The Crystallization Process in Petrochemical Industry
2021-06-29ZhangDejiangFanYingqiXieLiDingHuidianWangHao
Zhang Dejiang; Fan Yingqi; Xie Li; Ding Huidian; Wang Hao
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Crystallization is a widely used product refining process. Understanding the characteristics of the crystallization process in the petrochemical industry is helpful to selecting and designing new crystallization processes. This review briefly introduces the characteristics of the crystallization process, summarizes the crystallization processes applied in petrochemical industry, and discusses some points for crystallization process design. Crystallization in the petrochemical process is mainly used in the purification of polymer monomers and is often a unit operation in the whole refining process.Designing a reasonable crystallization process requires consideration of process safety, feasibility, and economy from an overall perspective.
Key words: crystallization; petrochemical; process design; purification; monomer
1 Introduction
Crystallization is a process in which solid substances precipitate from vapor or solution or melt in a crystalline state[1-2]. It is a basic unit operation that has long been used to produce various materials, such as crystalline salt from seawater and sugar from cane juice. With the development of technology, crystallization has been widely used in various chemical processes. Almost 70%of all solid chemical production processes involve a form of the crystallization process[3-4], typically in the production of fertilizer and pharmaceuticals.
The crystallization process has three main advantages:(1) It has low energy consumption because the heat to be removed during the crystallization process is usually the crystallization heat and the sensible heat of a solution,which are generally lower than the evaporation heat; (2)It has mild process conditions. The solubility of most substances decreases with a decreasing temperature.To obtain high yields, crystallization processes are often carried out under moderate conditions that are suitable for treating heat-sensitive products; (3) It has high product purity. A crystal is a solid substance with a regular arrangement of atoms, ions, or molecules in the internal structure[5]. The regularity of the internal structure of solid can result in a highly pure crystal. Therefore,crystallization is an important purification technology in the production of many value-added chemicals, especially the production of pharmaceuticals.The petrochemical products that use crystallization as a purification process include terephthalic acid, p-xylene (PX),caprolactam, adipic acid, and acrylic acid. This paper summarizes the typical crystallization processes, analyzes why these processes use crystallization for purification purpose, and provides a reference for the design of petrochemical processes that may use crystallization.
2 Purified Terephthalic Acid Crystallization
Purified terephthalic acid (PTA) is the main raw material used to manufacture high-performance, multipurpose plastics such as polybutyl terephthalate (PBT)and polyethylene terephthalate. As Figure 1 shows,terephthalic acid is mainly prepared by oxidation of PX in an acetic acid medium, using cobalt and manganese as catalysts and bromine as a promoter[6]. The oxidation by-product of PX contains water, but the solubility of terephthalic acid in a solvent with a molar ratio of acetic acid to an acetic acid–water solvent of 0.8507 at 423.15 K is 0.0372 mol/kg[7]. Therefore, the oxidation reaction product of p-xylene can crystallize crude terephthalic acid (CTA) through a flash evaporation process. Wang,et al.[8]studied the effects of seeding during the PX oxidation process on the crystallization of CTA. They attempted to control the crystallization process of CTA by seeding. The crystal growth kinetics of terephthalic acid and the incorporation rate of 4-carboxybenzaldehyde(4-CBA) from the solution into the crystal were determined experimentally. The experiments showed that the distribution coefficient of 4-CBA decreased with an increasing temperature. Aging is an operation that suspends crystals in its saturated solution and maintains it for a period of time at a small temperature amplitude[9].This operation repairs the irregular surface of the crystals generated during the growth process and dissolves the fine crystals. Wang, et al.[10]proved that the aging operation could significantly improve the crystal quality of CTA.After aging the terephthalic acid crystals at 507 K for 80 minutes, the content of 4-CBA in the crystals was reduced from nearly 1600 μg/g to nearly 550 μg/g.
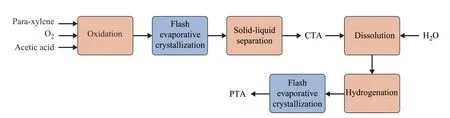
Figure 1 Flow chart of PTA production process
As the content of 4-CBA usually cannot meet the commercial specification of no more than 150 ppm, CTA needs further purification. The 4-CBA in CTA needs to be converted into p-toluic acid by hydrogenation, which is then removed by recrystallization. The solvent used in the recrystallization of terephthalic acid is water. This is because the single-pass yield of the PTA crystallization process using water as solvent is relatively high. Moreover,the solubility of hydrogen in water is relatively large, which increases the conversion rate of the hydrogenation reaction.The product temperature and pressure after hydrogenation are relatively high, reaching about 270 °C and 80 bar,respectively. The hydrogenation products then pass through a multi-stage crystallizer to be subject to gradual flash evaporation, yielding PTA crystals. Generally, the crystallization process of PTA is divided into multi-stages,and most commercial processes have five stages. Owing to concerns about the precipitation of p-phenylmethylbenzoic acid at low temperatures, many patents require the final temperature to be more than 120 °C[11]. For example,Olsen, et al.[12]suggested that the final temperature of crystallization should not be lower than 165 °C. The high final stage temperature means that the pressure of the solution is also high, which makes the downstream solid-liquid separation equipment difficult to operate. To overcome this problem, Lin, et al.[13]proposed a method of re-injecting the flashed solvent into the crystallizer. The idea is that reflux of the condensed solvent allows more impurities to be dissolved in the final stage mother liquor,so the pressure of the crystallization process in the final stage can be reduced to normal pressure.
There is not much difference in the crystallization process of PTA adopted by different companies. The main difference is the integration of energy. For example,Deming, et al.[14]introduced a method to remove residual water in CTA crystallization mother liquor by azeotropic distillation. Butyl acetate or propyl acetate was the entrainer used in this patent.
3 Caprolactam Crystallization
Caprolactam is an important petrochemical product that is mainly used for synthesis of compounds such as polycaprolactam fiber (nylon-6) and engineering resin PA6[15]. Caprolactam is mainly prepared from cyclohexanone oxime by the Beckman rearrangement.At present, there are two industrialized Beckman rearrangement processes. The main industrialized Beckmann rearrangement process is carried out in the liquid phase. Depending on the production process of raw materials, the crystallization process of caprolactam also varies. In the liquid-phase Beckman rearrangement process, the liquid-phase cyclohexanone oxime is converted into caprolactam by means of the catalysis of sulfuric acid. Subsequently, the reaction product is neutralized with ammonia, and caprolactam is obtained through water extraction, benzene extraction, ion exchange, hydrogenation, and distillation. Therefore, the study of the crystallization process for the purification of liquid-phase rearrangement products is often carried out by melt crystallization, with water acting as an impurity.Vapor-phase rearrangement is another caprolactam production process; the rearrangement reaction process is carried out directly in the vapor phase. The refining process of this technology mainly includes distillation,crystallization, and hydrogenation.
The liquid-phase rearrangement process is the first industrialized rearrangement process, so earlier studies on the crystallization of caprolactam often focused on the liquid-phase rearrangement products. Chianese, et al.[16]measured the mathematical model that was applied for predicting the growth of a crystal on a cylindrical cold surface that was in contact with a stirred melt.Poschmann, et al.[17]studied the use of suspension melt crystallization to remove water in a caprolactam aqueous solution. They investigated the suspension crystallization of caprolactam under the conditions of using 1.5%, 2.8%, and 4.8% of water, respectively. The conclusion was that caprolactam purified by suspension crystallization could meet the requirements. Jansens, et al.[18]found that the morphology of caprolactam crystals depended on the water content of the mother liquor and on the applied growth kinetics. Through single-crystal culture experiments, they determined two conditions under which the needle-like crystals were produced.One was at high growth rates in a water-rich mother liquor, and another one was at low growth rates in the absence of water. In subsequent work[19], they used a batch crystallization process, in which heat was removed by vacuum evaporation to study the crystallization process of caprolactam aqueous solution. In this study,the crystallization rate of caprolactam was controlled by the evaporation rate of water. According to the phase diagram, the optimized crystallization conditions included a water content of 5%, a temperature of 53 °C, and a pressure of 4.2 kPa. At present, the crystallization process of liquid phase rearrangement products has not yet been industrialized. This may be because the high solubility of caprolactam in water, resulting in a low single-pass yield.
As the reaction selectivity of the vapor-phase Beckmann rearrangement process is lower than that of the liquid-phase Beckmann rearrangement process, the crystallization of the vapor-phase rearrangement product requires the use of a special solvent system. Guo, et al.[20]measured the solubility data of caprolactam in different solvents. As shown in Table 1, since caprolactam is a polar molecule, in order to obtain a higher single-pass yield, non-polar solvents are usually used to purify caprolactam. Different companies generally use different solvents for the crystallization of caprolactam. The crystallization process of vapor-phase rearrangement products is currently industrialized by Sumitomo Corporation. Naoki, et al.[21]have mentioned in their patent that the crystallization process uses one or two aliphatic alkanes as solvents. The crystallization process consists of two parts. In the first part, the crystallizer is cooled via direct cooling. The cold solvent and the hot mother liquor are directly mixed to cool the solution and precipitate the crystal products. According to the operating condition, direct cooling crystallization can be divided into multiple stages. The crystal slurry is centrifuged to separate the solid phase and mother liquor.The solid phase is washed to obtain refined caprolactam.The mother liquor enters the second part of the crystallization. The residual caprolactam is recovered by evaporative crystallization and recycled back to the inlet of the direct cooling crystallization section. Chen, et al.[22]patented a control strategy to purify caprolactam with halogenated hydrocarbons acting as solvents. They made the crystallization process gentler by adding seed crystals to the metastable zone, and the crystal purity obtained was higher.

Table 1 Mole fraction solubility of caprolactam[20]
4 PX Crystallization
PX, which is produced by extraction and distillation from reformed petroleum fraction, wide-cut naphtha, or a steam-cracking product, is an aromatic hydrocarbon used primarily to produce intermediates for manufacturing polyester. It is the main feedstock for PTA production.
Xylene mixtures consisting of ortho-xylene, PX, metaxylene, and ethyl-benzene are mainly used as feedstocks for the separation and purification of PX. The boiling point of the individual xylene isomers is very close,which makes traditional distillation separation methods unsuitable. The crystallization method is one practical method for the commercial production of pure PX because the melting point difference between individual xylene isomers and PX exceeds 30 °C. A comparison of physical properties of individual xylene isomers is shown in Table 2.

Table 2 Comparison of physical properties of xylene isomers[23]
The crystallization methods widely used in industrial purification of PX include suspension melt crystallization and layer-based melt crystallization. Suspension melt crystallization is first used in the purification of PX. As shown in Figure 2, the suspension melt crystallization method is similar to cooling crystallization in solution.That is, the mother liquor containing PX is transported to a crystallizer with a rotating scraper, and then PX crystals are obtained by cooling[24]. The entire crystallization process is divided into two stages. The crystalline product of PX is obtained from the second stage. The final temperature of the first-stage crystallization process is about −55 °C to –75 °C, which maximizes the yield of the crystallization process. The crystal slurry is centrifuged or filtered to collect the crude PX crystals. The mother liquor separated by centrifugation is recycled in a xylene isomerization reactor. The crude PX crystals obtained by first-stage crystallization are then re-melted and injected into the second-stage crystallization process. Owing to the high content of PX in the feedstock, the temperature of the second-stage crystallization process is between −5 °C and −10 °C.

Figure 2 Schematic diagram for the two-stage suspension melt crystallization of PX
Solubility data for PX in xylene mixtures have a non-ideal behavior, and the reliable solubility data of PX in xylene mixtures were determined experimentally by Mohameed,et al.[23]The crystallization kinetic expressions under different crystallization condition can all be considered as empirical power laws in supersaturation. During the suspension-melt crystallization process, the crystal growth is considered to be particle-independent. The nucleation and growth kinetics can be described by:
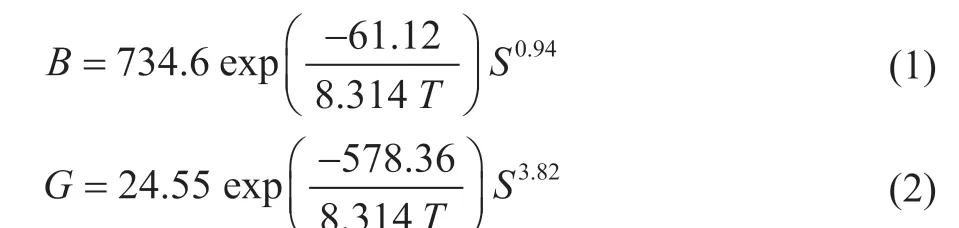
where the unit of B is number/m3/s, the unit of G is m/s, and S is relative supersaturation. In their later work[25],Mohameed, et al. determined the crystallization kinetics of PX in batch-pilot-scale scraped-surface crystallizers.They found that the nucleation occurred at the wall of the crystallizer, and growth occurred in the bulk solution.The crystal growth rate of PX was so fast that the supersaturation in the bulk solution was very low.
PX can also be produced by layer-based melt crystallization process developed by SULZER. In the layer-based melt crystallization process, the xylene mixtures and the melt circulation solution vertically enter the crystallizer. As a result of the high melting point of PX, it crystallizes on the tube-side surface first because the temperature drops, while xylene isomers flow out of the crystallizer in the form of droplets. When PX crystals of sufficient thickness are formed in the tube, the crystallizer must be heated to perform the sweating process. Because the crystals containing impurities have a relatively low melting point and are easier to melt, the solution first obtained during the sweating process is usually recycled.After the sweating, the remaining PX crystals are melted and collected as products. Such layered crystallization unit is often arranged in pairs and operated in batch mode.The obvious advantage of this crystallization method is that there is no need to use rotating equipment, and no solid-liquid separation equipment is required. However,the control of the crystallization process and the scaleup of the equipment have become obstacles that limit the application of this crystallization method. Figure 3 shows a schematic image of the layer-based melt crystallization.

Figure 3 Schematic diagram for the layer-based meltcrystallization of PX
The PX melt crystallization process does not add new solvents, so its energy consumption is relatively low. However, there are two drawbacks that limit the application of the PX crystallization process. The first is that the yield of the crystallization process is limited by the concentration of PX in the raw material. For example,when the PX concentration of the raw material is 20%,the yield of crystallization process is only about 60%. The second is that the equipment is complicated. Adsorption is a competitive technology for producing PX[26].Generally, when the PX concentration in feedstock is high, crystallization is appropriate; otherwise, adsorption is appropriate. With the development of technology,some studies have attempted to combine adsorptive separation and crystallization separation to achieve the best separation performance[27].
5 Adipic Acid Crystallization
As an important industrial material, adipic acid has many industrial uses, and the most common one is condensation with hexamethylene diamine to produce nylon 66, which is used to make daily necessities such as carpets, knitwear, and parachutes. Adipic acid products have multiple purity levels,but only adipic acid with a purity of higher than 99.8% can be used to produce polymers. Such high purity requirements make crystallization suited to purification of adipic acid.
Adipic acid is mainly produced by oxidizing KA oil, a mixture of cyclohexanone and cyclohexanol with nitric acid. The mass fraction of adipic acid in the oxidation product is about 14%, and the remaining substances are nitric acid, glutaric acid, succinic acid, water, and other substances. The solubility of glutaric acid and succinic acid in water is greater than that of adipic acid,so direct flash evaporation of the oxidation product can crystallize adipic acid and remove the main byproducts. Crude adipic acid crystals can be obtained by washing and centrifugation. Because the content of residual metal ions in the crude crystals often cannot meet the polyamide grade standard, the crude crystals need to be recrystallized. The recrystallization process of crude crystals still uses water as the solvent, and the crystallization method is also vacuum flash evaporation.As shown in Figure 4, to control the quality of the product, there can be as many as nine crystallizers connected in a series during the recrystallization process.Purified adipic acid can be obtained after centrifuging,washing, and drying the recrystallized product. The residual adipic acid in the mother liquor of the first crystallization and recrystallization stages is recovered by evaporative crystallization, and the recovered adipic acid is returned to the first flash evaporative crystallization equipment.Suren, et al.[28]measured the solubility of adipic acid in water and acetic acid. The mole fraction of adipic acid solubility in water at 303 K is 0.004, which indicates that the crystallization process of adipic acid can achieve a high yield. David, et al.[29]measured the nucleation,growth, and agglomeration of adipic acid during crystallization, and predicted the change in crystal size distribution with the stirring and feed concentration by using the population balance model. Costa, et al.[30]added the agglomeration process to the mathematical model of adipic acid crystallization, analyzed the influence of different process parameters on the particle size distribution of the final product, developed an optimal control strategy, and significantly improved the product quality.
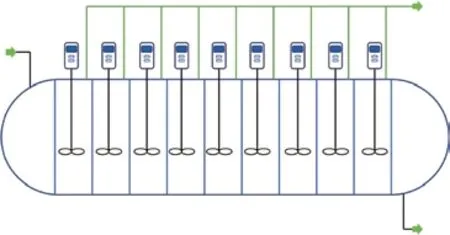
Figure 4 Schematic of adipic acid crystallizer
Some process intensification methods have been used to improve the quality of the products of adipic acid crystallization. Narducci, et al.[31]studied the effects of the continuous addition of ultrasonic radiation on the crystal product during the batch adipic acid crystallization process. Their results showed that spherical particles with increased and regular surface roughness could be obtained by continuous addition of ultrasonic radiation.In their later work, they studied the influence of ultrasonic addition on the continuous crystallization of adipic acid[32]and found that ultrasonic irradiation resulted in significantly smaller crystal sizes, reduced agglomeration,and an improved habit of crystals. Wohlgemuth, et al.[33]studied the influence mechanism of ultrasonic radiation on the quality of crystal products. They hypothesized that ultrasound changes the quality of crystal products by creating bubbles in the liquid and inducing heterogeneous nucleation at the gas-liquid interface. They found that the effect of gassing on the crystal product was similar to that of ultrasound. Moreover, the evaporative crystallization of adipic acid mother liquor consumes a lot of energy.In order to reduce the energy consumption, Kuhn, et al.[34]studied the membrane-assisted crystallization. The results showed that the energy conversion of membraneassisted crystallization was 6 times higher than that of evaporation.
In addition to process intensification, the use of carboxylic acid as a solvent for recrystallization was proposed in the patent CN 97195807[35]. Through this method and the continuous introduction of carbon monoxide into the solution, the residual trace metal ions in adipic acid crystals can be effectively reduced.
6 Acrylic Acid Crystallization
Acrylic acid (AA) is a large-volume petrochemical that is mainly derived from propylene. AA is a colorless and water-soluble carboxylic acid with a low melting temperature (13 °C). It has been widely used to prepare acrylate for the coating industry and superabsorbent polymers for baby diapers, fire foam, and thickeners.AA can be divided into two general grades based on its purity. One is the ester-grade AA, which is also called the crude AA (CAA, 85% — 95% purity); the other one is the glacial AA (GAA, 99.5%+purity).
Nearly all commercial AA is produced from propylene using two-stage oxidation. Crude AA has many impurities, such as acetic acid, propionic acid, acrolein,and allyl alcohol. Considering the low melting point and other physiochemical properties of AA and the purity requirements, distillation and crystallization are two main methods for purification.
The distillation process can obtain the ester-grade acrylic acid, but the glacial acrylic acid can only be purified by crystallization because acrylic acid is very easy to self-polymerize. Acrylic acid is generally purified by distillation into the ester-grade acrylic acid, and then the ester-grade acrylic acid is purified into the glacial acrylic acid by crystallization.
The main impurity removed during the acrylic acid crystallization process is water, which can deactivate the polymerization catalyst. Melt crystallization is the primary crystallization process. Currently, the most successful technology for the purification of AA is the falling-film crystallization, developed by Sulzer. Sulzer’s crystallization process contains static chilled surface crystallizer and dynamic chilled-surface crystallizer.The static chilled surface crystallizer is similar to the plate heat exchanger. The crystal is crystallized on the outside of the cold wall surface and is mainly used to recover the residual acrylic acid in the mother liquor[36].The crystals formed in the static crystallizer are returned to the dynamic crystallizer. The dynamic chilled surface crystallizer is similar to a shell-and-tube heat exchanger.The crystals can crystallize on the inside of the tube, and its main function is to ensure the purity of the product.Considering that the crystals of AA often contain impurities during melt crystallization, one crystallization process is not enough to produce high-purity GAA.Thus, the melt crystallization process usually has several“crystallization, sweating, crystallization…” cycles.As shown in Figure 5, Sulzer recommended to operate the apparatus with multi-stage dynamic crystallization through the falling-film crystallizer and multi-stage static crystallization[37]. GAA produced by BASF can reach a purity of 99.7%.
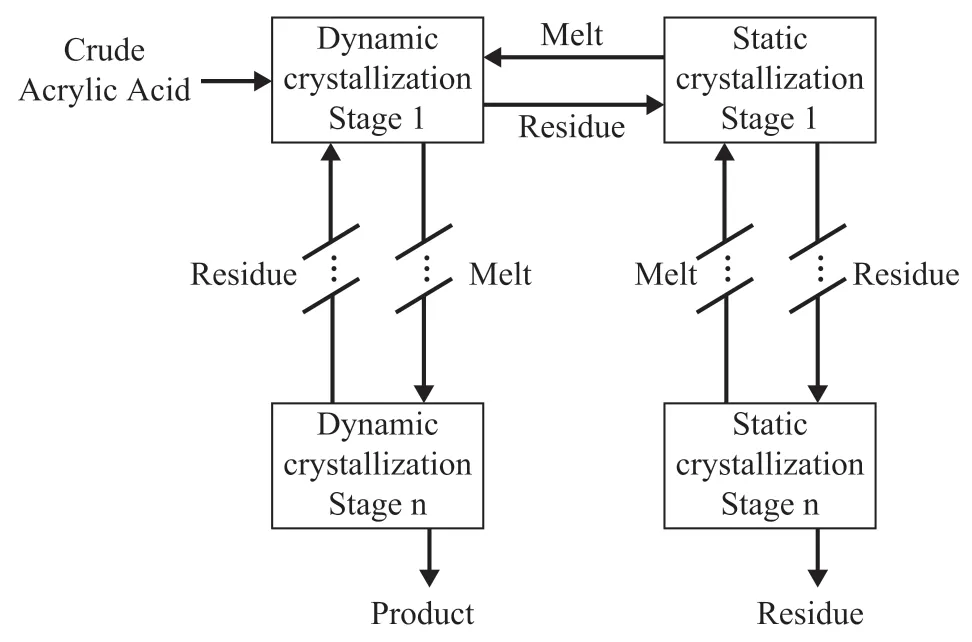
Figure 5 Schematic diagram for melt crystallization ofacrylic acid
Hengstermann, et al.[38]tried to determine the ternaryphase diagram by adding acetic acid, butanoic acid,or propionic acid into the aqueous melt of AA. They showed that it could increase the yield and improve the crystallization performance of AA by adding acetic acid or butanoic acid. Similarly, Le Page Mostefa, et al.[39]built the solid−liquid equilibrium diagram of the binary system acrylic acid (AA) + propionic acid (PA), and found that the AA and PA could form a peritectic compound, which narrowed the operating area for obtaining pure AA.
The crystal growth of AA is also very important in producing pure AA. Beilles S, et al.[40]found that in the presence of water, there were cavities in AA crystals that retained the impure liquid, preventing the formation of pure AA. Moreover, Hengstermann, et al.[41]found that the higher the supercooling and water content, the higher the tendency to form needle-like crystals, and the larger the length of the occurring cavity. Other impurities might also have effects on the crystal morphology of AA. There are also other crystallizers and more intense operations of falling-film crystallization, especially for the purification of bio-acrylic acid[42].
7 Selection and Design of Industrial Crystallization Process
Generally, the purification of a crude product often requires a combination of different purification processes. Therefore, we must arrange the order of the purification process so that it is based on a comprehensive understanding of the properties of products and impurity components in order to identify the impurities to be removed in each purification process, and optimize the purification process as a whole[43]. There are three factors that may affect whether a process will apply crystallization as a separation method. (1) Process safety:Some products are very sensitive to temperature, and special care should be taken when refining these products,such as glacial acrylic acid. (2) Process feasibility. Some impurities may form co-crystals or solid solution with the product. For these impurities, crystallization may not be an effective purification method. (3) Process economics. For most processes, the energy consumption of crystallization is relatively low. However, the premise of this conclusion is that no new solvent is added to the crystallization process. As the added solvents must be recovered, an energy consumption comparison between the crystallization process that needs to add new solvents and the other purification processes should be made.In addition, the solvent recovery system and the solid–liquid separation system will inevitably lead to increased investment in equipment. After clarifying the purpose of the crystallization in the overall refining process, a suitable crystallization method can be chosen.
For the crystallization process with large capacity,evaporation or cooling crystallization is generally used. As to which crystallization method is more suitable, it depends on the solubility of solute. Cooling crystallization is preferred when the solubility curve is relatively steep. Evaporative crystallization is the opposite. For substances that are prone to fouling on the heat exchange surface, flash crystallization is an effective method. Melt crystallization is mainly used for products with very strict purity requirements. The viscosity of the product has a great influence on the melt crystallization process because there may be a less mobile phase in the melt crystallization process. Therefore, highly viscous substances are not suitable for melt crystallization.Antisolvent crystallization is relatively complicated, and as far as we know, there are relatively few large-scale industrial applications in petrochemical industry.
After the crystallization method is chosen, the specific process operating parameters need to be determined.Large crystals are often preferred because a large particle size is beneficial to the downstream solid-liquid separation and helps improve the purity of the product. The control of the crystallization process needs to be based on a comprehensive understanding of the thermodynamics and crystallization kinetics of the product. Generally, to produce large crystals, the degree of supersaturation must be controlled within the metastable zone.
As mentioned above, purity is often the most important requirement for polymer monomers. Most of the impurities can be removed during the crystallization process, but some may remain in crystals. Generally,impurities remain in crystal products in the form of agglomerates, surface depositions, inclusions, co-crystals,and solid solution[4,44]. An agglomerate is the residue of impurities between crystal agglomerates. This form of impurity can be avoided by reducing the agglomeration of crystals during crystallization. Surface deposition refers to impurities that are resulted from the residual mother liquid or are absorbed onto the crystal surface.This form of impurity can be removed by washing the crystal surface. An inclusion is an impurity embedded in the lattice. The impurity inclusion rate is often a function of the crystal growth rate. Therefore, impurity inclusions can be reduced by adjusting the growth rate during the crystallization process. For impurities that could form cocrystal or solid-solutions with the product, the maximum recovery of products is limited by the eutectic point.In addition, there are still many factors that need to be considered in the actual crystallization process design,such as the heat tracing of the crystallization equipment and the solid-liquid suspension inside the crystallizer.Many design bases still need to be verified through experiments. Today, process analytical technologies(PAT) are widely used in the research of crystallization process[45]. The use of these instruments can effectively reduce the workload for experiments.
8 Conclusions
This review briefly introduces the crystallization process applied in the petrochemical industry. Because of the high requirement for purity, crystallization in the petrochemical industry is mainly used in the purification of polymer monomers. Being different from pharmaceutical crystallization process, the crystallization processes in petrochemical industry often require high capacity, high purity criteria and low energy consumption. For the design of a crystallization process, the key is to identify the impurities that can be removed by the crystallization process. Although crystallization is an efficient purification method, the complexity of its supporting processes, such as the solid-liquid separation process and the solvent circulation process, limits the application of crystallization to a certain extent. With improvements in product quality requirements and technological progress,crystallization can be used in more petrochemical processes.
杂志排行
中国炼油与石油化工的其它文章
- Heat Transfer and Kinetics Study of Moroccan Oil Shale Pyrolysis Process
- Structural and Textural Transitions of the Zn-BDC Materials During Dehydration-Rehydration Process
- Heat Exchanger Network Retrofit of Diesel Hydrotreating Unit Using Pinch Analysis
- Effect of Acidity on Methylation of Benzene with Methanol Catalyzed by HZSM-5: A DFT Study
- High-efficiency Extraction of Bitumen from Oil Sands Using Mixture of Ionic Liquid [Emim][BF4] and Dichloromethane
- Study on Reducing Injection Pressure of Low Permeability Reservoirs Characterized by High Temperature and High Salinity
