柠檬酸辅助可控制备花状银粒子及其表面增强拉曼散射性能
2021-07-10梅林玉邵云鹏张丛筠王俊元
梅林玉 李 莎 邵云鹏 张丛筠 王俊元*,
(1中北大学机械工程学院,太原 030051)
(2中北大学材料科学与工程学院,太原 030051)
Recently, noble metal materials with fine-tuned physicochemical and structural properties have drawn immense attention due to their potential applications in catalysis, electrically conductive component, biological and chemical sensing, especially for surface-enhanced Raman scattering (SERS)[1-4]. As is well known, SERS activity is closely related to size and shape of noble metal particles. Various investigations are focused on the influencing factors of size, shape and topography,or synthesis of specific Au/Ag nanostructures including nano star, nanospheres, hierarchical dendrites and flower-like nanoparticles,etc[5-10]. These structures can endow significant Raman enhancement in the“hot spot”area, showing excellent SERS performance[11].Among these structures, the flower-like Ag particles have complex morphology and high surface roughness,which are expected to show excellent performance as a single particle SERS detection substrate[12-13]. For synthesizing flower-like Ag,many means have been reported including seed-mediated approach, electrochemical deposition, wet - chemical approach or gas-solution interface technique,etc[6,14-16]. For instance, Xu[17]synthesized flower-like Ag mesoporous structures with diameters ranging from 200 to 700 nm by seed mediated method.Bian[18]successfully electrodeposited the flower-like silver structure with concave surface on indiumtin oxide (ITO) glass by double potentiostatic method,and its SERS signal enhancement was 3.3 times than that of the silver nanostructure without concave surface. Zheng[19]successfully synthesized flower-like Ag nanoparticles by liquid phase reduction using ascorbic acid as reducing agent. This unique flower-like structure had excellent sensitivity and stability.
Here, we developed an eco-friendly approach to synthesis a new class of hierarchical Ag microspheres in large quantities by extracting a natural small molecular organic acid (citric acid) as structure guiding agent innovatively. The flower-like Ag particles with uniform particle size and stable performance were synthesized in one step. The anisotropic growth of Ag microspheres was kinetically controlled by regulating reaction parameters: amount of citric acid and ascorbic acid. The formation mechanism was also proposed. Furthermore,the flower-like Ag particles had a highly roughed surface that could produce more active sites. The flowerlike Ag substrate prepared for detection of rhodamine 6G (R6G) had good sensitivity and stability, and the detection limit as low as 10-10mol·L-1was achieved.The results show that as a good performance substrate for SERS, the flower-like Ag has a broad application prospect.
1 Experimental
1.1 Materials
Analytical grade AgNO3(>99%), citric acid (AR),ascorbic acid (99%), polyvinylpyrrolidone (PVP,Mr=10 000) and R6G were purchased from Alfa Aesar(Ward Hill,MA,USA),and were used as received.Water used throughout all these experiments was purified with a Millipore system.
1.2 Synthesis of Ag flower-like microspheres
In a typical synthesis of flower-like Ag particles,at room temperature,1 mol·L-1AgNO3solution,1 mol·L-1ascorbic acid solution, 1 mol·L-1PVP solution and 0.1 mol·L-1citric acid solution were respectively prepared. Under the condition of ice water bath, 0.2 mL AgNO3solution and 2 mL PVP solution were added into a 25 mL beaker with 10 mL ultra-pure water with magnetically stirring. Then, different volumes (0.05~0.25 mL)of citric acid solution was added and continuously stirred for 5 min. After different volumes (0.1~0.3 mL) of ascorbic acid solution was rapidly injected,the solution quickly changed from colorless to black gray. Finally, the reaction solution was removed and centrifuged with ultrapure water for three times (2 000 r·min-1, 5 min), and the flower-like Ag particles were obtained.
1.3 Characterization and measurement
The morphologies of Ag particles were characterized by field-emission scanning electron microscopy(FE-SEM, ZEISS Supra 55 VP) at 10 kV. The crystalline structure was characterized by powder X-ray diffraction (PXRD) with a CuKαsource (Siemens D800,λ=0.154 nm) scanning from 10° to 90° (2θ) at the rate of 2(°)·min-1.The corresponding working voltage and current were 40 kV and 40 mA, respectively.The crystal structure was characterized by transmission electron microscopy (TEM, Hitachi H-9500 Highresolution,100 kV)and high resolution TEM (HRTEM,Hitachi H-9500 High-resolution, 200 kV). SERS spectra was recorded on a model confocal microscopy Raman system (Horiba Raman Spectrometer). The excitation wavelength was 532 nm. The integral time was 10 s and the laser power was 5 mW,using a 100×objective with laser spot of 1 μm diameter. For the detection of R6G, the Ag particles were mixed with different concentrations of R6G and sonicated for 30 min to reach the adsorption equilibrium. The resulting Ag particles were dispersed on glass substrates and dried in dark for SERS detection.
2 Results and discussion
2.1 Characterization of flower-like Ag nanostructures
It is able to witness the drastic change in morphologies of Ag particles with the assistance of citric acid.As shown in Fig.1a, hierarchical Ag particles resembling pompon shapes were perfectly assembled by lots of nanorods, arranging along different directions like petals,with the assistance of citric acid.The morphology and structure were further confirmed by TEM and XRD (Fig. 1b~1d). Fig. 1c shows a lattice-resolved HRTEM image of a petal. The lattice fringe distance of 0.24 nm is consistent with thed-spacing between (111)planes in face-centered cubic (fcc) structure of silver(0.235 nm from PDF No.89-3722)[2]. The XRD pattern of flower-like Ag exhibited five peaks around 38.0°,44.0°, 64.0°, 77.1° and 81.3°, corresponding to (111),(200),(220),(311)and(222)crystal plane,respectively,which also confirmed the crystal nature of pure fcc(PDF No.89-3722) (Fig.1d). The intensity ratios of diffraction peaks,I(111)/I(220), collected from citric acid directed system are higher than that of standard file for fcc silver (PDF), implying the hierarchical flower-like nanostructures are abundant in (111) facets and have the preferential growth orientation parallel to the (111)direction[2].
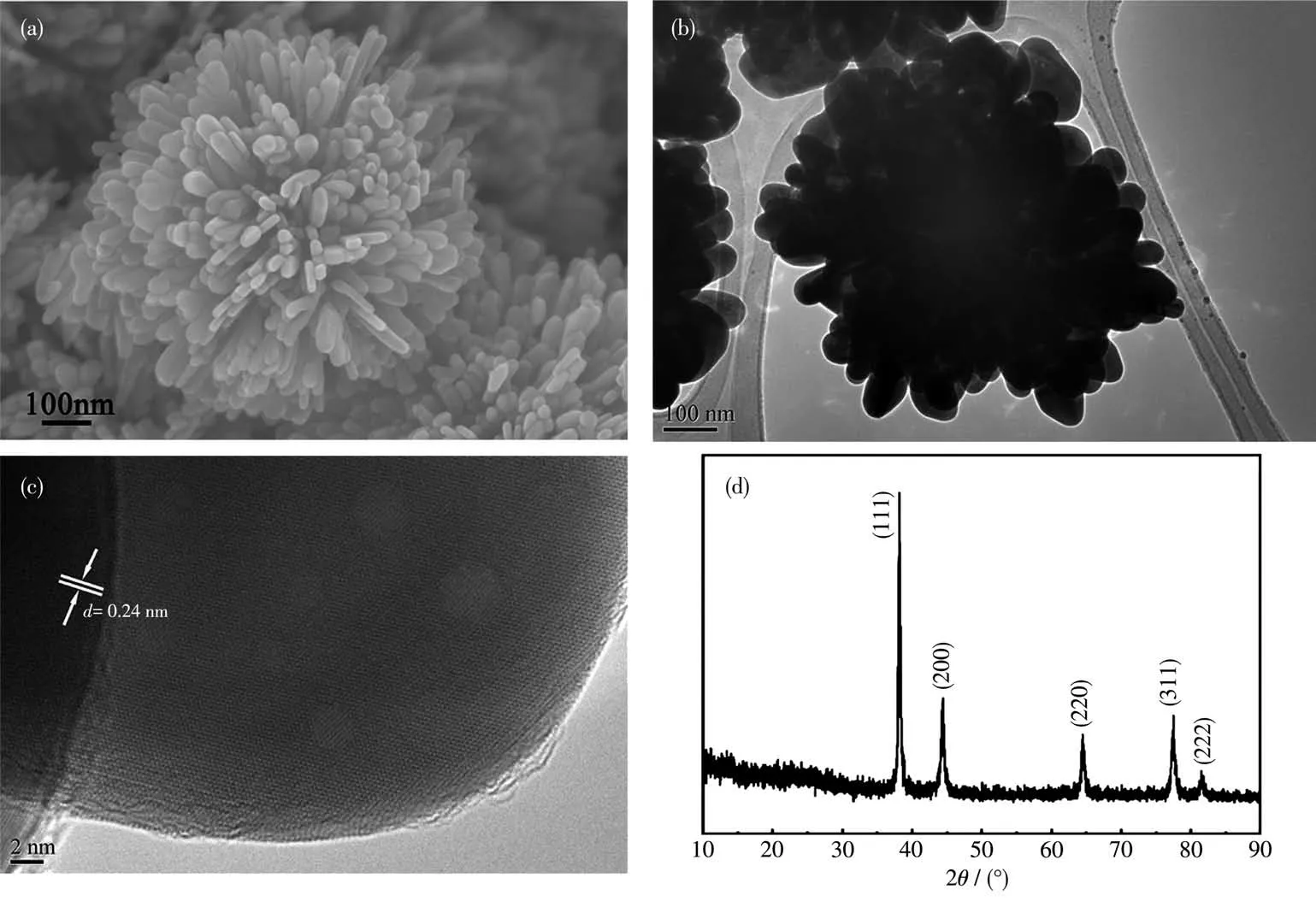
Fig.1 (a)SEM image,(b)TEM image,(c)HRTEM image and(d)XRD pattern of hierarchical flower-like Ag particles
2.2 Influence of amount of citric acid
To elucidate the influence of citric acid on the crystal nucleation and growth of Ag particles, a series of control experiments were performed by varying the amount of citric acid. The citric acid dosage plays a vital role in the nucleation and growth process of hierarchical Ag particles.Fig.2 clearly shows the morphology change of flower-like Ag particles with the increasing amount of citric acid. At low amount, incomplete flower-like particles assembled by irregular nanosheet were obtained (Fig.2a and 2b). Increasing the volume from 0.1 to 0.15 mL, the particles gradually grew into perfect pompon-like spheres, assembling by slender and vertically aligned rod-like nano-petals (Fig.2e and 2f). Further increase in the volume of citric acid adversely affected the formation of uniform flower-like structure. Rod-like nano-petals around the spherical Ag particles began to get shorter and blunter, as shown in Fig.2g and 2h.Especially with the volume up to 0.25 mL, the rod-like nano-petals almost disappeared and the remaining spherical Ag particles were assembled by loosely packed protuberances(Fig.2i and 2j).
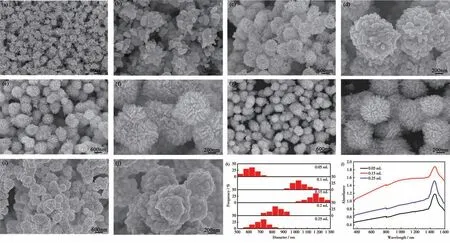
Fig.2 SEM images of flower-like Ag particles fabricated with different volumes of citric acid solution (0.1 mol·L-1):(a,b)0.05 mL,(c,d)0.1 mL,(e,f)0.15 mL,(g,h)0.2 mL,(i,j)0.25 mL;(k)Size distributions and(l)UV-Vis absorption spectra of Ag particles fabricated with different volumes of citric acid solution
When the volumes of citric acid were 0.05, 0.1,0.15, 0.2 and 0.25 mL, respectively, the diameter increased from 650 to 1 050, 1 150 nm and then decreased to 850, 750 nm (Fig.2k). This particle size change could be further confirmed by UV-Vis absorption spectra (Fig.2l), which showed a broadly extendable absorption bands in the visible region. By adding citric acid to 0.25 mL, the surface plasmon resonance(SPR) band of silver nanoparticles shifted to the lower wavelength. The results clearly indicate that the amount of citric acid has a significant influence on the nucleation and growth process. Proper amount of citric acid favores the formation of kinetically controlled anisotropic shapes.
2.3 Influence of amount of ascorbic acid
The low and high magnification SEM images of Ag particles synthesized with different ascorbic acid volumes are shown in Fig.3. With the increase volume of ascorbic acid, the Chinese rose-like spherical Ag particle composed of many interlaced nanosheets(Fig.3a and 3b) gradually transformed to pompon-like spherical Ag particles assembled by short and vertically aligned rod-like nano-petals(Fig.3c and 3d).However, the rod-like petals structure could not be maintained when the volume of ascorbic acid was too high(0.3 mL). The sharp flower structure was strikingly deformed due to excessive speed of reaction. As a result, abundant irregular blunt protuberances were observed around the spherical Ag particles,with significant reduction of surface roughness(Fig.3e and 3f).
2.4 SERS performance
In order to study the SERS properties of flowerlike silver particles, R6G with different concentrations as probe molecule were detected on the samples prepared from citric acid-directing systems under the optimal conditions. The Raman spectra (Fig.4) shows the strong bands at 1 310,1 364,1 508 cm-1,which are attributed to the aromatic C—C stretching vibrations.The vibration band at 1 185 cm-1is assigned to the C—H stretching vibration mode,and the bands at 610,776 cm-1should be ascribed to the C—C—C ring in-plane mode. Zhang[13]observed that flower-like Ag nanostructures prepared by chemical reduction method had similar Raman characteristic peaks, and the detection limit for R6G was 10-10mol·L-1. It is noteworthy that R6G molecules presented a good signal-to-noise ratio, even if the concentration downed to 10-10mol·L-1, and the distinctive and characteristic peaks of R6G could be still discernable at 10-10mol·L-1,indicating a great sensitivity in molecule detection. It can be interpreted that tiny interstitial sites and aligned oriented rod-like nano-petals on the roughened Ag surface generate numerous hot spots, inducing significant enhancement of electromagnetic fields.

Fig.3 SEM images of flower-like Ag particles synthesized with different volumes of ascorbic acid solution (1 mol·L-1):(a,b)0.1 mL,(c,d)0.2 mL,(e,f)0.3 mL
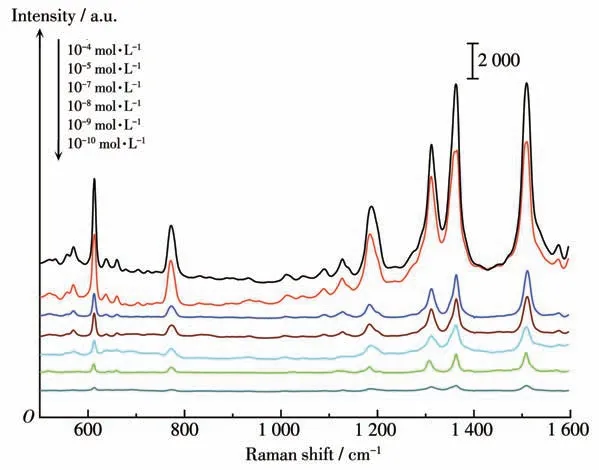
Fig.4 SERS spectra of flower-like Ag particles adsorbed to different concentrations of R6G
2.5 Growth mechanism
The growth mechanism of hierarchical flower-like Ag nanostructure was proposed. As shown in Fig.5, the functional groups of citric acid (carboxyl and hydroxyl)capture Ag+from the solution easily and coordinate with positively charged Ag+ions to form relatively stable complexes. When ascorbic acid is added, Ag+ions trapped in complexes are gradually released and reduced to Ag atoms. Under the suppress effect of citric acid-silver complexes on the reducing rate, the initial seeds start to aggregate into primary Ag crystals by capturing free Ag atoms from solution. With the incorporation of citric acid, the surface energy of (111) crystal surface is decreased, and Ag particles grow preferentially on (111) crystal surface, which favors to forming flower-like structure. Subsequently, citric acid is added, and free Ag atoms are deposited continuously,forming hierarchical Ag particles with different surface roughness. Accordingly, citric acid plays a role of structure-directing agent and paves a way for confining shapes of growing Ag particles.
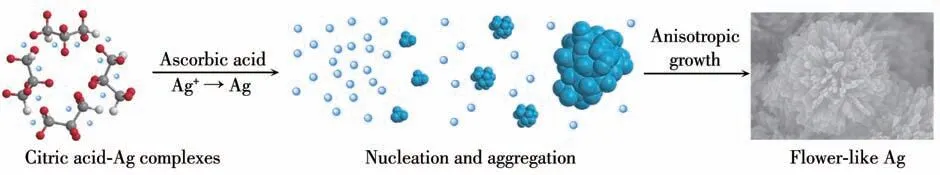
Fig.5 Illustration of proposed growth mechanism of formation of hierarchically flower-like Ag particles with assistance of citric acid
3 Conclusions
Hierarchical flower-like Ag particles with precisely controllable morphologies were successfully fabricated by a facile synthetic approach and acted as SERS substrates. The addition of citric acid can effectively control the diffusion of Ag+and consequently suppress the reduction rate, which induces the anisotropic growth of complex Ag assemblies under a kinetically controlled regime.The amount of citric acid or ascorbic acid can significantly affect the petal-like anisotropic morphologies. The as-synthesized substrates exhibit excellent SERS properties, which can still produce higher Raman signal for very low concentration probe molecules, and can potentially be used for trace detection of organic pollutants in environment.
Declaration of competing interest:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
