Effect of Fruit Color and Ripeness on Volatile Distribution in Cherry Tomato (Solanum lycopersicum var. cerasiforme) Fruit
2021-07-08CHENGGuotingCHANGPeipeiWANGXinyuLOUQianqiAhmedElSAPPAHZHANGFeiLIANGYan
CHENG Guoting, CHANG Peipei, WANG Xinyu, LOU Qianqi,Ahmed H.El-SAPPAH,4, ZHANG Fei,*, LIANG Yan,*
(1.College of Horticulture, Northwest A&F University, Yangling 712100, China; 2.State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University, Yangling 712100, China; 3.Dezhou Academy of Agricultural Science, Dezhou 253015, China;4.Genetics Department, Faculty of Agriculture, Zagazig University, Zagazig 44511, Egypt)
Abstract: To analyze the effect of fruit color and ripening on the volatile profile of cherry tomatoes, the volatile compositions of the inbred lines Jinzhu (orange), Heiyingtao 1 (purple), 1 Hao (pink), and Hongzhenzhu (red), were measured at the mature green, turning and red stages by headspace solid-phase micro-extraction (HS-SPME) and gas chromatography-mass spectroscopy (GC-MS).The volatile content of orange tomato fruit was significantly higher than that of red and pink tomato fruit at each stage.The highest content of volatiles appeared in the orange tomato fruit at the mature green stage and in purple tomato fruits at the turning and red stages, and the lowest content of volatiles was always observed in pink tomato fruits during the whole period.The intensity of volatile odor was determined by the concentration and olfactory threshold of volatiles.3-Methyl-butanal, (E,E)-24-heptadienal, 1-penten-3-one, hexanal,benzaldehyde, and (Z)-3-hexen-1-ol contributed significantly to tomato odor.The strongest odor was observed in red, orange, and purple tomato fruit at the mature green, turning and red stages, respectively.With fruit ripening, the concentration of total volatile components was increased, and the odor became stronger.In conclusion, fruit color and ripeness have a significant effect on the volatile profile of cherry tomatoes.
Keywords: Solanum lycopersicum var. cerasiforme; aromatic compounds; odor; fruit color; maturity
Cherry tomato (Solanum lycopersicumvar.cerasiforme)being a good source of vitamins, flavonoinds, and aroma,which is becoming increasingly popular due to its attractive color and delicious flavor[1].As the specific contribution of volatile compound to the flavor was gradually recognized, the enthusiasm to improve the flavor was stimulated.After hard work, the regulation of volatile accumulation was gradually clarified[2-5].It was found that many factors were affecting the metabolism of volatiles, including the supply of volatile precursors, gene transcription levels, enzyme activity[6-9],management measures, plant growth conditions, biological and abiotic stresses, and postharvest treatment, ect.[6-7,10-13].Both fruit color and harvest ripeness are important quality characteristics.Furtherly, the fruit color had substantial impact on consumers’ expectations about sensory and functional properties[14-16].Although lots of studies focused on the effects of fruit color and ripeness on the volatiles and flavor[9,13-14,17], driven by commercial interests, previous studies mainly paid more attention to the tomato fruit with red or pink color at the red ripe stage[18-19].Not only that, tomato fruit could be picked at any stage of ripening for different purposes.Few studies were comprehensively considered about the regulation mechanisms of fruit color and ripeness to volatile metabolism.
To investigate the effect of fruit color and ripeness on volatiles, the volatiles and aroma profiles were compared and analyzed systematically among the four colors at three ripening stages of tomato fruit.This study would lay a foundation for breeding tomato germplasm with excellent flavor and guide suitable harvest.
1 Materials and Methods
1.1 Materials and reagents
The breeding materials of cherry tomato tested in this study were named Jinzhu, Heiyingtao 1, 1 Hao, and Hongzhenzhu.The uniform codes of the four inbred lines were CI3008, CI5004, CI2010, and CI1009, whose colors of ripe fruit were orange, purple, pink, and red, respectively (Fig.1).The seeds of tomato were provided by the research group on Tomato Genetic Breeding and Quality Improvement of Northwest A&F University.

Fig.1 Cherry tomato fruit at different ripening stages
Cherry tomato seed was sown in January of 2018, and the seeding was planted two months later in the standardized research greenhouse of the College of Horticulture,Northwest A&F University.According to the color change on the surface of tomato fruit, the ripening process was divided into six stages, including mature green stage, breaker stage,turning stage, pink stage, light red stage and red stage[20].In this study, the mature green stage (S1), turning stage (S2), and red stage (S3) were chosen.According to the above sampling criteria, the orange tomato reached the mature green, turning and red stages 28, 44 and 53 days after-anthesis, respectively.The pink tomato reached the mature green, turning and red stages 25, 40 and 45 days after-anthesis, respectively.The red tomato reached the mature green, turning and red stages 26,42 and 50 days after-anthesis, respectively.The purple tomato reached the mature green, turning and red stages 31, 48 and 59 days after-anthesis, respectively.The tomato fruit were selected from the third infloresence.Thirty fruit were taken for analysis from each color of tomato at each stage of ripening.
The reagents used were anhydrous Na2SO4and 2-nonanone (chromatographic pure), were purchased from Tokyo Chemical Co.Ltd., Japan.
1.2 Instruments and equipments
0.04 L headspace bottle, ten-thousandthof the analytical balance, thermostatic magnetic stirrer (Troemner LLC.,USA); 75 μm CRA/PDMS extraction head, manual injection handle of SPME (Supelco Co.Ltd., USA); TRACE ISQ gas chromatography-mass spectrography (Thermo Scientific Co.Ltd., USA); FJ200-SH digital high-speed dispersion homogenizer (Specimen model factory in Shanghai, China).
1.3 Methods
1.3.1 Headspace solid-phase microextraction (SPME)sampling
Tomato was homogenized with a FJ200-SH digital high-speed dispersion homogenizer.0.005 kg homogenate of tomato fruit was weighed from each sample using one ten-thousandthof the analytical balance.Anhydrous Na2SO4(0.003 kg) and 10-5L of 2.5 × 10-5kg/L 2-nonanone(chromatographic pure) as the internal standard were added to the headspace bottle of 0.04 L.Three biological replicates were run.Placed the headspace bottle on a thermostatic magnetic stirrer keeping 50 ℃, 500 ×gfor 600 s.Then,volatile aromatic compounds were adsorbed by 75 μm CRA/PDMS extraction head with manual injection handle of SPME for 1 800 s.The extraction head was inserted immediately into the gas chromatography-mass spectrography (GC-MS)chamber of TRACE ISQ.The volatile aromatic compounds should be desorbed for 180 s, then GC-MS for 2 280 s.
1.3.2 GC-MS operating conditions
The GC-MS conditions referenced the literature[21-22],which were modified slightly.
The conditions of GC: 230 ℃ of inlet temperature, over 99.999% helium of carrier gas, no split injection, 1.67 × 10-5L/s of column flow rate, split ratio 20:1.The sample injection mode was splitless sample injection.Then, the diverter valve opened after 180 s.The column heat-up programs were as follows:the initial temperature was kept at 40 ℃ for 180 s, and increased to 110 ℃ at a rate of 0.17 ℃/s.Then, the temperature rose to 230 ℃ at a rate of 0.1 ℃/s, which was kept for 480 s.
The conditions of MS: ionization method of electron bombardment ion source, 70 eV of electron energy, a full scan of scanning mode,m/z35~500 of scanning mass range,230 ℃ of an ion source and transmission line temperature.
1.3.3 Qualitative and quantitative analysis
After the tomato samples beding identified by GC-MS,kinds of aromatic volatile compounds were evaluated with the Xcalibur software (version 2.0, Thermo Scientific,USA), which were searched by computer and compared with the standard mass spectrum of the library (NIST2011,USA).The volatile aromatic compounds were identified by reference to the registry number (CAS#) of the compound,similarity index (SI), reverse similarity index (RSI) of mass spectrometry and related literature[23].Only the aromatic volatile compounds, whose SI and RSI both higher than 800(1 000 as the highest) were analyzed.
The concentrations of various aromatic volatile compounds were calculated by the internal standard peak area normalization method.That was the peak area of each component compared with the peak area of the internal standard (2-nonaone)[24].The calculation formula was as follows:
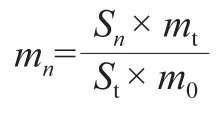
Wheremnis the concentration of the volatile compound(namedn), whileSnindicates the peak area of volatile compound (namedn).mtmeans the internal standard concentration (2-nonanone), whileStshows the internal standard peak area.m0is the mass of sample.
Based on the calculation formula of the internal standard peak area normalization method, the response curve of volatile concentration (μg/kg) (y) and peak area (x) fitted a simple linear regression equation:y= 364.17x+ 0.141 6,R2= 0.954.
1.4 Statistical analysis
WPS Office 2019 and SPSS 17.0 were used for statistical analysis.Three biological replicates of each sample were taken and used mean values.The error among the three biological replicates was analyzed with the standard deviation (SD).The significant difference among samples was performed by one-way analysis of variance (ANOVA)(P< 0.05) and Tukey test.The correlation was expressed by pearson correlation coefficient.Then the corresponding tables and figures were made.
2 Results and Analysis
2.1 Differences in volatile components among four colors of tomatoes
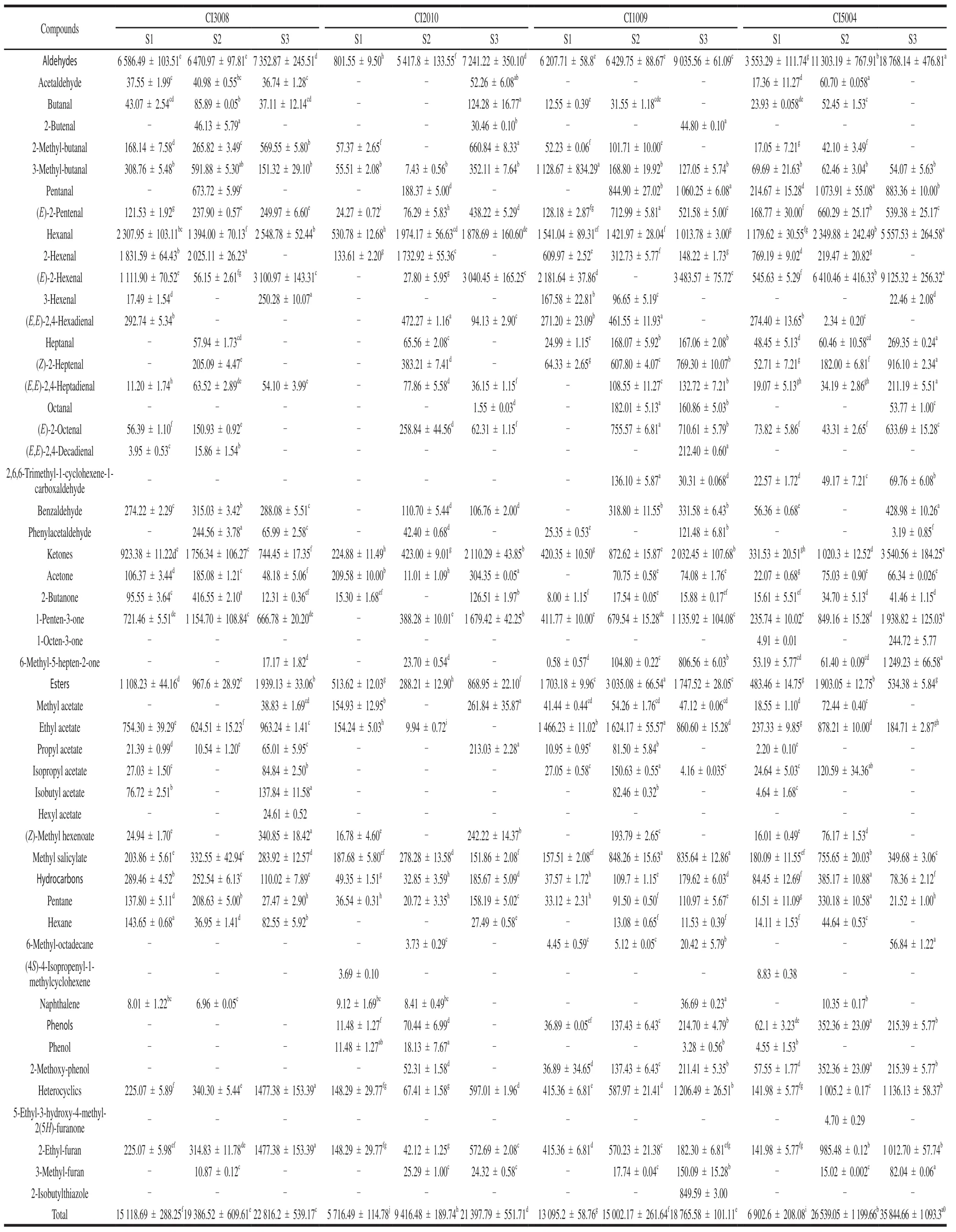
The properties of detected volatile compounds were shown in Table 1.A total of 59 volatiles were detected from the fruit of tomatoes in 4 colors at 3 ripe stages, including 22 aldehydes, 13 alcohols, 5 hydrocarbons, 8 esters, 5 ketones,4 heterocyclics, and 2 phenols (Table 2).There were 47, 49,54, and 54 volatiles in the fruit of CI3008, CI2010, CI1009,and CI5004, respectively.The component of volatileswas always as the least in CI2010.At S1, the component of aldehydes was 17 in CI5004, whereas it was only 5 in CI2010.With the fruit ripening, the components of volatiles increased in general.Tomato would have better flavor with more prosperous volatile components[18].
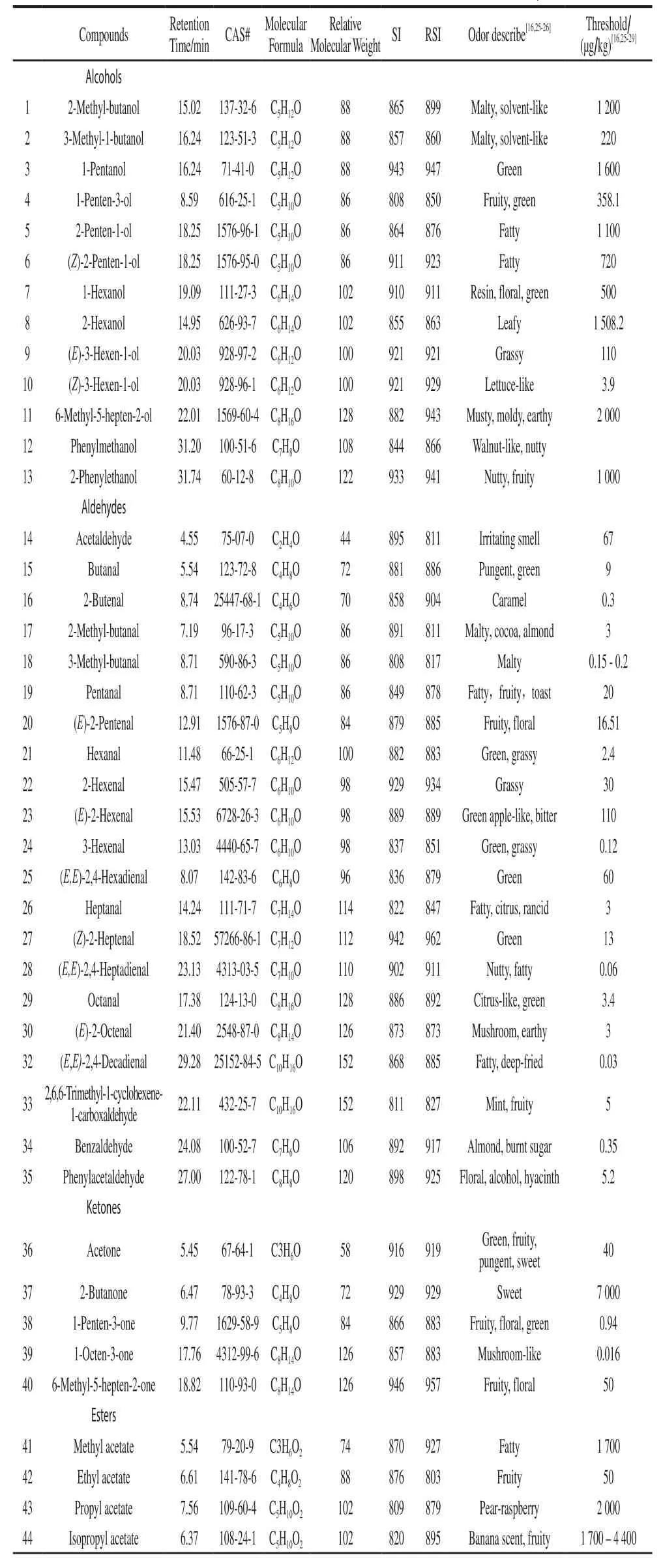
Table 1Properties of volatile components detected in cherry tomato fruit
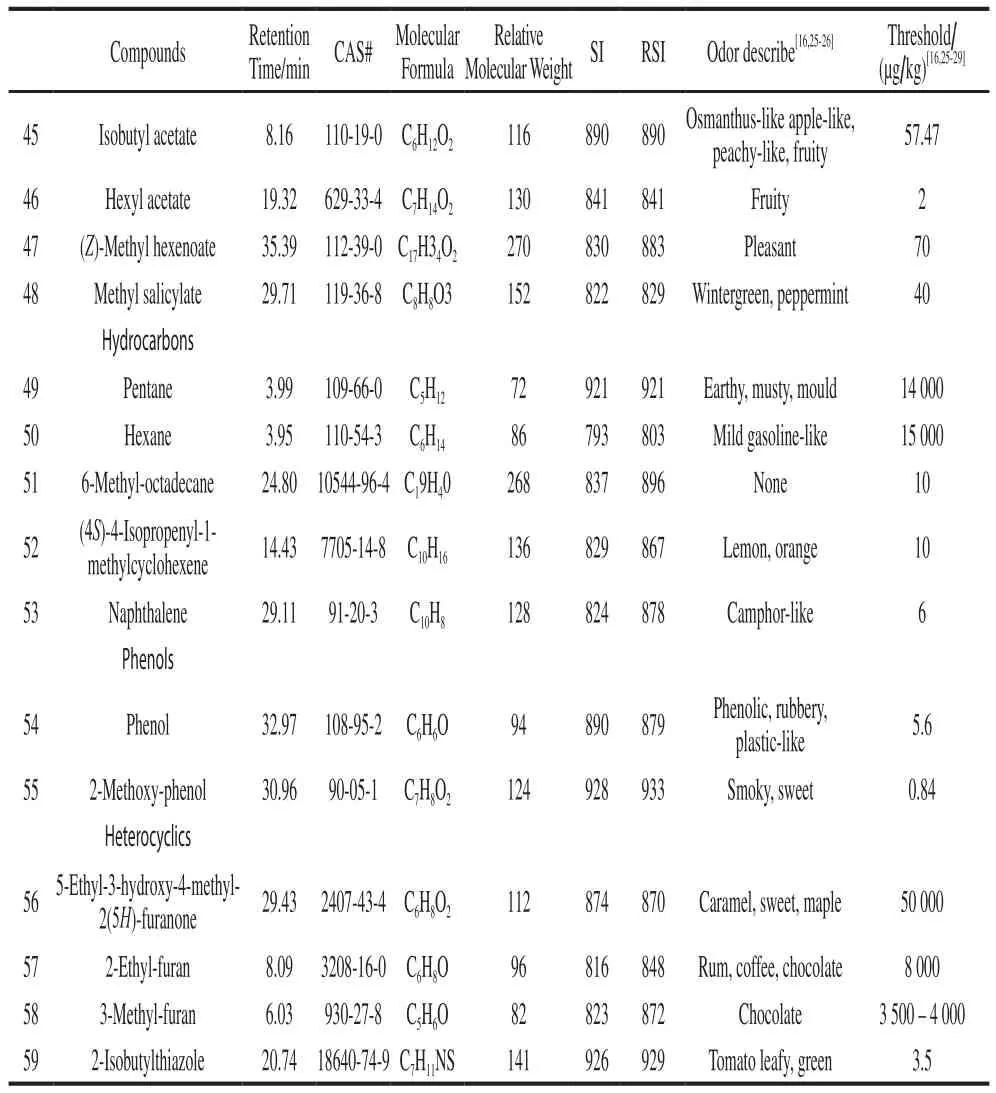
Continued Table 1
Characteristic effect volatiles have important contribution to flavor.14 of 29 primary volatiles[27,30-31]were detected; namely, hexanal, (E)-2-hexenal, (Z)-2-heptenal,1-hexanol, (Z)-3-hexen-1-ol, 1-pentanol, 1-penten-3-ol,3-methyl-1-butanol, 1-penten-3-one, 6-methyl-5-hepten-2-one, phenylacetaldehyde, 3-methyl-butanal, methyl salicylate, and 2-isobutylthiazole.Besides, 2-methyl-butanol,2-methyl-butanal, isobutyl acetate, (E)-2-pentenal, (E,E)-2,4-decadienal, 1-octen-3-one, and 2-methoxy-phenol were detected, which were important volatiles for tomato[32].The components of key volatiles were the most in CI1009 and CI5004, followed by CI3008, where as CI2010 the least.2-isobutylthiazole was only detected in CI1009.1-Octen-3-one was only detected in CI5004.Hexyl acetate was only detected in CI3008.However, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde was not detected in CI3008 and CI2010.(E,E)-2,4-Decadienal was not detected in CI5004 and CI2010.3-Hexenal was not detected in CI2010.
2.2 Differences in volatile concentrations among four colors of tomatoes
The concentration of volatile is an important factor affecting the flavor intensity.According to the formula, the concentration of each volatile compound was calculated(Table 2).The concentration of total volatiles had a significant difference among the four colors fruit.The highest concentration was observed in CI3008 (15 118.69 μg/kg) atS1, and it appeared in CI5004 at S2 (26 539.05 μg/kg) and S3 (35 844.66 μg/kg).Properties of volatiles are affected by functional groups.According to the concentrations of volatiles with different functional groups, two principal components (PC) can be extracted (Fig.2), accounting for 68.63% of the total variance.The contributions to the PC1 mainly came from aldehydes, alcohols, ketones, and heterocyclics volatiles, while the PC2 mainly depended on esters volatiles.The four inbred lines of different colors could be separated by the scatter plot of principal component,which means the volatiles concentrations had a significant difference among four colors of tomatoes.In CI5004, the score of PC1 increased most from S1 to S2, and the score of PC2 decreased most from S2 to S3.This suggested that aldehydes, alcohols, ketones, and heterocyclics increased,and esters decreased, significantly.The highest score of PC2 appeared in CI1009.The mapping points of four colors tomatoes were more dispersed at S2 (not S3), which indicated the volatile concentration had a larger variation coefficient at S2.With the fruit ripening, the scores increased continuously in PC1.
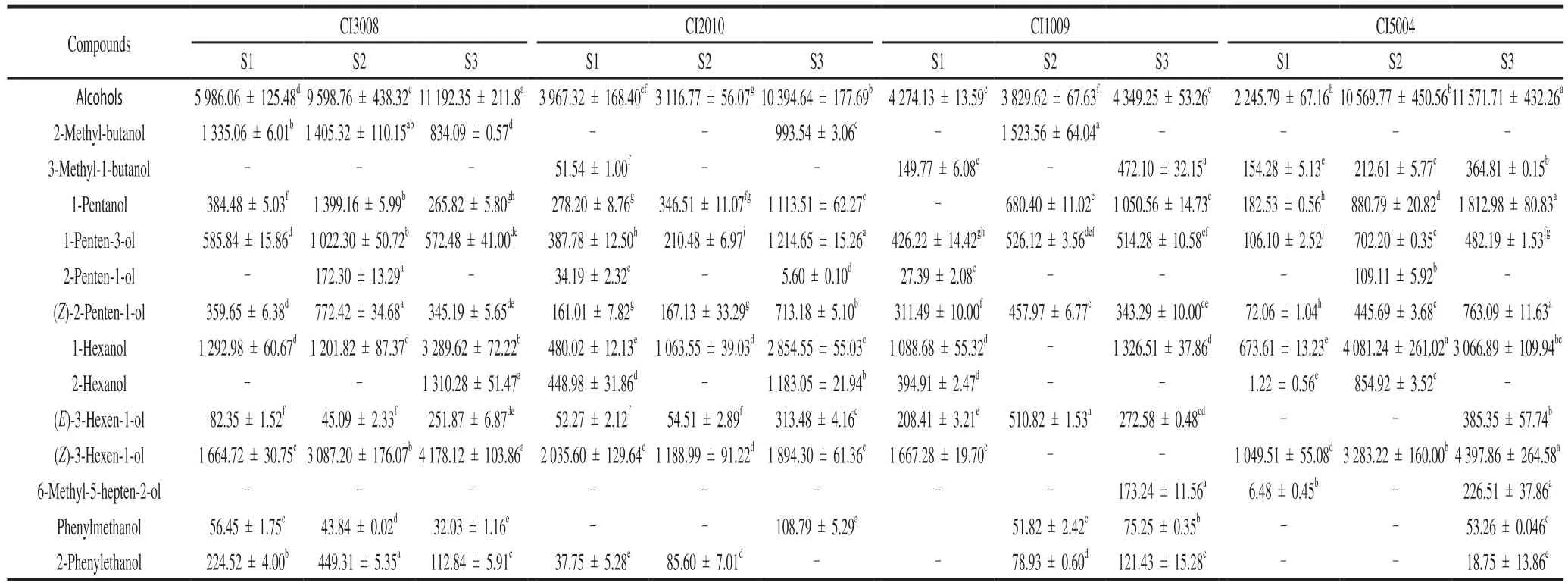
Table 2Concentrations of volatile components (n = 3, 2-nonanone equivalent) detected in cherry tomato fruit with different colors at three ripening stages μg/kg
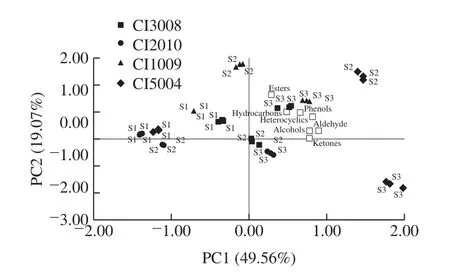
Fig.2 Principal component analysis (PCA) plots for discrimination of cherry tomato fruit with different colors at three ripening stages
Essential nutrients are the main sources of tomato volatiles, including the fatty acids, branched chain amino acid(BCAA), phenylalanine, and carotenoid[33].The concentration of 14 characteristic effect volatiles could account for 64.05%of the total volatiles.The C6volatiles were more abundant;namely, (E)-2-hexenal (2 944.69 μg/kg), (Z)-3-hexen-1-ol(2 444.68 μg/kg), hexanal (1 974.89 μg/kg), and 1-hexanol(1 856.32 μg/kg).
In CI5004, the fatty acids derivatives were the most at S2 and S3, such as hexanal (2 349.88 and 5 557.53 μg/kg),(E)-2-hexenal (6 410.46 and 9 125.32 μg/kg), heptanal,(Z)-2-heptenal, (E,E)-2,4-heptadienal, 1-pentanol, 1-hexanol(4 081.24 and 3 066.89 μg/kg), and (E)-3-hexen-1-ol.Hexanal and (E)-2-hexenal had an essential role for tomato flavor[17].The fatty acids metabolism was more active in purple tea leaves than the green ones[34].Because the conversion rate of chloroplasts into chromoplast was low in purple tomato,keeping high chloroplasts concentration at the later stage[1].Besides, 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde and 6-methyl-5-hepten-2-one were more abundant in CI5004 at S3.They were from lycopene andβ-carotenoid, catalyzed by carotenoid cleavage dioxygenase (CCD1 and CCD4) and lipoxygenase (TomLoxC)[14,35-38].The level of 2-methoxyphenol was the highest in CI5004, because there was a higher concentration of anthocyanin in purple tomato[19].
In CI3008, alcohols, aldehydes and ketones were more abundant at S1 than other colors of tomatoes.They were mainly the fatty acids derivatives (1-pentanol, 1-penten-3-ol, (Z)-2-penten-1-ol) and phenylalanine derivatives(phenylmethanol, 2-phenylethanol, benzaldehyde).The phenylalanine derivatives had a significantly positive correlation with phenols.The synthesis of 2-phenylethanol and 2-phenylacetaldehyde were catalyzed by aromatic amino acid decarboxylases[39].Benzaldehyde, phenylmethanol,and methyl benzoate were derived fromL-phenylalanine,catalyzed by aromatic amino acid amino transferases[40].The branch amino acids were also important precursors of volatile compounds[11,41].The BCAAs derivatives were more in CI3008 at S3.They were mainly ethyl acetate, isopropyl acetate, isobutyl acetate.Besides, the concentrations of total volatiles were always higher in CI3008 than that in CI1009 and CI2010; especially, 1-hexanol (1 928.14 μg/kg), (Z)-3-hexen-1-ol (2 976.68 μg/kg), and 2-phenylethanol.Lycopene accumulation in orange tomato was inhibited and fatty acids content was higher[1].Orange tomato varieties had excellent nutritional properties and sensorial characteristics[42].
Continued Table 2
In CI1009, esters (ethyl acetate 1 317.00 μg/kg)were the highest among the four colors of tomatoes.The phenylalanine derivatives, methyl salicylate (835.64 μg/kg)and phenylacetaldehyde were abundant at S3.Red color tomato contained more lycopene[43].But the carotenoid derivatives in CI1009 were less than that in CI5004 at S3.Because the accumulation of volatile was not only related to the concentrations of the precursors, but also affected by the activity of enzymes and the specificity of enzymes to substrate[25].The inner biochemical reaction, sampling criterion, and fruit color could all affect the volatiles accumulation[44].
In CI2010, the concentrations of volatiles were the least among the 4 colors of tomatoes.The fatty acids derivatives were lower, and 3-methyl-1-butanol, (Z)-2-heptenal,phenylacetaldehyde, and 6-methyl-5-hepten-2-one were not detected at S3.
2.3 The changes of volatile compound concentrations with the fruit ripening
With the fruit ripening, chloroplast converted to chromoplastand, which caused a series of biochemical changes, e.g.altering the volatile compounds profiles[45].Since the concentrations of linoleic and linolenic, carotenoid,and amino acids increased continuously with fruit ripening,the components and concentrations of total aromatic volatile compounds increased ceaselessly[13,17].In this study, the mapping points of volatiles moved forward along PC1 with the fruit ripening (Fig.2), which were consistent with the increase in total volatiles, aldehydes, heterocyclics, and the fatty acids derivatives (Table 2).Total volatiles concentration was 2.97 fold as high as S1 at S3.Total volatiles in CI5004 increased by 19 636.45 μg/kg from S1 to S2, and by 11 981.31 μg/kg in CI2010 from S2 to S3 as the most.Because the precursors were abundant in supply, with enhanced enzymes activity and increased cell membrane permeability,enzyme and substrate could contact more easily, many nonvolatile compounds were hydrolyzed to their free form during fruit ripening[17,46].From S1 to S2, the fatty acids derivatives including (1-pentanol, (Z)-2-penten-1-ol, pentanal, (E)-2-pentenal, heptanal, (Z)-2-heptenal, (E,E)-2,4-heptadienal,(E)-2-octenal, 1-penten-3-one, 3-methyl-furan), phenylalanine derivatives (methyl salicylate) were all increased in their correspending concentration.Alcohols, aldehydes, heterocyclics increased, which mainly derived from fatty acids, e.g., the average value of (E)-2-hexenal increased by 3 012.65 μg/kg from S2 to S3 in both CI5004 and CI1009.Similarly, Buttery et al.[47]had found that (E)-2-hexenal increased in tomato from S1 to S3.Because abscisic acid and ethylene concentrations increased along with the fruit ripening, which could promote almost all volatile synthesis pathways[45,48].In contrast, the exogenous auxin could change the volatiles accumulation via inhibiting the carotenoids accumulation, the chlorophyll degradation and altering the genes expression of volatile synthetases[49].
Besides, both in CI1009 and CI5004, the carotenoid derivatives (6-methyl-5-hepten-2-one), 1-penten-3-one,(Z)-2-heptenal, and 3-methyl-furan were increasing with the fruit ripening.Since the synthesis of the carotenoid derivatives mainly occured at the late stage[37].Whereas, the concentrations of the branch chain amino acid derivatives(ethyl acetate, isopropyl acetate, butanal, 2-methyl-butanal)deceased from S2 to S3.Since alcohol acyltransferases(SlAAT) had low activity in modern tomato and fatty acids desaturases (SlFAD), SlAAT could reverse esters to alcohols at the late stage[50].On the other hand, the concentrations of the fatty acids derivatives volatiles decreased in CI1009,while increased in CI5004, such as 3-methyl-1-butanol,1-hexanol, (Z)-3-hexen-1-ol, hexanal and (E)-2-hexenal from S1 to S2, and octanal, (E)-2-octenal and hexanal from S2 to S3.Because along with fruit ripening, more chlorophyll was transformed into lycopene in CI1009, while the transformation was restricted in CI5004.Concentrations of the phenylalanine derivatives in CI1009 and the fatty acids derivatives in CI5004 increased continuously with the fruit ripening.The concentrations of the fatty acids derivatives including ((E)-2-pentenal, hexanal, 3-hexenal,octanal, (E)-2-octenal, (E)-3-hexen-1-ol) deceased from S2 to S3 in CI1009.Since lipoxygenase (SlLoxC) activity increased at early stage and decreased at ripe stage of tomatofruit[4].The lipoxygenase, hydroperoxide lyase showed the highest activity at the pink stage of ripeness, and alcohol dehydrogenase was most active at the red stage[3].
Both in CI3008 and CI2010, the concentrations of the phenylalanine derivatives (2-phenylethanol,phenylacetaldehyde, methyl salicylate) increased from S1 to S2, while decreased from S2 to S3.The change trend of esters was in opposition to this.Differently, the fatty acids derived volatiles, ((Z)-3-hexen-1-ol and 1-penten-3-ol) increased in CI3008, while decreased in CI2010 from S1 to S2.The levels of following volatiles decreased in CI3008, while increased in CI2010; namely, hexanal and (E)-2-hexenal from S1 to S2, and 1-penten-3-one,1-pentanol and 1-penten-3-ol from S2 to S3.
From the above, ripeness could affect tomato volatiles by increasing the concentrations of the C6compounds , not the volatile components, mainly.It was only at the late stages that the volatiles accumulated in large quantities.Some primary volatiles peaked at the turning (CI3008), pink, or red stages[51].So, it was unwise to harvest tomato before the mature green stage.Because the concentrations of volatiles were less, and the odors were terrible in the early picked tomato[6,52].
2.4 Odor active values of volatile compounds
The flavor intensity of volatiles could be reflected by odor active values (OAVs).Flavor characteristics of tomato could be described as herbaceous, floral, fruity, fatty, cocoalike, and irritating odors[53].In this study, the four colors of tomatoes had the average OAVs of 3 208.56, 2 543.92,1 553.67, 943.07, and 128.44 in the herbaceous, fatty, cocoalike, fruity and floral, and irritating odors, respectively.Similarly, Italian vine-ripe tomatoes had intense grassy and green odors with red color[16].The sum OAV of 14 primary volatiles accounted for 51.84% of the overall flavor.Among all the detected volatiles, the most OAVs were 3-methylbutanal (OAV=1 465.59, malty), (E,E)-2,4-heptadienal(OAV=1 247.60, nutty, fatty), 1-penten-3-one (OAV=953.73,fruity and floral), hexanal (OAV=822.87, green and grassy),benzaldehyde (OAV=709.00, almond, burnt sugar), and (Z)-3-hexen-1-ol (OAV=626.84, lettuce-like), which contributed the most to tomato flavor.Differently, former studies show that (2E)-3-(3-pentyl-2-oxiranyl)acrylaldehyde and (Z)-3-hexenal had the highest OAVs in fresh tomato[16,54].Based on the flavour dilution (FD) factor, (Z)-3-hexenal (FD=1 024)and (E)-2-hexenal (FD=256) were the most powerful aromaactive compounds in cherry tomato[23].Due to the different tomato materials used, 3-methyl-butanal was more abundant than (Z)-3-hexenal in this study.
Among the four colors of tomatoes, the strongest odor appeared in CI1009, CI3008, and CI5004 at S1, S2, and S3,respectively.The following odors were significantly intense, such as the overall flavor, herbaceous (3-hexenal, OAV=1 396.46),cocoa-like odors (3-methyl-butanal, OAV=6 449.54) at S1,the fatty odor ((E,E)-2,4-heptadienal, OAV=1 809.14) at S2,((E,E)-2,4-decadienal, OAV=7 866.5), ((E,E)-2,4-heptadienal,OAV=2 211.93) at S3 in CI1009; the fatty, floral and fruity odors at S1, the overall flavor, floral and fruity, cocoa-like odors at S2 in CI3008; the overall flavor, herbaceous (1-octen-3-one, OAV=15 294.80; hexanal, OAV=2 315.64 and(Z)-3-hexen-1-ol, OAV=1 127.66), floral and fruity (1-penten-3-one, OAV=2 062.58) odors at S3 in CI5004.Garden Gem and Roma tomato with red color had higher overall liking, tomato flavor, and fruity odor (3,7-dimethyl-1,6-octadien-3-ol, (3E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one, and 6-methyl-5-hepten-2-one)[55].Because the fatty acids derivatives, flavonol and anthocyanin were more in the purple fruit[1,56], and carotenoid derivatives were abundant in the red tomato at the later stage[23].
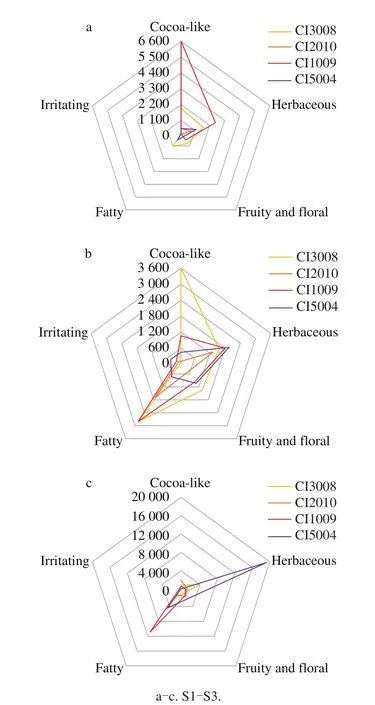
Fig.3 Flavor characteristics of tomato fruit at different ripening stages
With the fruit ripening, the odors enhanced as a whole.For a better flavor, it is best to pick tomatoes at the red stage of tomato fruit[6].If tomatoes were harvested at S1, their aroma intensity would be lost by 80%.At S3, the overall aroma was the strongest in CI5004, followed by CI1009,and the least were in CI3008 and CI2010.During the fruit ripening, the aroma increased in CI5004 and CI2010.The strongest aroma appeared at S2 in CI3008, while the aroma was lighter than other stages in CI1009 at S2.It is suggested to harvest the tomato of CI3008, instead of CI1009 at S2.
2.5 The correlation between volatile and aroma of tomato fruits
The odor of aldehydes was described as grassy, while alcohols emitted light and sweet fruit odor[57].Ketones were essential contributors to fruity and floral odor[16].Unlike the above, some aldehydes had fruity, floral, fatty, and cocoa-like odors.(E)-3-Hexen-1-ol and (Z)-3-hexen-1-ol released herbaceous odor.2-Methyl-butanol and 3-methyl-1-butanol emitted cocoa-like odor.1-Octen-3-one had mushroom-like odor.The esters had fruity, sweet, fresh or fatty odors[16].Similarly to this study, phenols were described as a smoky odor[57].However, the furans had an attractive malty aroma that could soften the smoky odor of phenols[10].
In order to represent the relationship between the volatile and aroma more accurately, pearson correlation analysis was conducted in this study.The concentrations of total volatiles,aldehydes and ketones had very significantly positive correlations with the odors of overall flavor, herbaceous,fruity and floral, respectively.There was a significantly positive correlation between alcohols concentrations and fruity and floral odor.The phenols concentrations and irritating odor had a very significantly positive correlation.The concentrations of fatty acids and carotenoid derivatives had very significantly positive correlations with the overall aroma, herbaceous, fruity and floral odors, respectively.The concentrations of carotenoid and phenylalanine derivatives had a significantly positive correlation with fatty odor.It seems that the tomato aroma was mainly herbaceous, fruity and floral odors, contributing mainly from fatty acids and carotenoid-derived aldehydes and ketones volatiles.
3 Conclusion
Both fruit color and ripeness had a significant effect on volatiles and flavors profiles of tomato.Total volatiles concentrations were the most in CI5004, followed by CI3008,CI1009, and CI2010 in turn.Similarly, the overall aroma,herbaceous, fruity, and floral odors were the strongest in CI5004.The fatty odor was the strongest in CI1009.With the fruit ripening, the volatiles and OAVs were increasing.At S1 and S2, the cocoa-like odor was the strongest in CI1009 (OAV=6 466.96) and CI3008 (OAV=3 625.71),respectively.At S3, the herbaceous odor (OAV=19 302.20)was the strongest in CI5004.The volatiles that contribute a lot to tomato aroma were 3-methyl-butanal (OAV=1 465.59,malty), 1-penten-3-one (OAV=874.26, fruity and floral),hexanal (OAV=822.87, green and grassy), benzaldehyde(OAV=708.10, almond, burnt sugar), and (Z)-3-hexen-1-ol(OAV=522.37, lettuce-like).
