, evidenced as the coexistence of Co2+and Co3+species as Co3O4in the silica[12].In conjunction with the results extracted from the above characteristic of O1s,Cu2p, and Co2pbinding energy, we speculate the presence of Cu2+and Co3+/Co2+, demonstrating the existed obvious influence of Cu strongly interacts with the loaded Co of surfaces of silica matrix. This further inspires us that the existed Coギand Coバspecies could accelerate the electron transfer between the above two Co species and promote the reaction.
2.3 Toluene adsorption performance and catalytic activity
Additionally, the adsorption test of toluene and toluene-TPD experiment was carried out to investigate the adsorption behavior of these samples for VOCs.
Fig.8a exhibits the toluene adsorption isotherms of calcined 1CuyCo/NS-MS catalyst. As the proportion of Co varied from 1 to 2 and then to 3(relative to copper),the adsorption capacity for toluene gradually decreased to some extent, which implies that the cobalt oxides have been imparted into mesopore and occupied the space of mesopore thereby affecting the toluene adsorption ability in this case. Notably, the decreased trend of toluene adsorption performance from 1Cu2Co/NS-MS-s to 1Cu3Co/NS-MS-s was weakened compared to 1Cu2Co/NS-MS-s and 1Cu1Co/NS-MS-s, which agrees with the above - mentioned structural character of materials.However, the curve declined sharply with respect to 1Cu4Co/NS-MS-s. This phenomenon indicates probably the fact of too much cobalt content leading to a blockage of mesopore to some extent because of toluene molecules with a larger kinetic diameter[52]. Such a phenomenon also accounts for the poor catalytic activity for later toluene degradation.
The results of TPD measurements mainly depend on the interactions between the adsorbate molecules and the adsorbent surface, which belongs to chemical adsorption.The desorption amount detected in the TPD experiment is a function of the number of adsorption sites available in each adsorbent, and the desorption temperature can be regarded as a relative measurement of the strength of adsorbate-adsorbent interaction. The toluene-TPD profile of 1Cu3Co/NS-MS-s catalyst is shown in Fig.8b. Obviously, the desorption peak temperature of the catalyst was about 110 ℃,which is consistent with that of similar metal oxide catalysts reported in relevant literature[53]. However, toluene over the catalyst was almost completely desorbed after 200 ℃,so the adsorption of toluene on the catalyst was inappreciable when the temperature was equal to or higher than 200 ℃. In addition, only one peak can be observed in the TPD profile, which indicate that there is only one type of adsorption site for toluene. What′s more, the high desorption amount of toluene by 1Cu3Co/NS-MS-s indicates an excellent toluene adsorption capacity, which provides abundant active sites and well reserved mesopores for catalytic oxidation of toluene.
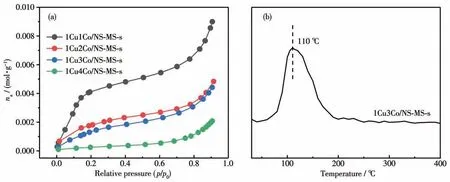
Fig.8 Adsorption isotherms of toluene of 1CuyCo/NS-MS-s catalysts at 25 ℃(a)and toluene-TPD profile of 1Cu3Co/NS-MS-s catalyst(b)
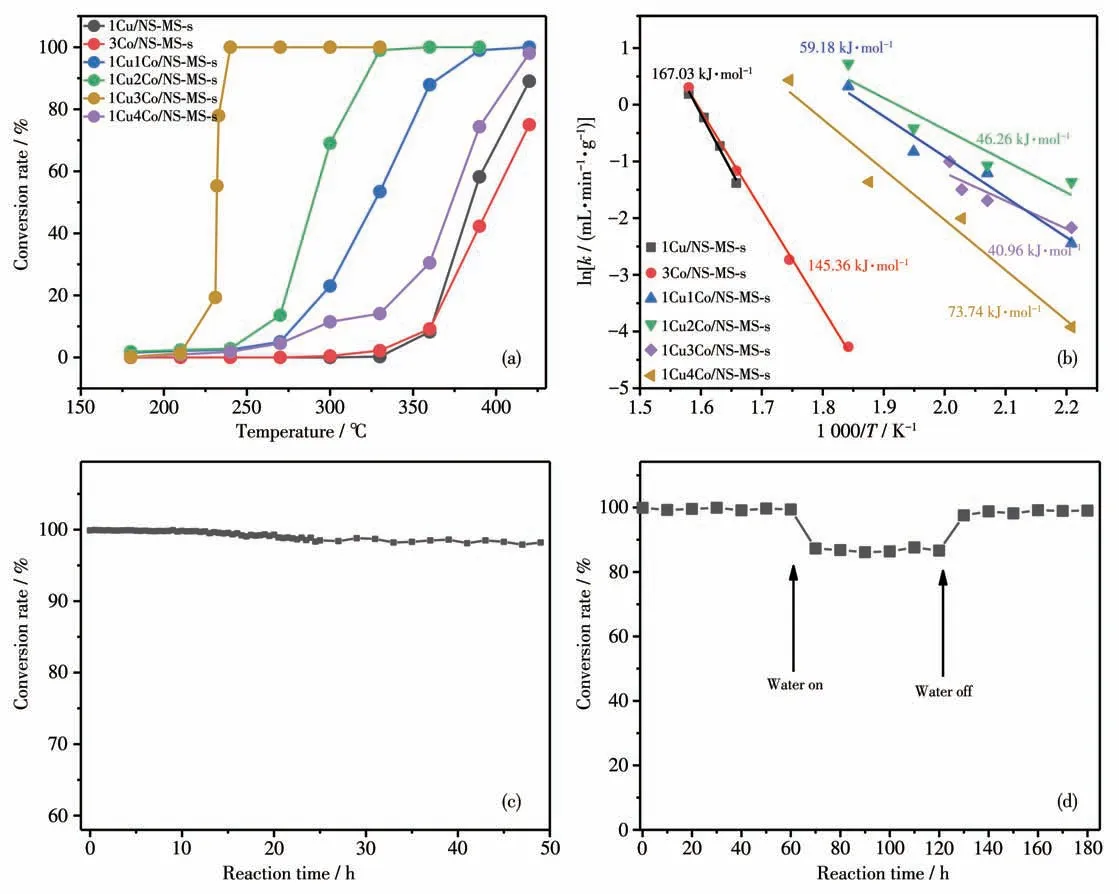
Fig.9 (a)Catalytic results of series of xCuyCo/NS-MS-s catalysts for total oxidation of toluene as a function of reaction temperature;(b)Arrhenius plots for total oxidation of toluene over xCuyCo/NS-MS-s catalysts;(c)Catalytic life test and(d)water resistance of 1Cu3Co/NS-MS-s catalyst at 240 ℃
The catalytic behavior towards toluene VOCs decomposition was tested in a temperature range of 180~420 ℃. In the low temperature range of 25~210 ℃,all the catalysts exhibited almost no conversion over toluene (Fig.9a). However, in the higher reaction temperature range,except CO2and H2O,no CO,benzaldehyde, or benzoic acid were detected under the conducted experimental conditions, hinting at the complete degradation of toluene under the assistance of a catalyst. Besides, Fig.9a shows the toluene conversion rate as a function of the temperature forxCuyCo/NS-MS-s catalysts.The Co loading affected the catalytic efficiency of toluene degradation, especially,the enhanced Co loading contributed to the increment of catalytic efficiency of toluene degradation. But excessive Co loading in the Cu-bearing samples resulted in the worse catalytic activity taking the example of 1Cu4Co/NS-MS-s, which should ascribe to the pore block and poor structure-property and morphology of catalyst inducing the limited diffusion behavior of toluene molecule. Taken together, the sample 1Cu3Co/NS-MS-s exhibited obviously improved catalytic performance for toluene compared to other counterparts, which should thank to the high synergy of dispersed Cu-Co oxides in the strong interaction state on mesopore in great reaction-preferred catalyst microenvironment without limited pore block. In addition, the catalytic conversion of toluene increased gradually with the rising reaction temperature. In particular,when the reaction temperature reached above 230 ℃,the toluene conversion began to zoom rapidly. TheT50andT99reached 232 and 239 ℃,respectively(T50is the reaction temperature when the toluene conversion is 50%;T99is the reaction temperature when the toluene conversion is 99%). The complete oxidation to CO2occurred at 240 ℃for 1Cu3Co/NS-MS-s catalyst.What′s more, the toluene conversion of 1Cu4Co/NS-MS-s,1Cu/NS-MS-s, and 3Co/NS-MS-s were less than 10%in the temperature range of 180~300 ℃but their catalytic conversion began to remarkably increase over 330 ℃. Also, the following reactivity trend was clearly observed:1Cu3Co/NS-MS-s>1Cu2Co/NS-MS-s>1Cu1Co/NS-MS-s>1Cu4Co/NS-MS-s>1Cu/NS-MS-s>3Co/NS-MS-s.
In addition, we also noticed that the catalytic degradation efficiency of toluene over the bimetals modified catalyst was obviously superior to that of the single metal modified catalyst. To our knowledge, the introduction of metal species will cause certain aggregation of metal species in the pore under high-temperature treatment, even the blockage in the case of excessive introduction of metal (Scheme 2a). This explains the result that catalyst 3Co/NS-MS-s with relatively poor structural property exhibited a lower catalytic performance compared with low copper loading of 1Cu/NSMS-s possessing greater structural property (Scheme 2c). Whereas in this study, the introduction of two metal Cu-Co species did not have an adverse effect on the catalytic performance of 1Cu3Co/NS-MS-s catalyst for toluene, also reflecting the synergistic effect (synergetic adsorption and activation) of neighboring contact copper and cobalt active sites on the catalytic process(Scheme 2b). Besides, the introduction of bimetals impedes the further aggregation of Co species owing to the strong interplay of Cu-Co oxides, thereby providing superior structural properties compared to the monometal Co modified one even in the same Co loading condition.
Table S2 displays a comparison of the catalytic activity of 1Cu3Co/NS-MS-s with reported transition metal-based catalysts on different supports. The catalytic performance of catalyst 1Cu3Co/NS-MS-s for toluene removal in this work was comparable with noble catalyst Pd-Pt-0.8/CeO2-10-γ-Al2O3[55],which is also higher than those of 1Co3Cu/sep[54], MnO2[56], Cu-Mn/β-zeolite,Cu-Mn/MCM-41,and Cu-Mn/ZSM-5-25[59].Additionally, the observed catalytic activity of 1Cu3Co/NS-MS-s was slightly less than 4Cu0Co/H[57], Cu6Co2/AC, and Cu4Co4/AC[58], probably because of the lower toluene inlet concentration or GHSV in the case of these two compared catalysts. Therefore, taken together, the developed catalyst 1Cu3Co/NS-MS-s still affords a higher advantage in toluene degradation.
In fact, we also tried catalytic degradation experiments for other VOCs including methanol and ethyl acetate, which could be more easily degraded into nontoxic small molecules than toluene at a lower temperature(ca.150,215 ℃,respectively).The results indicated that the catalyst 1Cu3Co/NS-MS-s still revealed the best catalytic ability compared to other counterparts and the variation trend of performance was also consistent with that we have discussed above.
The activity of the catalysts can also be evaluated by comparing the apparent activation energy (Ea), and the lowerEaof the sample indicates the higher activity[60]. When the toluene oxidation reaction was controlled within the dynamic range (toluene conversion rate <20%),the slope of Arrhenius curve was used to evaluate the value ofEa. According to the slope of Arrhenius curve, theEaof the catalyst can be calculated and the fitting result is shown in Fig.9b. The variation tendency ofEaof all catalysts is as follows:1Cu3Co/NS-MS-s<1Cu2Co/NS-MS-s<1Cu1Co/NS-MS-s<1Cu4Co/NS-MS-s<3Co/NS-MS-s<1Cu/NS-MS-s.This result is inversely proportional to the variation trend of performance of the catalysts we obtained above (except the two catalysts loaded with single metal oxides),which verifies that 1Cu3Co/NS-MS-s possessed best activity in all the prepared samples and modification of the original support by active species such as composite metal oxides reduces the energy barrier of catalytic oxidation of toluene.
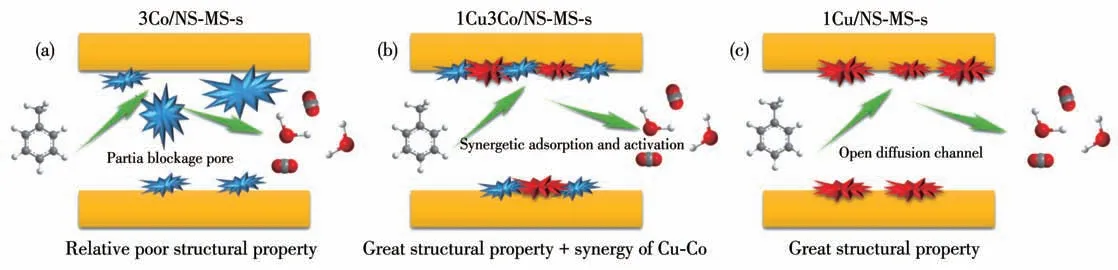
Scheme 2 Rational illustration for enhanced catalytic performance of 1Cu3Co/NS-MS-s compared to monometal counterparts
Catalytic life is one of the significant factors to evaluate the performance of the catalyst in practical applications[61-62]. Fig.9c displays the life test result of 1Cu3Co/NS-MS-s at 240 ℃for 49 h.Clearly,the activity of the catalyst remained excellent during the first 20 h and the conversion rate of toluene remained above 99%. With further prolonged reaction time up to 25 h,there was nearly no loss of activity for 1Cu3Co/NS-MS-s,which still made the conversion rate of toluene up to 98.5%. The slight decrease could be attributed to the fact that the carbon dioxide products gathered and adsorbed on the surface of the catalyst during the catalytic reaction have not been desorbed completely so that the contact surface between toluene and the active species decreases. The developed catalyst also was attempted for use at higher applied temperature(~700 ℃), which exhibited good thermal stability and~100% conversion rate of toluene for 2~3 d. The above results confirmed the long-lived catalytic property of 1Cu3Co/NS-MS-s catalyst.Besides,as we all know,water vapor is not beneficial to the shifts of the reaction equilibrium[12]. In order to roughly simulate the actual situation of industrial production, a few amounts of water vapor were introduced into the toluene stream and the effect on the catalytic performance at 240 ℃was tested (Fig.9d). The results indicated that the introduction of water vapor restrained the toluene oxidation over 1Cu3Co/NS-MS-s to some extent, which led to the phenomenon that the conversion rate of toluene was reduced by about 15%. The existence of water vapor triggers the weakening of catalytic activity, which could associate the competitive adsorption of the water molecule and toluene molecule on the reactive active metal atoms[63]. Especially, the polar water molecule is more easily adsorbed on the metal oxides compared to the toluene molecule, which could account for the downshift of activity after introducing the water vapor.However, the toluene conversion was well returned to the initial level after excluding water vapor from the feed gas. The above results suggest that the waterresistance of the 1Cu3Co/NS-MS-s catalyst was basically satisfactory. Taken together, obtained catalyst bearing bimetal Cu-Co showed excellent catalytic efficiency for the toluene degradation by virtue of efficient synergy of bimetals.
3 Conclusions
In summary, we successfully prepared bimetallic Cu-Co oxides modified HMS nanosphere catalysts using an improved micelle template method. During the assembly process of the nanosphere, Cu-Co bimetals werein-situloaded on the mesoporous surface. A series ofxCuyCo/NS-MS-s materials were obtained by adding different proportions of cobalt and copper in the synthetic process, which not only took advantage of the synergetic effect of bimetals but also the high dispersion of active species. The influence of the bimetallic ratio on the morphology and catalysis performance of the material was in detail studied. It has been found that 1Cu3Co/NS-MS-s exhibited the highest activity among all the samples,on which toluene could be completely converted at 240 ℃. The result is considerable compared with other counterparts and has the encouraging potential for the practical remediation of VOCs pollutants.
Acknowledgments:This work was financially supported by National Natural Science Foundation of China (Grants No.51672114, 21908085, 21806077), Natural Science Foundation of Jiangsu Province, China (Grant No.BK20161357), Postdoctoral Research Foundation of Jiangsu Province (Grant No.2020Z291), Foundation from Marine Equipment and Technology Institute for Jiangsu University of Science and Technology,China (Grant No.HZ20190004), and High-tech Ship Research Project of the Ministry of Industry and Information Technology,China(Grant No.[2017]614).
Supporting information is available at http://www.wjhxxb.cn
