Validating the deep time carbonate carbon isotope records:effect of benthic f lux on seaf loor carbonate
2021-07-02WeimingDingTingNieYongboPengYuanlinSunJinzhuangXueBingShen
Weiming Ding·Ting Nie·Yongbo Peng·Yuanlin Sun·Jinzhuang Xue·Bing Shen
Abstract It is a consensus that marine carbonate archives the isotopic composition of seawater dissolved inorganic carbon(DIC,δ13Csw),the largest active C reservoir in the hydrosphere.Carbonate carbon isotope(δ13Ccarb)excursions have been used to ref lect perturbations of the global carbon cycle and related environmental change.However,the deep timeδ13Ccarb records indicate faster and more pronounced perturbations of the carbon cycle compared to the present day.Here,we reportδ13Ccarb and elemental compositions of Late Paleozoic carbonate sections from South China,showing negative correlations betweenδ13-Ccarb and Fe2+content of carbonate(Fecarb).We suggest that,because Late Paleozoic carbonate was mainly produced by benthic carbonate-secreting organisms,δ13Ccarb recorded the isotopic composition near the seaf loor,where benthic flux derived from anaerobic organic matter degradation delivers both Fe2+and 13C-depleted DIC from porewater.The binary mixing between seawater and benthic flux would result in the deviation ofδ13Ccarb from δ13Csw.The negative correlation implies thatδ13Ccarb is inf luenced by benthic f lux and is affected by the seaf loor redox and sedimentation rate.The deep time spatially heterogeneous and temporally oscillatoryδ13Ccarb records in the basin-scale could be alternatively attributed to the variations of local environmental factors rather than a δ13Csw depth-gradient.Thus,the seaf loor carbonate precipitation is continuously affected by diagenetic reactions in sediments,suggesting thatδ13Ccarb recording the seawater DIC composition is conditional.Our study urges that the interpretation ofδ13Ccarb should also consider the sedimentary process and depositional environment of marine carbonate.
Keywords Fe2+content of carbonate(Fecarb)·Benthic f lux·Sediment·Paleozoic·South China
1 Introduction
The global carbon(C)cycle bridges inorganic reactions and biological processes in the Earth system(Houghton 2014;Sundquist and Ackerman 2014).The surface Earth long-term carbon cycle can be expressed as volcanic COdegassing(the ultimate C source)counterbalanced by carbonate precipitation and organic carbon burial(the ultimate C sinks)(Houghton 2014;Kump and Arthur 1999).On the other hand,the short-term carbon cycle is primarily fueled by organic matter production and destruction.In the modern ocean,organic matter is mainly produced by oxygenic photosynthesis in the surface ocean,which consumes atmospheric COand generates oxygen(O).Organic matter degradation mainly occurs in the ocean interior and converts organic matter(CHO)to COor HCOby consuming oxidants(mostly O).Thus,organic matter production and degradation directly link to the atmosphericp
Oandp
COlevels,and directly or indirectly modulate the marine redox landscape and global climate(Fike et al.2006;Hoffman et al.1998).The perturbation of the carbon cycle due to change in C source or sink is normally associated with environmental change,some of which might have a severe impact on the biosphere,e.g.,mass extinctions(Berner 2002).Thus,unraveling the deep time carbon cycle would provide the f irst-order constraint on the evolution of habitable planet Earth.Reconstruction of the deep time carbon cycle has been approached with carbon isotopes,particularly carbonate carbon isotopes(δC).It is widely accepted that δCof marine carbonate records the isotopic composition of dissolved inorganic carbon in seawater(DIC,δC),the largest active C reservoir in the Earth system(Sundquist and Ackerman 2014).In the context of the steady-state carbon cycle model(Kump and Arthur 1999),δCis mainly controlled by organic carbon burial,i.e.an increase of organic carbon burial would lead to a positive excursion inδC,and vice versa.Thus,a positive or negativeδCexcursion may ref lect the change of marine DIC composition,indicating a signif icant perturbation of the global carbon cycle.Furthermore,because the residence time of the marine DIC pool(-10years)is two orders of magnitude longer than the ocean mixing time(-10years),δCcould be regarded as homogeneous in the global ocean(Kump and Arthur 1999).Therefore,it has long been held that the chemostratigraphic variation of δCref lects the f luctuation ofδCand accordingly can be correlated globally(Higgins et al.2009;Sundquist and Ackerman 2014;Swanson-Hysell et al.2010).
However,the deep time carbon cycle derived from δCchemostratigraphic data differs from the modern marine carbon cycle in the following aspects.First,δCmay display high frequency(centimeter to meter scale)stratigraphic variations(Husson et al.2020;Lang et al.2016;Xiao et al.2020;Zhou et al.2016),ref lecting dramaticδCoscillation in 10to 10years of duration.More importantly,most of this high frequencyδCstratigraphic variation cannot be correlated in either basinal or regional scales(Lang et al.2016;Wang et al.2016),and thus may not be attributed to the f luctuations of δC.Second,large onshore-offshoreδCgradients(>3%)are commonly observed in ancient sedimentary basins(Jiang et al.2007;Lang et al.2016;Shen et al.2011),which is in sharp contrast to the subtle(<2%)depthδCgradient in the modern open ocean(Kroopnick 1985;Tagliabue and Bopp 2008).Large depthδCgradients only occur in modern anoxic basins(Volkov 2000).By using the modern anoxic basin as an analog,the widespread largeδCgradient in a deep time sedimentary basin may imply pervasive oceanic anoxia throughout the Earth’s history.Although this model may explain some Proterozoic and Early Paleozoic data(Chang et al.2017;Jiang et al.2007;Wang et al.2016)when the atmospheric Olevel was not high enough to oxidize the whole ocean(Sperling et al.2015),it cannot explain large δCgradients after Late Paleozoic(Qie et al.2015)when the ocean was fully oxygenated(Krause et al.2018)except for several marine anoxia events(Song et al.2013).These observations indicate thatδCcould signif icantly deviate fromδC.
Given the assumption of marine carbonate recording the seawater geochemical composition,deviation fromδChas been attributed to diagenetic alteration of carbonate,including water–rock or water–sediment interactions at various stages of diagenesis or authigenic carbonate precipitation in sediments(Ahm et al.2018;Derry 2010;Jacobsen and Kaufman 1999;Schrag et al.2013).Therefore,diagenetic evaluation of carbonate rocks is a standard protocol before data interpretation(Kaufman and Knoll 1995).However,current petrographic and geochemical methodologies can only apply to meteoric diagenesis and burial diagenesis(Melim et al.2001).There is a knowledge gap in recognition of diagenetic reactions occurring in the shallow depth of sediments,where carbonate diagenesis is most active(Ahm et al.2018;Higgins et al.2018;Malone et al.2001).
The assumption of carbonate recordingδCmight be violated at least for carbonate deposited during a certain period.It is well known that direct seawater carbonate precipitation was not common after the Paleoarchean era(Grotzinger and James 2000);instead most marine carbonate was produced at seaf loor via biologically-induced or biologically-controlled processes(Tucker and Wright 1990),especially before the evolution of planktonic calcif iers in Mesozoic(Martin 1995;Ridgwell 2005).Once deposited at the seaf loor,carbonate precipitation is inevitably affected by seaf loor benthic f lux that deliversCdepleted DIC,Fe,Mnfrom sediment porewater(Dale et al.2015;McManus et al.2012;Schroller-Lomnitz et al.2019).This is ref lected by the lowδCof the modern Bahama Banks carbonate platform,which is attributed to the diffusive DIC f lux from the sediment porewater(Patterson and Walter 1994).Thus,it is reasonable to speculate that seaf loor carbonate precipitation might record both the seawater and sediment porewater signals(Bergmann et al.2013;Ding et al.2019).Therefore,it is a timely contribution to evaluate howδCof deep time carbonate is affected by seaf loor benthic f lux.
In this study,we report high-resolution biostratigraphic and chemostratigraphic data from four carbonate sections transecting the Devonian-Carboniferous(D-C)boundary in South China.A high-resolution conodont biostratigraphic framework allows precise chemostratigraphic correlation among sections.Our data indicate that theδCprof iles cannot be correlated between different sections,andδCis signif icantly affected by the benthic f lux that is dependent on the seaf loor Ofugacity.
2 Geological background
The Late Paleozoic paleogeography of the Yangtze Block is characterized by the development of multiple carbonate ramps or isolated carbonate platforms,surrounded by intraplatform basins(Chen et al.2001a,2001b)(Fig.1).The sampling intervals include the lower Tangbagou Formation in the Qilinzhai(QLZ)section that was deposited in the nearshore platform environment,and the equivalent Wuzhishan Formation in the Daposhang(DPS),Xiada(XD),and Duli(DL)sections that were located in the intraplatform basin environments(Fig.1).The lower part of the Tangbagou Formation is mainly composed of thinbedded lime mudstone or wackstone with rare occurrences of bioclastic packstone/grainstone(Fig.2),whereas the Wuzhishan Formation is dominated by thin-bedded dark lime mudstone and wackstone with 1 to 2 black shale layers near the top(Fig.2).Conodont biostratigraphy indicates that the sampling interval of the three intraplatform basin sections is bracketed between the middleSiphonodella
praesulcata
to the upperSiphonodella duplicata
conodont zones,transecting the Devonian-Carboniferous(D-C)boundary(-359.5 Ma)that is def ined at the base ofSiphonodella sulcata
zone(Fig.3)(Davydov et al.2012).No conodont of biostratigraphic signif icance is recovered from the near-shore QLZ section.Instead,the foraminifera biostratigraphy indicates that the QLZ section also transects the D-C boundary(Hance et al.2011;Wu and Liao 2001).
Fig.1 Late Paleozoic paleogeography of Guizhou and Guangxi Provinces on the Yangtze Platform,South China.Sample localities are marked by red asterisks.The Qilinzhai section was deposited in the nearshore platform,whereas the Daposhang,Xiada,and Duli sections were located in the intraplatform basin

Fig.2 Field photographs and photomicrographs.a-c Field photograph showing the Wuzhishan Formation in the Duli,Xiada,and Daposhang sections.d Field photograph showing the Tangbagou Formation in the Qilinzhai section.e-g Photomicrograph showing the micrite limestone of the Wuzhishan Formation,with rare occurrences of bioclasts.h Photomicrograph showing the micrite limestone of the Tangbagou Formation in the Qilinzhai section
3 Methods
3.1 Sample preparation
Mirrored thin and thick sections were prepared for micromill sampling.Sample powders were drilled from the polished thick section,and the sampling procedure was guided by thin section observation under an optical microscope.Only micrite was sampled,while hydrothermal veins,if any,biogenic clasts and recrystallized regions were avoided.Powder samples were collected for elemental composition analyses and carbonate carbon and oxygen isotope measurements.
3.2 Elemental composition analyses
The sample dissolution procedure was modif ied from the sequential extraction procedure for carbonate-associated Fe(Fe)of Poulton and Canf ield(2005),which could avoid dissolution of iron oxides/oxyhydroxides or silicate minerals(such clay).A buffer solution consisting of acetic acid(HAc)and ammonium acetate(NHAc)was prepared by adjusting the ratio of HAc and NHAc to achieve a pH of 4.5.For each sample,about 30 mg of powdered sample was dissolved in a 10 ml buffer solution.To guarantee complete dissolution of carbonate,centrifuge tubes were placed on a shaking table at 50°C for 48 h.After centrifugation,0.5 ml supernatant was dried down in a hotplate,and the residue was dissolved in 5 ml 2%nitric acid(HNO)ready for elemental composition analyses.
Major and minor elemental compositions were measured by a Spectra Blue Sop Inductively Coupled Plasma Optical Emission Spectrometer(ICP-OES)at Peking University.Analyses were calibrated by a series of gravimetric standards with concentrations between 0.1 to 10 ppm.Elemental concentrations were reported concerning the carbonate content,i.e.to sum up CaCOand MgCOcontents to 100%,while the Mg/Ca ratios are reported in a molar ratio(Wang et al.2019).The analytical precisions for all reported elements are better than 5%by the weight percent.
3.3 Carbonate carbon(δ13Ccarb)and oxygen(δ18Ocarb)isotope analyses
Carbonate carbon and oxygen isotopes were measured by a MAT253 isotope ratio mass spectrometry(IRMS)at Louisiana State University.The detailed analytical procedure has been extensively reported in previous studies(Li et al.2016),and thus will not be reiterated here.Isotope ratios are reported inδnotation as per mil(%)deviation relative to VPDB standard.The analytical precisions for δCandδOare 0.1%and 0.3%(1 standard deviation),respectively.
4 Results
The elemental and isotope data are listed in Tables S1-S4.There is a wide range of variation inδCamong different sections(Figs.4 and 5).δOvalues are indistinguishable among different sections(Fig.5).All the studied samples have low Mg/Ca ratios(<0.05)but show wide ranges of variation in both Feand Mn(Fe and Mn contents in carbonate,respectively)(Fig.6&see supplementary materials).
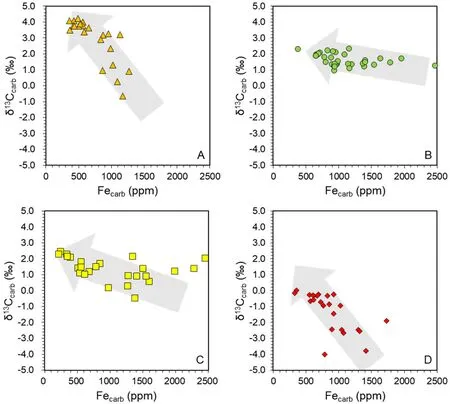
Fig.6 Cross-plots showing the negative relationship betweenδ13Ccarb(y-axis)and Fecarb(x-axis)for different sections.a Qilinzhai Section.b Daposhang Section.c Xiada Section.d Duli Section.The scales of the x-axis and y-axis are the same for all of the f igures.The data points in each cross-plot represent all measured samples in each section.The grey arrows represent the general negative trend ofδ13Ccarb and Fecarb.Daposhang Section shows a weak correlation due to the limited variation ofδ13Ccarb
5 Discussion
5.1 Diagenesis consideration ofδ13Ccarb
It is widely accepted that geochemical compositions of carbonate could be altered in diagenesis,resulting in the deviation ofδCvalues from the pristineδC of ambient seawater.It is a standard protocol to evaluate the potential diagenetic alteration of carbonate samples before data interpretation(Swart 2015).By definition,diagenesis brackets all reactions occurring within sediment porewater after deposition.Diagenesis can be classified into three categories,(1)meteoric diagenesis referring to meteoric fluids interacting with sediments during subaerial exposure(Melim et al.2001),(2)early marine diagenesis involving interactions of seawater and sediments at a shallow depth of sediments(Fantle and Higgins 2014;Higgins et al.2018;Hoffman and Lamothe 2019),and(3)burial diagenesis characterized by porewater-rock interactions in deep burial(Derry 2010;Jacobsen and Kaufman 1999;Marshall 1992).
Petrographic observations could provide the first order constraint on the burial diagenesis. Commonly,recrystallizationofcarbonatemineralsindeepburialwould obliteratetheprimaryrockfabric.Assuch,preservationof originalrockfabricandabsenceofrecrystallizationmay indicateinsignificantburialdiagenesis.Itishighlyplausiblethatmicrite(limemud)maynotundergosignificant burialdiagenesis.Inpractice,micro-millsamplingunder theguidanceofpetrographicobservationcouldavoidcollectingrecrystallizedrockfabric.Thestudiedcarbonate samplesaremainlycomposedoflimemudstoneorwackstone(Fig.2a–d),andpetrographicobservationsindicate negligibleecrystallization(Fig.2e–h),rulingoutdiageneticalterationindeepburial.
Potentialmeteoricdiagenesisisnormallyevaluatedby geochemicalproxies.ItissuggestedthatδOismore susceptibletowater–rockinteractionsduetofastand thoroughexchangewithsedimentporewater,whileδCtendstobebufferedbyhighCcontentofcarbonate(Marshall1992).However,ifthediageneticprocess involvesorganicmatterdegradationthatgeneratesCdepletedDIC,δCwouldbecomelowerandshowa positivecorrelationwithδO(KaufmanandKnoll 1995).ExceptfortheDPSsection,nocorrelationbetween δCandδOisobserved(Fig.5).BothδCandδOoftheDPSsamplesshownarrowrangesof variation (δC:+0.96% to+2.33%; δO:--7.37%to-6.24%,respectively),reducingthesignificanceofcorrelation.
Traditionally,itisproposedthatextremelylowδOvalues(<-10%)mayindicateδCbeingsignificantlyaltered,i.e.neithertheabsolutevaluenorthe stratigraphictrendisreliable,whereassampleswithmoderateδOvalues(-10%to-5%)areregardedas leastaltered,i.e.althoughtheabsoluteδCvalues mightbealtered,thestratigraphictrendmaystillrecordthe original seawater trend, and could be used for chemostratigraphiccorrelation(Derry2010;Kaufmanand Knoll1995).AllthestudiedsampleshaveδOranging from-10%to-5%(TablesS1–S4),suggestingthe preservation of the stratigraphic trend but unreliable absolute values.However,we will argue that although δOis slightly low,the systematic difference inδCobserved in the studied sections may not result from meteoric diagenesis(Fig.5).Despite intersectional variations inδC(+2.78%,+1.64%,+1.28%,-1.04%;all p-values are below 0.01.T-test results in Table 1),the four sections have similar meanδOvalues with a high extent of overlapping (-7.02%,-6.88%,-6.44%,-7.39%for the QLZ,DPS,XD,and DL,respectively;p
values varies from<0.01 to 0.254.T-test results in Table 1)(Fig.5).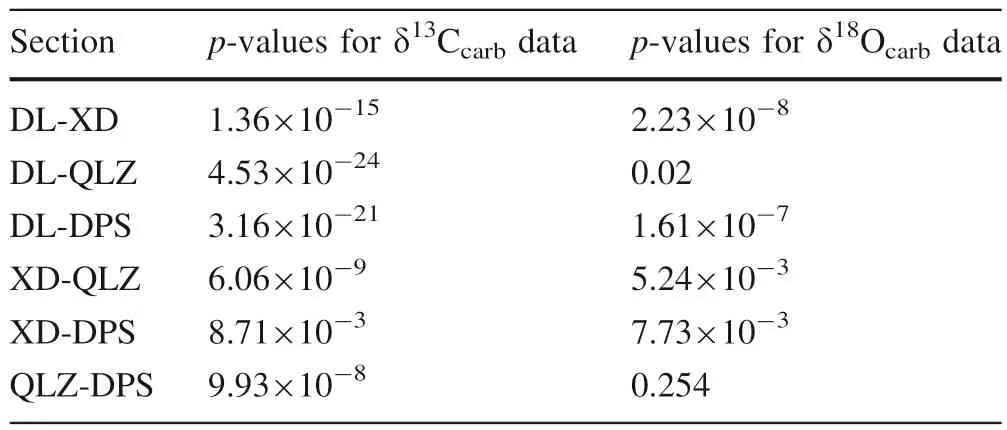
Table 1 P values of T-tests forδ13Ccarb,δ18Ocarb of studied sections
As discussed above,possible burial and meteoric diagenesis could be ruled out.Yet,we cannot argue thatδCrecords the seawater composition due to the possible inf luence of early marine diagenesis,which is diff icult to be evaluated by either petrographic observation or geochemical proxies.δCcould be inf luenced by f luidbuffered conditions rather than the long-held sedimentbuffered conditions(Higgins et al.2018),although neither recrystallization nor neomorphism is included or identif ied in the petrographic study.Furthermore,authigenic carbonate precipitation would modifyδC,but authigenic micrite cannot be differentiated from original marine micrite in the geological records(Schrag et al.2013).
Therefore,the traditional diagenetic evaluations only focus on meteoric and burial diagenesis.There is a knowledge gap about marine diagenesis that involves the interactions of seawater,porewater,and calcareous sediment.In other words,sedimentation and early marine diagenesis cannot be completely differentiated by either petrographic or geochemical analyses.
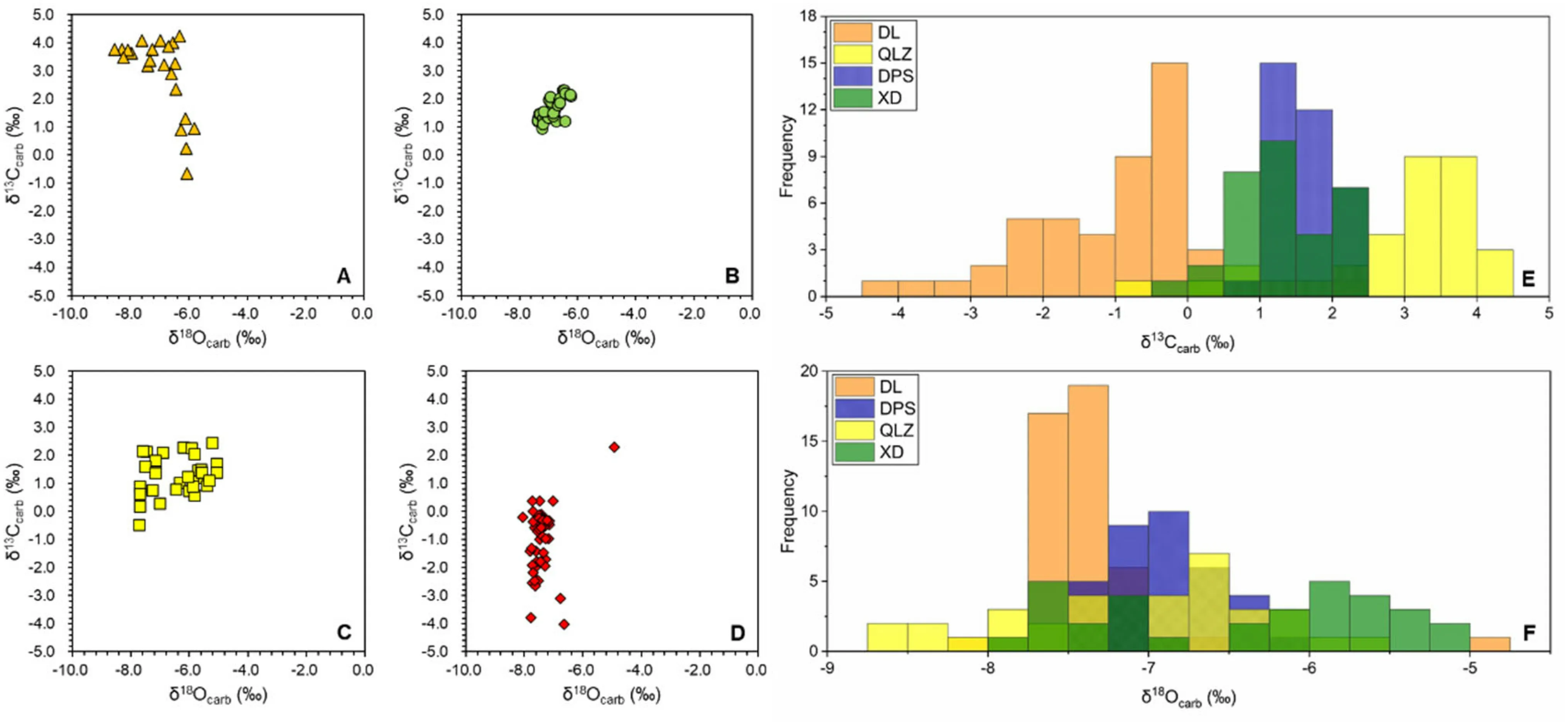
Fig.5δ13Ccarb versusδ18Ocarb cross-plots and histograms of studied sections.aδ13Ccarb vs.δ18Ocarb cross-plot of Qilinzhai section,bδ13Ccarb vs.δ18Ocarb cross-plot of Daposhang section,cδ13Ccarb vs.δ18Ocarb cross-plot of Xiada section,and dδ13Ccarb vs.δ18Ocarb cross-plot of Duli section.eδ13Ccarb Histogram of four sections.fδ18Ocarb Histogram of four sections.Absence or poor correlation betweenδ13Ccarb andδ18Ocarb is shown except for the DPS section,which has limited variations in bothδ13Ccarb andδ18Ocarb.δ13Ccarb of studied sections displays systematic difference whileδ18Ocarb overlaps with each other
5.2 Interpretation of the stratigraphic variation ofδ13Ccarb
In this study,δCshows a wide range of stratigraphic variation in the same section,and the stratigraphicδCprof iles cannot be correlated among different sections within the high-resolution biostratigraphic framework(Fig.4).Although the potential diagenetic alteration of δCcannot be completely ruled out,neither could the stratigraphicδCvariation be attributed to the burial and meteoric diagenesis(seeSect.5.1
).One possible interpretation of the stratigraphicδCvariation is the temporal oscillation of seawater DIC composition.This interpretation can be conf idently ruled out by the long residence time of seawater DIC(Zeebe 2012)and short duration of the studied sections(praesulcata
zone:0.74 million years,kockeli
zone:0.16 million years,sulcata
zone:0.5 million years,duplicata
zone:0.9 million years)(Davydov et al.2012).Thus,the only interpretation is that δCis not inf luenced by the seawater DIC composition alone.Carbonate-secreting organisms account for more than 90%of carbonate production in Phanerozoic(Tucker and Wright 1990),and most carbonate-secreting organisms possessed the epi-benthic or in-fauna life-styles before the Mesozoic evolution of planktonic calcifers,such as planktonic foraminifera,bivalves,crustaceous,and coccolithophora(phytoplanktons)(Ridgwell and Zeebe 2005;Vachard et al.2010).The typical Paleozoic fauna,e.g.brachiopod,corral,echinoderm,and benthic foraminifera(e.g.fusulinids)(Sepkoski and Miller 1985),would be responsible for the majority of carbonate production in the Late Paleozoic ocean,suggesting predominant carbonate precipitation at the seaf loor.
Unlike carbonate formed in the water column,carbonate precipitating at the seaf loor is also affected by elements diffusion from sediment porewater,i.e.the benthic f lux(Fig.7).Benthic f lux is common in the modern continental margins and enriches in reduced components,such as Fe,Mn,NH,as well asC-depleted DIC(Cai et al.2015;Dale et al.2014;McManus et al.2012;Schroller-Lomnitz et al.2019;Severmann et al.2010).Benthic f lux is driven by the porewater-seawater concentration gradients due to various anaerobic organic matter degradation in sediment porewater,e.g.iron reduction,sulfate reduction.With the consideration of the potential inf luence of benthic f lux,the geochemical composition of carbonate might deviate from that of seawater signals.Below,we will constrain the inf luence of benthic f lux onδCby using ferrous Fe content in carbonate(Fe)as a proxy.

Fig.7 Schematic model of carbonate formation in the seaf loor.a Carbonate precipitation on the seaf loor with deeper redox boundary of Fe2+within the sediments.The more oxic condition in the SWI might be linked to low productivity in the surface ocean and reduces the intensity of Fe2+and DIC f lux derived from anaerobic respiration.Therefore,carbonate is characterized by highδ13Ccarb but low Fecarb.b Carbonate precipitation on the seaf loor with shallower redox boundary of Fe2+within the sediments.Due to the enhanced primary productivity or stagnation of ocean cycling,O2 fugacity on the seaf loor would decrease.Thus,the redox boundary of Fe2+would be closer to the SWI.Higher Fe2+and DIC f lux derived from anaerobic respiration within the sediments could be recorded in carbonate,showing lowδ13Ccarb but low Fecarb
Total dissolved Fe remains at an extremely low level in the modern open ocean(Tagliabue et al.2017).In contrast,Fe oxides/oxyhydroxides in sediment could be reduced by dissimilatory iron reduction(DIR).DIR can be expressed by the following chemical reaction:

DIR elevates porewater Feand HCOconcentrations(Morford et al.2009),generating Feand DIC concentration gradients between seawater and porewater.Besides,porewater DIC could also be produced by other anaerobic respirations within sediments,such as manganese reduction and sulfate reduction(Meister et al.2007).DIC or Fein benthic f lux could be incorporated into carbonate minerals when precipitation at the seaf loor.Thus,the upward diffusion of porewater FeandC-depleted DIC(benthic f lux components)might add to the carbonate deposition at the seaf loor,elevating the ferrous Fe content in carbonate(Fe)and loweringδCvalues.Due to negligible dissolved Fe in seawater(<1.5 nM)(Tagliabue et al.2017)and large benthic Fef lux ranging from 0.02 to 568μmol md(Dale et al.2015),Fecould be used as a tracer for benthic f lux.Thus,carbonate precipitating at the seaf loor records the signal of both seawater and benthic f lux.The seawater component is characterized by negligible dissolved FeandC-enriched DIC,while the benthic f lux component has high Fecontent andCdepleted DIC.The binary mixing between the seawater and benthic f lux component would cause a negative correlation betweenδCand Fe(Fig.7).
Indeed,we observe negative correlations betweenδCand Fein XD,DL,and QLZ sections,while the poor correlation in DPS samples might be attributed to limited variation inδC(Fig.6).We develop a binary mixing model to quantify the relationship between FeandδCfor seaf loor carbonate precipitation.On the one hand,because of a large Feconcentration gradient between sediment porewater and seawater,Feis mainly determined by the benthic Fef lux,which can be expressed by the following equation:
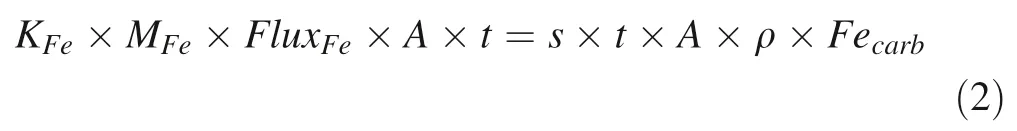
A
is the area,t
is the sedimentation time,s
is the sedimentation rate,ρis the density of carbonates(2.71 g cm).Benthic Fe f lux is sensitive to Ofugacity at the seaf loor(Ding et al.2019),while Feis not only affected by benthic Fe f lux but also sedimentation rate when the supply of Fe(III)is suff icient for DIR reactions(Wang et al.2019).A high sedimentation rate would dilute Fein carbonate lattice,lowering Fevalues(Eq.2).Thus,high Femay indicate a large benthic f lux and/or low sedimentation rate.On the other hand,seaf loor carbonate would have both seawater and porewater C sources,the latter of which derives from benthic DIC f lux.We assume that seawater within heighth
above the seaf loor could contribute to the carbonate formation.Therefore,the carbon mass balance of seaf loor carbonate can be expressed by the following equation:
M
is the molecular weight of CaCO(100 g mol).Therefore,δCcould be calculated by a binary mixing between seawater DIC(δC)and benthic DIC f lux(δC):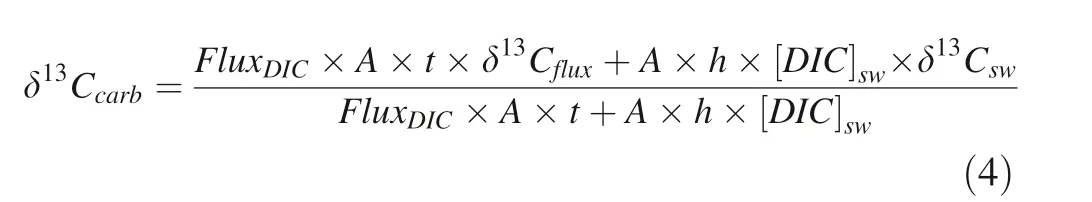
δCis similar to theδC in sediment porewater,which is determined by the mixing ratio of seawater DIC and DIC derived from organic matter degradation.Modern sediment porewater is characterized by a wide range of δC values(-2%to-25%).In our simulation,δC= -5%is set as the default value(Meister et al.2007).Rearranging Eqs.(2)–(4),we can arrive at:

We can further simplify Eq.(5)by def ining the ratio of Fluxand Fluxasα,which can be expressed as follows:

Considering the magnitude of the Flux(mmol md)and Flux(μmol md)in the modern ocean,αshows a wide range of variation(10–10)due to the following reasons.First,Fluxis not produced solely by DIR.Other anaerobic microbial processes,such as microbial manganese reduction and microbial sulfate reduction,would also generateC-depleted DIC.The different abundance of electron acceptors,which may vary locally,would result in differentαamong sections.Second,porewater Fetends to be oxidized to rather insoluble FeOOH or converted to pyrite by reacting with HS.As such,the loss of Feduring the diffusion process would be redox-controlled locally.
We can establish the negative relationship between δCand Feas follows:

praesulcata
zone+kockeli
zone,sulcata
zone,andduplicata
zone,respectively).This assumption is reasonable,given the relatively short time duration of these conodont zones(Gradstein et al.2012).In a simulation,we assign a series ofδCvalues of+4%for theduplicata
zone,+5%for thesulcata
zone,and+3%for thepraesulcata
zone andkockeli
zone,respectively.Differentαvalues are set to f it the trend of the data of different sections and could be compared within the individual biostratigraphic zone.The modeling results indicate thatαranges from 150 to 1200 for theduplicata
zone,150 to 1600 for thesulcata
zone,150 to 1800 for thepraesulcata
zone andkockeli
zone,respectively(Fig.8).
Fig.8 Binary mixing models simulating the negative relationship betweenδ13Ccarb and Fecarb by considering the effect of benthic f lux when the sameδ13Csw value is applied for different sections within the same conodont zone.a Samples from the duplicata zone.b Samples from the sulcata zone.c Samples from the praesulcata zone and kockeli zone.The contour lines indicate the different values ofα,which represents the different ratios of DIC f lux and Fe2+f lux.KFe=2.32 andδ13Cf lux=-5%
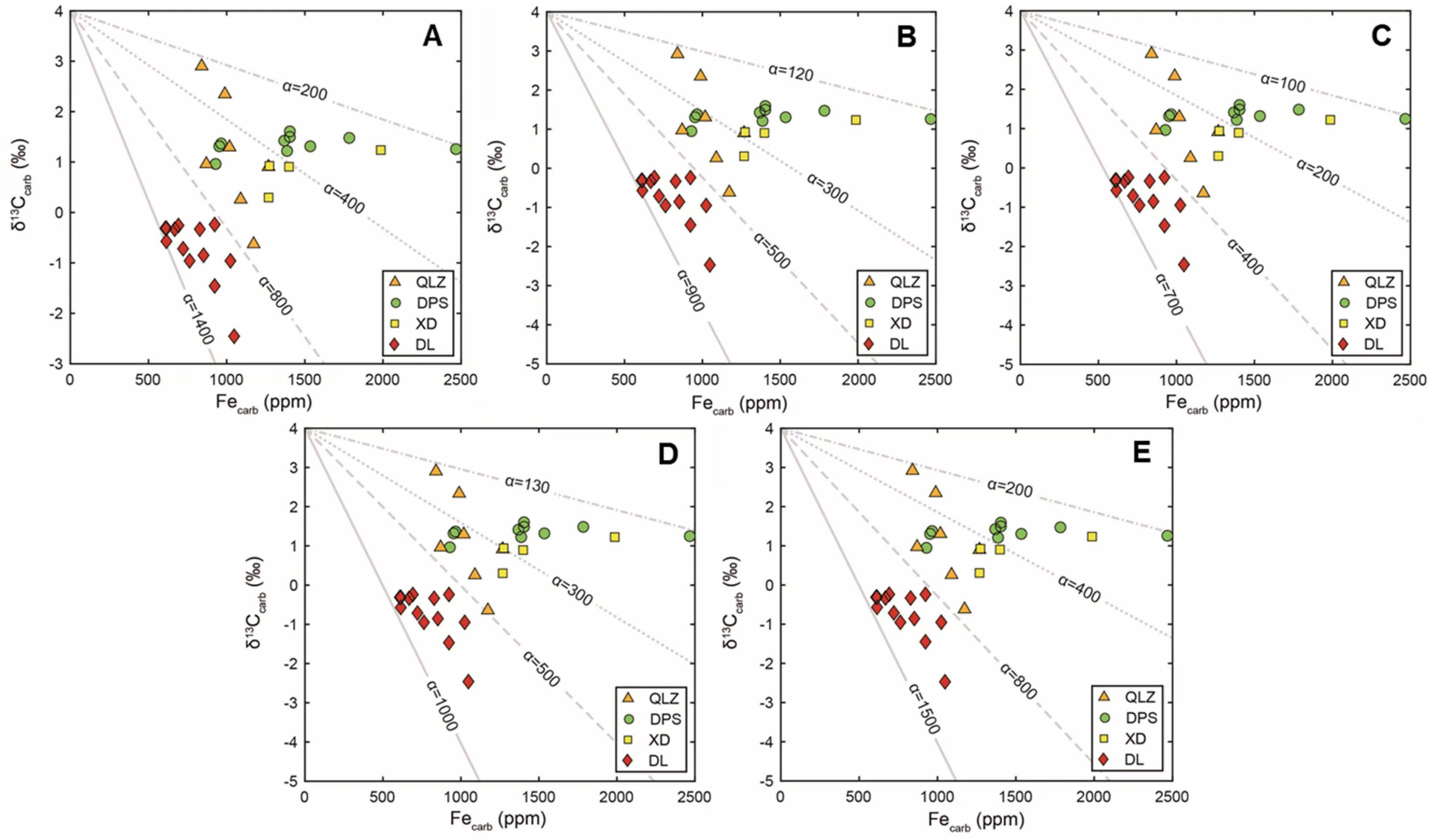
Fig.9 Sensitivity tests of parameters KFe andδ13Cf lux of the samples within the duplicata zone.a KFe=2.32 andδ13Cf lux=-3%.b KFe=2.32 andδ13Cf lux=-7%.c KFe=2.32 andδ13Cf lux=-10%.d KFe=2 andδ13Cf lux=-5%.e KFe=3 andδ13Cf lux=-5%

Fig.10 Sensitivity tests of parameters KFe andδ13Cf lux of the samples within the sulcata zone.a KFe=2.32 andδ13Cf lux=-3%.b KFe=2.32 andδ13Cf lux=-7%.c KFe=2.32 andδ13Cf lux=-10%.d KFe=2 andδ13Cf lux=-5%.e KFe=3 andδ13Cf lux=-5%.The blue dashed line indicates theδ13Cf lux value of the end member—FluxDIC
The values ofα(10–10)are reasonable considering the magnitudes of DIC f luxes and Fef luxes in the modern ocean.We set K=2.32 andδC=-5%as the default values according to the previous studies.The sensitivity tests about KandδCare shown in Figs.9,10 and 11,where Kvaries from 2 to 3 andδCvaries from-10%to-3%.The exact values ofαstill within the acceptable range.IfδCvalues are lower,lowerα values are required.Overall,the values of KandδCdo not disturb the negative relationship betweenδCand Fe.
The existence of shale interlayers among carbonate strata as well as siliciclastic components in carbonate thinsections(Figs.2,3 and 4)suggest abundant Fe(III)input for organic matter degradation within sediments.Thus,variations of Fein different sections could partly result from the f luctuations of seaf loor Ofugacity(Ding et al.2019).The seaf loor redox condition is directly controlled by organic matter input.Higher surface ocean productivity would consume more Oby aerobic oxidation in the water column,lowering Ofugacity in the seaf loor.Low seaf loor Ofugacity would lead to the shallower depth of redox boundary(Ding et al.2019;Wang et al.2019),resulting in a higher benthic Fef lux.As a result,carbonate with more negativeδCand high Fe content may indicate precipitation under a more reduced condition with the possibly higher inf lux of organic matter from the surface ocean,and vice versa.
In addition,δCmay also be affected by sedimentation rate as illustrated in the following equation by rearranging Eqs.(3)and(4):

sulcata
zone(Fig.8b)may be partly attributed to rapid sedimentation of carbonate on the platform environment(Figs.1 and 3),reducing the inf luence of the benthic f lux on the geochemical signals.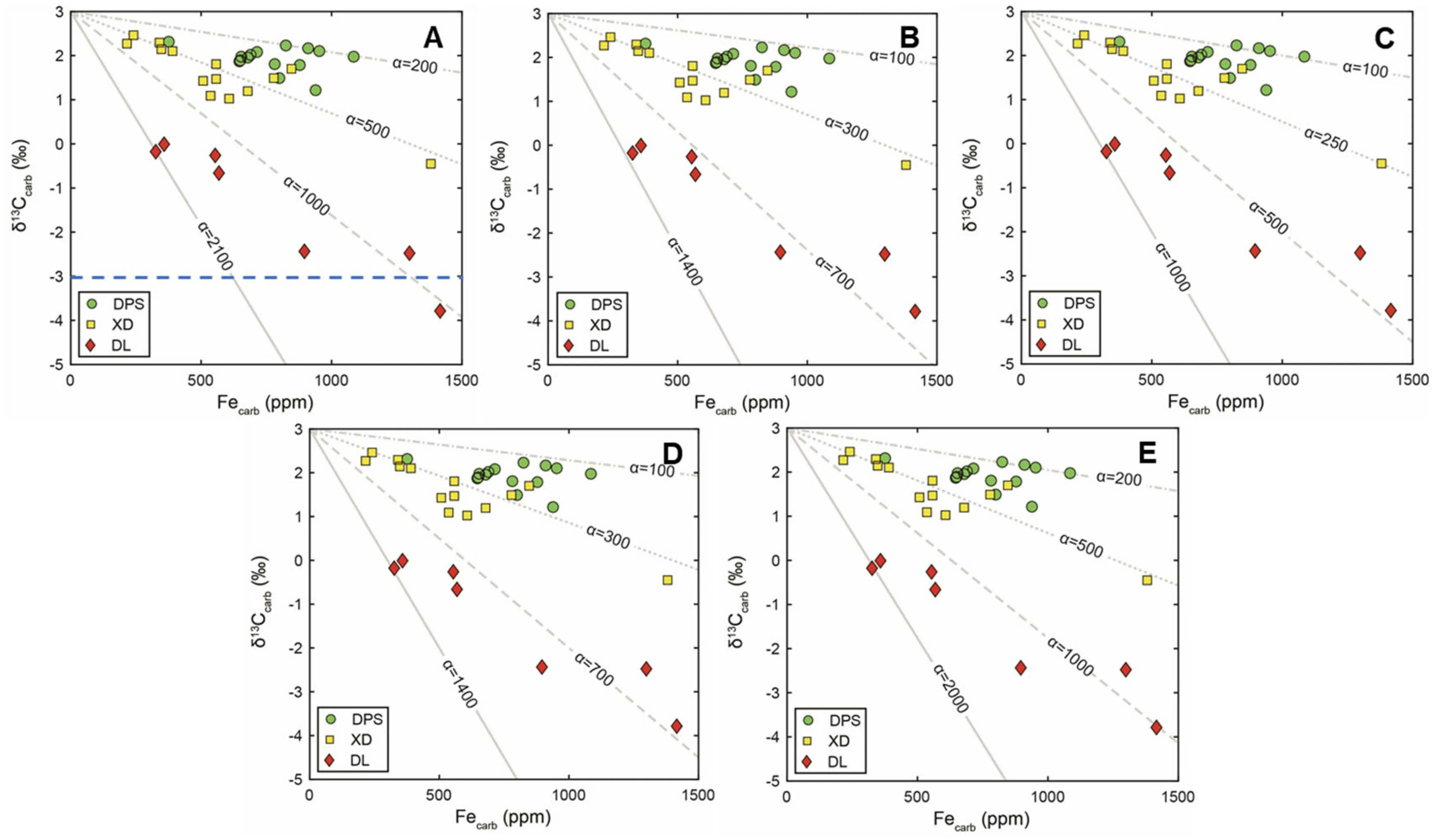
Fig.11 Sensitivity tests of parameters KFe andδ13Cf lux of the samples within praesulcata zone and kockeli zone.a KFe=2.32 andδ13Cf lux=-3%.b KFe=2.32 andδ13Cf lux=-7%.c KFe=2.32 andδ13Cf lux=-10%.d KFe=2 andδ13Cf lux=-5%.e KFe=3 andδ13Cf lux=-5%.The blue dashed line indicates theδ13Cf lux value of the end member—FluxDIC
5.3 Reappraisal the deep timeδ13Ccarb records
The negative correlation betweenδCand Feis in accordance with previous studies of Archean seaf loor carbonate precipitation(Bergmann et al.2013;Higgins et al.2009),highlighting the importance of anaerobic organic matter degradation on seaf loor carbonate precipitation.To take a step forward,we propose that theδCand Ferelationship might apply to all carbonate precipitated at the seaf loor.Benthic DIC f lux is controlled by anaerobic organic matter degradation in sediment porewater and is also sensitive to the Ofugacity in the seaf loor.Suff icient supplies of organic matter and oxidants such as Fe,Mn oxides,and oxyhydroxides are the prerequisite of generating benthic f lux.For seaf loor carbonate precipitation at high benthic f lux,its geochemical composition would likely deviate from the seawater composition.Thus,δCcannot ref lect seawaterδC.
However,we do not argue against the chemostratigraphicδCcorrelation on either a regional or global scale(Xiao et al.2016;Zhu et al.2007).Large surface-todeep oceanδCgradient may still indicate systematic variations in the carbonate depositional environment(Jiang et al.2007).However,we stress that such isotopic gradient may not necessarily ref lect heterogeneous marine DIC composition.Instead,we propose thatδCvariation could also be explained by the f luctuation of benthic f lux or change of sedimentation rate.Identif ication and a better understanding of the inf luence of benthic f lux on the carbonate depositional process are of great importance because anaerobic organic matter respiration is ubiquitous within the sediments.
Nevertheless,we should consider the effect of the benthic f lux on other elements or isotopic signals individually.Benthic f lux is driven by a concentration gradient between porewater and seawater,requiring porewater enrichment of certain elements/isotope.Only carbonate deposition inf luenced by benthic f lux could its composition deviate from the seawater composition.On the contrary,some elements could diffuse from the seawater into the sediment due to reaction consumptions such as Mg during the dolomitization process(Higgins and Schrag 2010;Huang et al.2015),which is reversed to the diffusion direction of the benthic f lux.Thus,these types of elements in carbonate should record the information of seawater source.Although the process of carbonate precipitation is similar,the inf luential factors of different elements can vary according to the specif ic geochemical cycle.
Therefore,we argue that the assumption that carbonate records the seawater chemical composition could be violated if carbonate is precipitated at the seaf loor and benthic f lux is not negligible.With the consideration of benthic f lux,seaf loor carbonate precipitation is continuously affected by diagenetic reactions in sediments.In this sense,sedimentation and early marine diagenesis are continuous processes, and their effects cannot be differentiated.
6 Conclusions
The negative correlation betweenδCand Feof Late Paleozoic carbonate implies thatδCcould be affected by benthic f lux when carbonate precipitates at the seaf loor.With the consideration of benthic f lux,in addition toδC,δCis also affected by the surface ocean primary productivity,sedimentation rate,and ocean circulation that controls the Oinf lux in the deep water.Thus,the assumption that marine carbonate archives the seawater DIC composition could be violated for carbonate precipitation at the seaf loor.Nevertheless,δCmight be close toδC,when carbonate is precipitated in mixed shallow water,where the benthic f lux is negligible.We also suggest thatδCcould be highly heterogeneous in the basin scale,but such isotopic difference may not necessarily imply the sustained geochemical gradients of the seawater.High-frequency f luctuations ofδCcould not result from the rapid reorganization of the global carbon cycle.Instead,such variations ofδCmay ref lect the local oscillations of sedimentation rate,seaf loor redox condition,or surface ocean primary productivity.Accordingly,the application ofδCchemostratigraphic correlation needs to differentiate whether the observed δCexcursions were driven by the variation of seawater DIC composition(i.e.the global signal)or the change of local environmental factors.
Acknowledgement
This study was supported by National Science Foundation of China(No.41772015 and No.41772359).Funding
This study was supported by the National Science Foundation of China(No.41772015 to Sun and No.41772359 to Shen).Data availability
All data and materials can be found in the main text and accompanying supplementary information.Declarations
Conf licts of interest
The authors declare no competing interests.杂志排行
Acta Geochimica的其它文章
- Petrology and geochemical framework of dolerites dykes of Temte´,North Cameroon,Central Africa
- Interaction of Ca2+and soil humic acid characterized by a joint experimental platform of potentiometric titration,UV-visible spectroscopy,and f luorescence spectroscopy
- Trace and rare earth element geochemistry of the black and grey shales of the Calabar Flank,Southeastern Nigeria:constraints on the depositional environment and the degree of metal enrichment
- Geochronological and geochemical comparison of Precambrian meta-maf ic volcanics in Zhongtiaoshan and Lu¨liangshan Regions in Shanxi,North China Craton
- Mineralogy and geochemistry of sands of the lower course of the Sanaga River,Cameroon:implications for weathering,provenance,and tectonic setting
- Episodic crustal growth and reworking at the southeastern margin of the North China Craton:evidence from zircon U-Pb and Lu-Hf isotopes of Archean tonalite-trondhjemite-granodiorite gneisses in the Bengbu-Wuhe area
