Effect of Acidity on Methylation of Benzene with Methanol Catalyzed by HZSM-5: A DFT Study
2021-06-29WeiPifengFuGuangbinMuShanliangGaoJichaoWenZhenhaoZhuXuedong
Wei Pifeng; Fu Guangbin; Mu Shanliang; Gao Jichao; Wen Zhenhao; Zhu Xuedong
(1. School of Chemistry & Chemical Engineering, Linyi University, Linyi 276000;2. SINOPEC Qingdao Refining and Chemical Company, Ltd., Qingdao 266500;3. School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Abstract: Different acidic HZSM-5 zeolites were constructed by doping with Al, Ga, or In. The effect of acidity on the adsorption of methylbenzenes and the reaction energy barriers for the methylation of benzene with methanol catalyzed by HZSM-5 zeolite were investigated by using the density functional theory. The results show that acidity exhibits less effect in the adsorption of methylbenzenes, while linear relationships are observed between the acidity and reaction energy barriers. As the acidity increases, the reaction energy barrier decreases linearly, and the stepwise pathway becomes dominant in strong acidity environment, while weak acidity is conducive to the concerted pathway. The calculation results could contribute to understanding the relationship between the acidity and the zeolite-catalyzed alkylation reaction of methylbenzenes.
Key words: benzene; methanol; methylation; acidity; HZSM-5
1 Introduction
Acidity and pore structure are critical indicators that can affect the catalytic performance for the zeolite-catalyzed reactions. The 10-membered-ring HZSM-5, a solid acid zeolite catalyst, has been widely used in aromatic transformation reactions, since its pore size is similar to the dynamic diameter of aromatics. However, the acidity can strongly influence the catalytic performance, and fine-tuning of acidity is a difficult point in the zeolitecatalyzed reactions.
The effect of acidity on the zeolite-catalyzed reactions has been investigated extensively by experiments. Wang,et al.[1]systematically investigated the effect of acidity on the methanol-to-hydrocarbon process over HZSM-22 and SAPO-11, and the results indicated that the acidic strength of the two one-dimensional 10-membered-ring catalysts had an important influence on the catalytic activity and stability, as well as the product distribution and deactivation mechanism. Mores, et al.[2]have revealed that higher Brønsted acid sites density enhances the formation of larger coke species as verified by insitu UV/Vis and confocal fluorescence microscopy, while the increased weak acid sites can facilitate the catalytic stability[3]. Low density of Brønsted/Lewis acid sites has been verified to be beneficial to increasing the paraxylene selectivity in toluene alkylation with methanol[4]. A consensus has been reached that an appropriate Brønsted acid/Lewis acid ratio and the number of acid sites are quite critical for zeolite-catalyzed reactions.
The alkylation of benzene with methanol is regarded as an alternative route for producing high value-added products such as toluene and xylene[5-6]. Therefore, the methylation of benzene by methanol is extensively studied in recent years, and the 10-membered-ring zeolite HZSM-5 has been demonstrated to be a selective catalyst for this reaction. Efforts have been made by modifying the acidity to improve the selectivity and lifetime of HZSM-5, and it has been reported that nitridation[7], metal oxide modification[8-12], and post-treatment[13]are effective methods to tailor acidity and increase the catalytic performance of HZSM-5 in the methylation of benzene with methanol.
Some DFT calculations have also been focused on the effect of acidity on the zeolite-catalyzed reactions.Morses, et al.[14]investigated the influence of acid strength on the methanol to dimethyl ether theoretically by Al-, Ga-, or In-induced Brønsted acid sites, with the ammonium ion formation energy serving as a measure of acidity. The results have shown a linear correlation between acidity and activation energy, and the stepwise pathway would be dominating for weaker acids. Li, et al.[15]investigated the effect of the Brønsted acid strength on the methylation of benzene with methanol over 5T,12T, and 104T HZSM-5 models by applying the ONIOM method. The different Brønsted acid strength was established by changing the size of the cluster model, and the calculation results indicated that benzene was readily methylated by methanol over stronger Brønsted acidic sites with lower energy barriers.
It has been demonstrated experimentally that acidity plays an important role in the zeolite-catalyzed methylation of benzene with methanol, and a great deal of work have been made to tailoring acidity of HZSM-5 to increase its catalytic performance. But the effect of acidity on this reaction has not been investigated at the molecular level,and what is the effect of acidity on the adsorption energy of reactants and reaction energy barriers is still unknown.In this paper, the effect of acidity on the reaction energy barrier for the methylation of benzene with methanol is investigated by periodic density functional theory (DFT),which can provide insights into this reaction from the molecular scale and direct catalyst design.
2 Computational Methods
The calculations were performed via the VASP software[16],and the detailed computational methods have been depicted in our previous works[17-18]. The projected augmented wave method[19]and the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional[20]were used, with the cut-off energy set at 500 eV. Periodic boundary conditions were applied, and the Brillouin zone sampling was restricted to theΓ-point in the calculations. The transition state configurations located by the improved dimer method[21]were considered as converged when forces on the atoms were less than 0.03 eV/Å.The total energy was corrected with zero-pointenergy (ZPE), and the van der Waals interactions (Edisp)were calculated by the DFT-D3 method[22]. Thus, the total energy (Etotal) can be obtained by the following equation:Etotal=EDFT+Edisp+ZPE, whereEDFTis the electronic energy computed by VASP package.
As presented in our previous works[17,23], different acidic HZSM-5 were constructed via replacing the Si atom in T12 site by Al, Ga, or In atom of the purely siliceous version of ZSM-5 (i.e. silicalite-1), and the so-caused negative charge was neutralized by adding a proton to the adjacent oxygen atom. Specifically, the M12-O (H)-Si (3) (with M representing Al, Ga, or In) was selected as the acid site of HZSM-5, since it was located in the intersection of the straight channel and sinusoidal channel,which could provide most space for the guest molecules.Because of the difference in electronegativity of Al,Ga, and In atoms, HZSM-5, which has various acidity values, was formed, while keeping the pore structure almost unchanged, as shown in Figure 1 (a)―(c). The adsorption energy of pyridine was calculated to evaluate the difference in acidity of HZSM-5, and the computed values were -2.16 eV, -2.04 eV, and -1.90 eV for the Al-,Ga- and In-induced HZSM-5, respectively, indicating a decreased acidity. The adsorption structures of pyridine are displayed in Figure 1 (d)―(f), and the different values in adsorption energy illustrate the various acidity.

Figure 1 Construction of different acidic HZSM-5 by Al, Ga, and In substitution and the adsorption of pyridine over Al-ZSM-5, Ga-ZSM-5, and In-ZSM-5, with the values in the parentheses representing the adsorption energy(Yellow: silicon; red: oxygen; purple: aluminum; white: hydrogen; gray: carbon; blue: nitrogen; similarly hereinafter)
3 Results and Discussion
The adsorption energy of methylbenzene molecules over Al-, Ga- and In-induced HZSM-5 is calculated with the adsorption energy of pyridine serving as a measure of acidity, which is summarized in Table 1. The results show similar adsorption energy of methylbenzene molecules,which means that the acidity exhibits less effect on the adsorption of methylbenzenes under the same pore structure conditions.
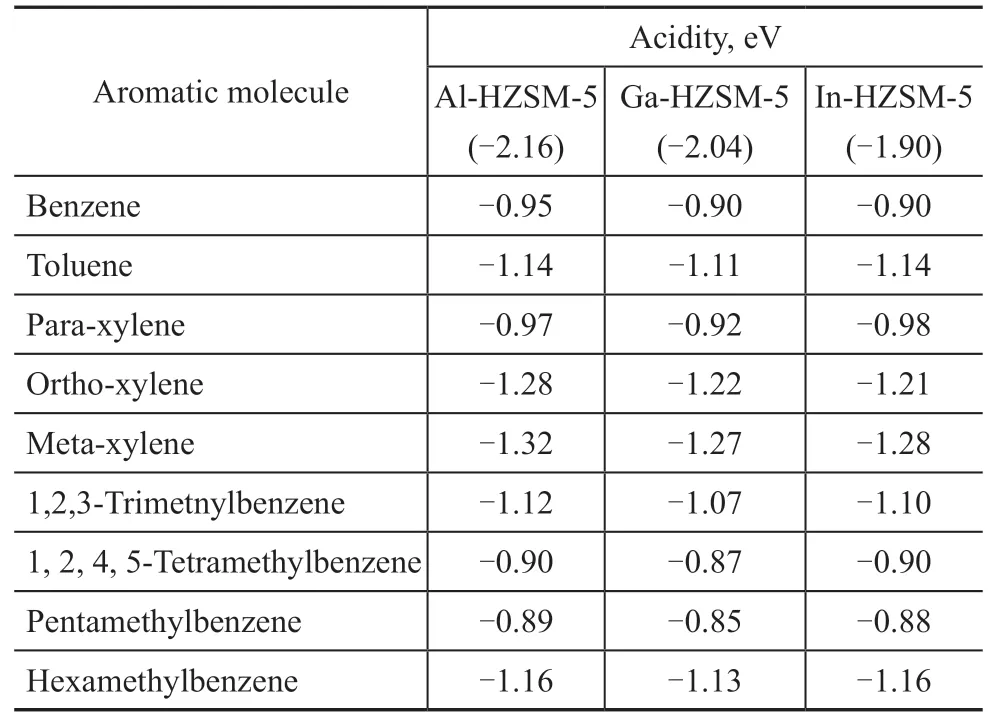
Table 1 Adsorption energy of several aromatic molecules over different acidic HZSM-5
In our previous studies[17-18,24], two distinct reaction routes have been proposed for the zeolite catalyzed methylation of benzene with methanol, i.e.theconcerted pathway and the stepwise pathway. The effect of the acidity on the reaction energy barrier is investigated based on the two reaction pathways. In the concerted pathway,benzene and methanol molecules are firstly co-adsorbed in the vicinity of acidic sites, which are shown in Figure 2 (a)―(c). In these geometric configurations, methanol molecule is combined with the acidic site by forming two hydrogen bonds with the neighboring oxygen atoms of the zeolite framework, while benzene molecule is located in the center of the straight channel. In the geometric configurations of the transition states demonstrated in Figure 2 (d)―(f), water molecule has been produced by forming hydrogen bonds with the neighboring oxygen atoms of the zeolite framework, whereas the methyl group becomes planar and is located between the water and benzene molecules. The distances of C-O bond being broken and the C-C bond being formed are 2.18 Å and 2.09 Å in the Al-doped HZSM-5, while they are 2.20 Å and 2.07 Å in the Ga-doped HZSM-5, and 2.23 Å and 2.04 Å in the In-doped HZSM-5. An interesting result could be found that even though the distances are diverse, but the sum of the distances of the C-O bond being broken and the C-C bond being formed are the same (4.27 Å) over the three different acidic HZSM-5 catalysts. The obtained reaction energy barriers for the concerted pathway over Al-, Ga- and In-induced HZSM-5 are 149 kJ/mol, 152 kJ/mol, and 158 kJ/mol, respectively. As the acidity increases, the energy barrier decreases, which is consistent with the calculation results reported by Li, et al.[15].
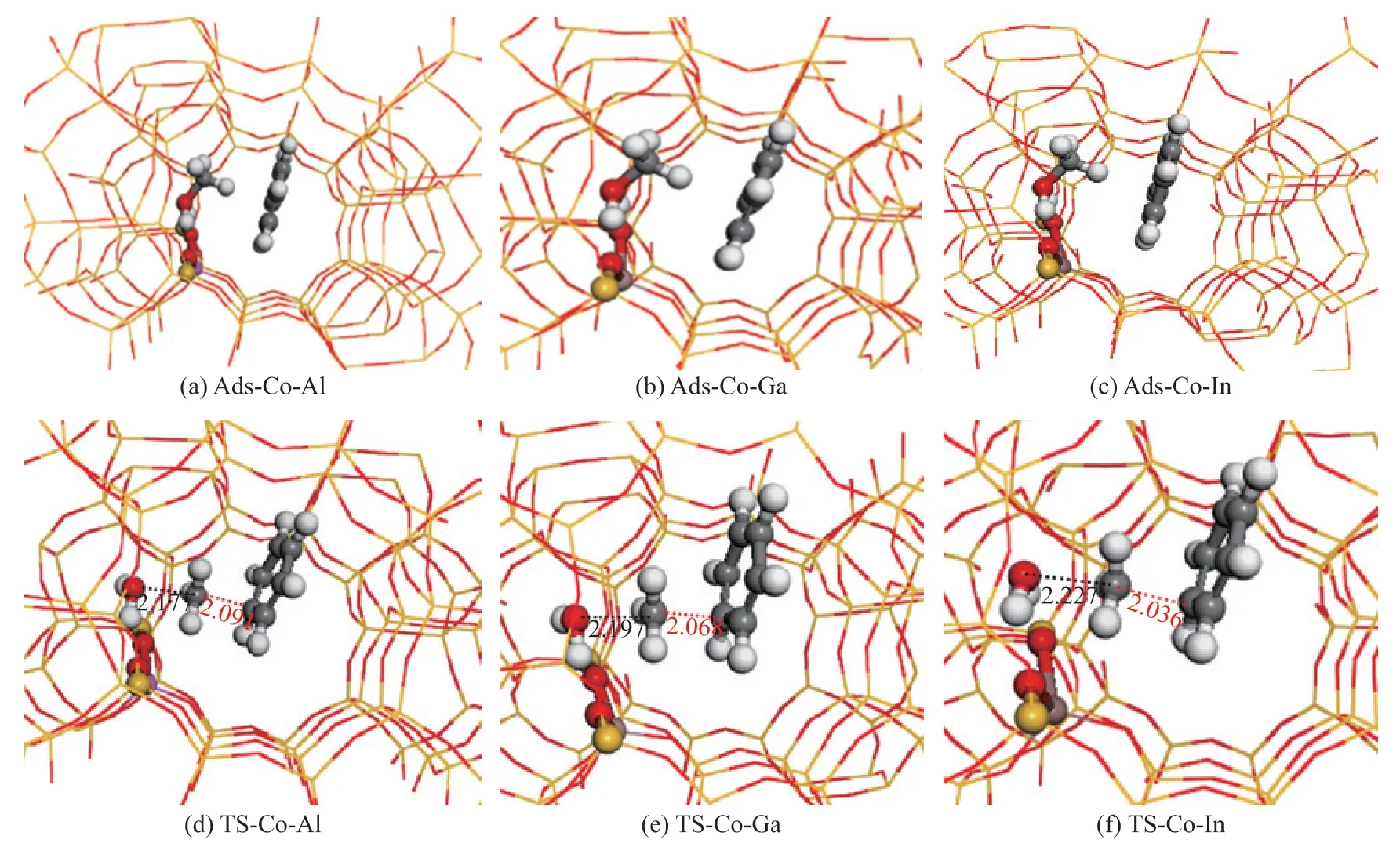
Figure 2 Co-adsorption structures of benzene and methanol over Al-, Ga-, and In- induced HZSM-5, and the corresponding transition states for forming toluene over Al-, Ga-, and In- induced HZSM-5(The bond lengths are in Å, similarly hereinafter)
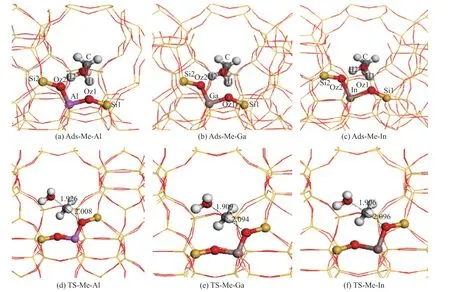
Figure 3 Adsorption structures of methanol over Al-, Ga- and In- induced HZSM-5, and the corresponding transition states of methanol dehydration over Al-, Ga-, and In- induced HZSM-5
In the stepwise pathway, methanol molecule is firstly adsorbed in the vicinity of the acidic sites to produce surface-methoxy group, and the corresponding adsorption geometries are shown in Figure 3 (a)―(c). The structures of the transition states for forming surface-methoxy group, as well as the important bond length, are depicted in Figure 3 (d)―(f). It can be seen that the methyl group is planar and is located between the water molecule and the oxygen atom of the framework. The computed reaction energy barriers for forming the surface-methoxy over Al-,Ga-, and In-induced HZSM-5 are 149 kJ/mol, 153 kJ/mol,and 161 kJ/mol, respectively. The newly generated surfacemethoxy group is alkylated with benzene molecule rapidly.As described in Figure 4 (a)―(c), benzene molecule is adsorbed in the vicinity of the newly formed methoxy group, and then a transition state is formed, as elaborated in Figure 4 (d)―(f), in which the benzene molecule is parallel to the planar methyl group. The distances of C-O bond being broken and the C-C bond being formed are both 2.15 Å in Al-doped HZSM-5, while they are 2.15 Å and 2.12 Å in both Ga- and In-doped HZSM-5. The calculated reaction energy barriers for the methylation of benzene with surface-methoxy group to produce toluene over Al-,Ga-, and In-induced HZSM-5 are 97 kJ/mol, 106 kJ/mol,and 114 kJ/mol, respectively.
The correlations between the acidity and the reaction energy barriers for the methylation of benzene with methanol are drawn up by using acidity as the X-axis and energy barriers as the Y-axis, as illustrated in Figure 5. Obviously, we can see that as the acidity decreases,the reaction energy barriers increase linearly in both the concerted pathway and the stepwise pathway. This relationship could be quantified by a linear equation, as shown in Figure 5. The results show that the formation of methoxy group is a controlling step in the stepwise pathway, however, the energy barriers for the formation of methoxy group are similar with those in the concerted pathway, implying that it is difficult to distinguish which pathway is the dominant one in this acidity scope. Judging from the slope of the linear equations, it can be seen that the energy barrier for the concerted pathway will be lower than that in the stepwise pathway as the acidity decreases,signifying that the concerted pathway prevails in the weak acidity environment. In contrast, strong acidity is more conducive to lowering the energy barrier for forming methoxy group, implying that the stepwise pathway becomes dominant in the strong acidity environment,which is in agreement with what Li, et al.[15]have obtained by cluster model. As a result, acidity could influence the reaction route by changing the reaction energy barrier. It is quite challenging to increase properly the selectivity of para-xylene and limit the unwanted by-products, such as ortho-xylene and meta-xylene. It could be speculated that if the unwanted by-products have a different correlation with acidity, then an optimal acidity for the methylation of benzene with methanol should be found. Therefore, further investigation should be focused on the influence of acidity on inhibiting the formation of by-products during zeolitecatalyzed methylation of benzene with methanol.
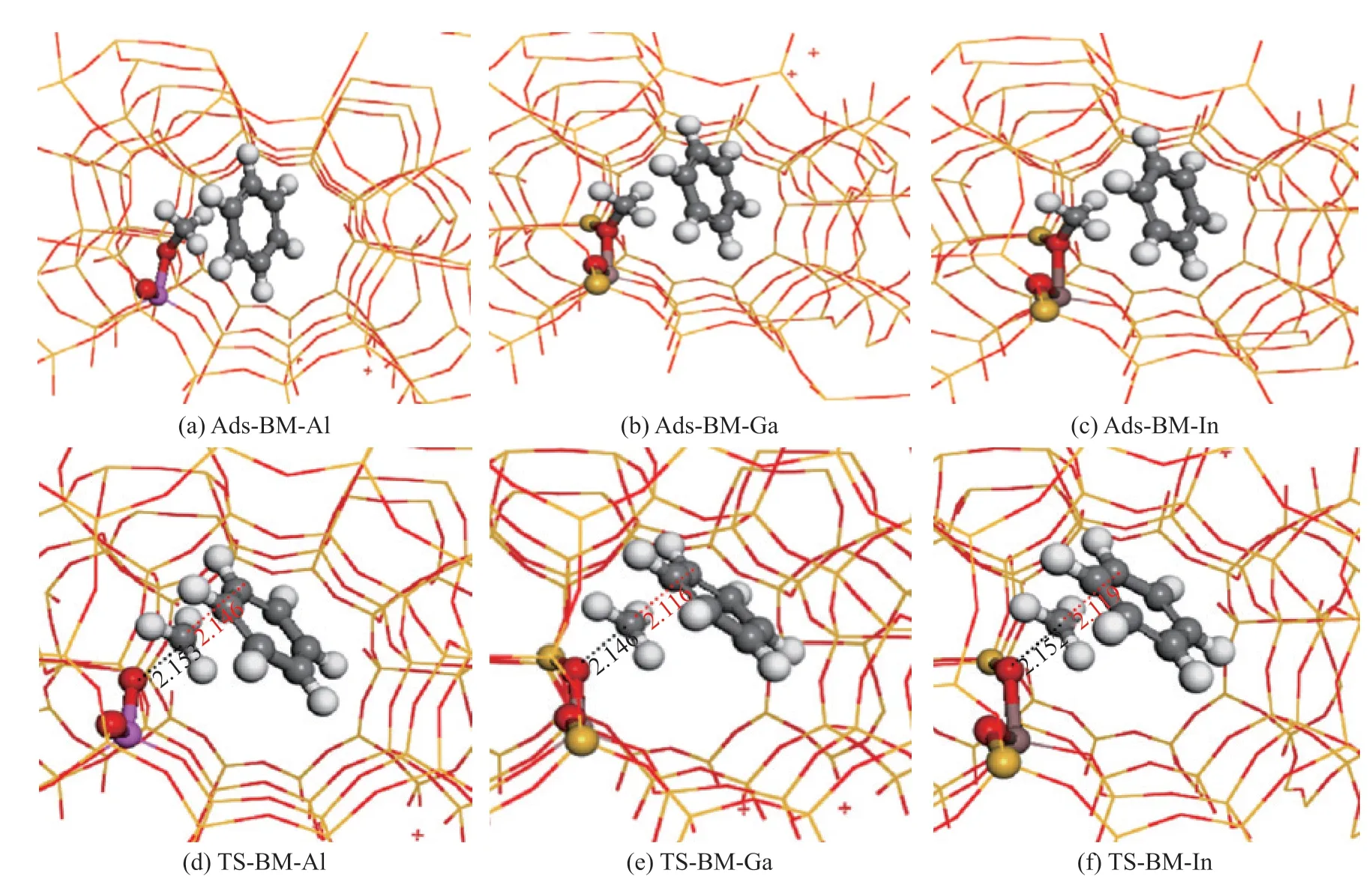
Figure 4 Adsorption structures of benzene in methoxy group over Al-, Ga-, and In- induced HZSM-5, and the corresponding transition states for forming toluene over Al-, Ga-, and In- induced HZSM-5
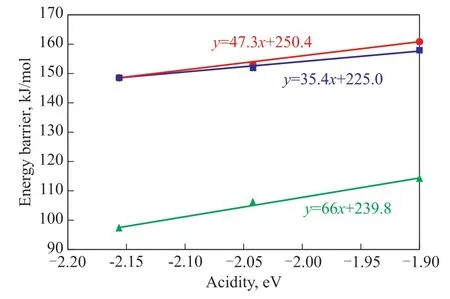
Figure 5 The correlation between acidity and energy barriers for the methylation of benzene by methanol
4 Conclusions
In this work, different acidic HZSM-5 zeolites were firstly prepared by substituting Si atom with Al, Ga,and In atoms. Based on the constructed different acidic HZSM-5 models, the effect of acidity on the adsorption of methylbenzenes and the reaction energy barriers for the methylation of benzene with methanol, by both the concerted pathway and the stepwise pathway, were investigated by periodic density functional theory.The results show that acidity exhibits less effect on the adsorption of methylbenzenes, and the calculated ratedetermining step in the stepwise pathway is the formation of methoxy group. The correlation between acidity and reaction energy barriers could be described by linear equations for both the concerted pathway and the stepwise pathway. As the acidity increases, the reaction energy barrier decreases. The results show that weak acidity is conducive to the concerted pathway, while the stepwise pathway becomes dominant in strong acidity environment.Our calculated results indicate that acidity could influence the reaction route by changing the reaction energy barrier.The calculation results could contribute to understanding the relationship between acidity and the zeolite-catalyzed alkylation reaction of methylbenzenes.
Acknowledgements:We gratefully acknowledge the financial support from the Natural Science Foundation of Shandong Province (ZR2018LB028).
杂志排行
中国炼油与石油化工的其它文章
- Heat Transfer and Kinetics Study of Moroccan Oil Shale Pyrolysis Process
- Structural and Textural Transitions of the Zn-BDC Materials During Dehydration-Rehydration Process
- Heat Exchanger Network Retrofit of Diesel Hydrotreating Unit Using Pinch Analysis
- Application of a New Catalyst Deactivation Model for Residue Hydrotreating
- High-efficiency Extraction of Bitumen from Oil Sands Using Mixture of Ionic Liquid [Emim][BF4] and Dichloromethane
- Study on Reducing Injection Pressure of Low Permeability Reservoirs Characterized by High Temperature and High Salinity
