Meta-Analysis on the associations between Prenatal Perfluoroalkyl Substances Exposure and Adverse Birth Outcomes
2021-06-22LIFangZHONGZhehui钟哲辉XUChenye徐晨烨ZHANGBeibei张贝贝MUHAMMADAamirSHENChensi沈忱思LIUShuren刘树仁YINShanshan尹杉杉
LI Fang(李 方), ZHONG Zhehui(钟哲辉), XU Chenye (徐晨烨)*, ZHANG Beibei (张贝贝), MUHAMMAD Aamir, SHEN Chensi (沈忱思), LIU Shuren (刘树仁), YIN Shanshan (尹杉杉)
1 College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
2 Water-Energy Resilience Research Laboratory, School of Engineering, Westlake University, Hangzhou 310024, China
3 College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310058, China
4 Interdisciplinary Research Academy (IRA), Zhejiang Shuren University, Hangzhou, 310015, China
Abstract: The epidemiological associations between the prenatal perfluoroalkyl substances (PFASs) exposure and the reproductive outcomes remain controversial. A continuous evaluation is needed to combine the inconsistent results. In this study, we explored the associations between PFASs exposure and the low birth weight (LBW), preterm birth and small for gestational age (SGA). The quality of selected literature, quantitative estimates, publication bias and subgroup analysis were performed on the basis of 17 retrieved articles published before December 2020. The results showed a significant positive association between the perfluorooctane sulfonate (PFOS) exposure and the risk of LBW [Odds ratio (OR)=1.17; 95% confidence interval (CI): 1.01, 1.36; heterogeneity: P=0.30, I2=17%]. The positive association was also observed between the PFOS and the risk of preterm birth (OR=1.19; 95% CI: 1.01, 1.39, P=0.007; I2=62%). There was a paucity of evidence regarding the negative effects of perfluorooctanoic acid (PFOA), perfluorohexanesulfonic acid (PFHxS) and perfluorononanoic acid (PFNA) on the pregnancy outcomes. The findings from the subgroup analysis (the sampling period, the birth gender and biologic specimens) did not substantially altered the results of the overall pooled estimate ORs. The increased prevalence of negative birth outcomes with gestational PFASs exposure warrants further explorations from biological process perspective.
Key words: perfluoroalkyl substances (PFASs); meta-analysis; low birth weight (LBW); small for gestational age (SGA); preterm birth
Introduction
Perfluoroalkyl substances (PFASs) are a group of persistent organic pollutants with excellent water, oil resistance and chemical, thermal stability[1-3]. These unique properties of PFASs are widely applied in a large number of commercial and consumer products, including grease-resistant paper, non-stick cookware, packaging products, anti-fouling and grease-repellent coatings for textiles, and aqueous film-forming foam (AFFF) used in military and fire training[4-5]. They are ubiquitously found in different environmental matrices and foods, having strong persistence, bioaccumulation capability, and potential toxicity[6-8]. The accumulation of PFASs in human through drinking water, diet, air and dust can lead to intractable endless health issues[9-11], such as cancer, immune system dysfunction, hormone destruction, developmental and reproductive hazards[12-13].
Among all the populations, women and their newborns are quite susceptible to chemical exposure.Inuteroexposure to PFASs is of great concern to maternal-neonatal populations[14-15]. They may increase the risks of gestational hypertension, preeclampsia[16-17], preterm birth[18], miscarriage[19]for pregnant women and have negative effects on the birth weight, thyroid hormone level[20-21], cognitive level[22], early immune function[23]and adiposity[24]for newborns. The previous studies proved that PFASs were able to transport from mothers to the fetus through the placenta[25-26]. The transplacental efficiencies of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) were approximately 70% and 60%, respectively[27]. An increasing number of studies have observed the associations between PFASs exposure and the birth weight, birth length, head circumferences and length of gestations[25, 28-33]. In the long-term, the impairment of reproductive outcomes at birth is associated with adiposity in childhood[24], the higher mean age of pubertal development[34], cardiovascular diseases[35], attention deficit and hyperactivity disorder at 3, 6, 12, 24 months of age[15]. The previousinvitroand animal studies addressed the mechanisms by which PFASs might interfere with the endocrine disruption[36-38]. Metabolomics analysis also proved the role of metabolism in altering birth outcomes due to prenatal exposure to xenobiotics[39].
Although numerous investigations focused on the PFASs transplacental exposure, but the associations between the PFASs exposure and impairment of reproductive outcomes remained inconsistent across studies[33, 40-43]. Meta-analysis has become an ideal model to quantitatively consolidate a weighted average of the point estimates and provides a clear interpretation of existing information[44]. However, to the best of our knowledge, there are only three meta-analysis studies exist regarding the PFASs exposure and fetal growth (two for human cohorts, and another one for mice), and comprehensive knowledge of meta-analysis in association with PFASs is still lacking. Negrietal.[45]combined results from 8 studies and found a change of birth weight of -46.1 g [95% confidence interval (CI): -80.3, -11.9] for an increase of 1 ln ng/mL of maternal or cord PFOS exposure. Steenlandetal.[46]conducted a meta-analysis of 24 studies and found a decrease of birth weight of 10.5 g (95% CI: -16.7, -4.4) for an increase of 1 ng/mL maternal or cord PFOA exposure. Koustasetal.[47]further estimated increasing concentrations of PFOA in pregnant mice (per unit mg/kg) which was associated with a change in mean pup birth weight of -0.023 g (95% CI: -0.029, -0.016). The previous papers only emphasized on the birth weight as pregnancy outcomes. The analysis of decreased birth weight largely focused on the continuous birth weight which seemed “insufficient” for maternal-neonatal cohorts. In fact, the low birth weight (LBW), the small for gestational age (SGA) and preterm birth also explain up to 30% of neonatal mortality and are predictors of lifelong health[48]. Nevertheless, no review has considered the consolidation of associations between the PFASs exposure and SGA or preterm birth. Thus, an updated and multi-layered meta-analysis is urgently needed to comprehensively evaluate the associations and bridge the knowledge gap of epidemiological evidences.
Therefore, due to the wide spread contamination of PFASs and their potential risks to neonates, we have conducted a meta-analysis to evaluate the associations between prenatal PFASs exposure and adverse birth outcomes. The LBW, SGA and preterm birth were selected as the main indicators for birth outcomes[26]. This study aimed to give an overarching evaluation of the existing epidemiological evidences to answer the pragmatic challenges of PFASs exposure association with the fetal growth and development.
1 Methods
1.1 Search strategy
In this study, the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) group were followed for information gathering. The PubMed, Web of Science and ScienceDirect were also searched for all relevant references published before December 2020, using keywords including “PFASs”, “PFOA”, “PFOS”, “birth outcome”, “pregnancy outcome”, “fetal growth”, “SGA”, “LBW” and “preterm birth”. The LBW was defined as 2 499 g or less at birth and might be associated with neonatal mortality and morbidity[49]. Gestational age was determined based on the last menstrual period. Neonates were categorized as preterm birth when gestational age was less than 37 weeks. SGA was defined as a weight below the 10thpercentile of the gestational age and gender[30].
1.2 Selection criteria
In the collected articles’ database, the titles and abstracts of the articles were screened to select the most relevant articles, and the studies that did not focus on the PFASs (PFOS and PFOA) exposure on fetal growth were excluded. The full text of the most relevant studies were then examined, the selected publications should fulfill the following selection criteria.
(1) The epidemiologic study design was cross-sectional, case-control, prospective, or cohort. (2) Studies must be written in English language. (3) PFASs levels had to be measured in actual tissue samples including maternal blood, breast milk, cord serum, cord blood, or serum lipid rather than environmental data or other indirect ways. (4) Studies must be clearly reported these information: a clear study area, year of publication, the number of participants, the OR or RR of LBW, SGA and preterm birth with their 95% CI values. (5) Studies must include complete original data. Case reports, reviews, letters, editorials, and abstract articles were excluded.
1.3 Data extraction and quality assessment
Two independent investigators extracted the data by a standardized collection form. All differences were resolved by discussions. The following information were extracted from the selected articles including study ID, study design, PFAS level, OR/RR and its corresponding 95% CI, selection, comparability, exposure and Newcastle-Ottawa Scale (NOS) score.
The widely accepted NOS was used to evaluate the quality of selected literatures[50]. The full score of this method was 9 points. The quality of the literature was high when the evaluation score was ≥ 8, and the score of medium-quality literature were 5-7 points. All the extracted data and information were organized in Table S1.
1.4 Statistical analysis
The heterogeneity among the studies was quantified using Cochran’s Q test andI2test. TheP<0.10 was considered as statistically significant for Q test, and then we used random-effect analysis. Otherwise, theI2represents the percentage of total variation across studies due to heterogeneity rather than chance. TheI2values of 25%, 50% and 75% interpreted as low, moderate and high degrees of heterogeneity, respectively. The fixed-effect model was conducted whenI2<25% in the absence of significant heterogeneity. TheP<0.10 orI2>25% was usually considered as significant heterogeneity which questioned the validity of pooled estimates[44, 51].
The effect estimates considered for pooling the data were OR and RR for LBW, SGA and preterm birth incidence. The OR and RR were assumed to be having similarity in this study. For the used binary variables, we aimed to consolidate OR and RR from primary studies. For some studies between the highest exposure group and the references one, raw outcome data were pooled to yield unadjusted OR.
All the meta-analyses were carried out by using Stata 14 (Stata Corp, College Station, TX, USA) and Review Manager (RevMan) 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). The LBW, preterm birth or SGA were binary variables. The Mantel-Haenszel test was applied in the inverse of variance for the fixed-effect model, assuming that the results among the studies differ only by sampling error. Dersimonian and Laird method was applied in random-effect model to consolidate the overall binaries with 95% CI values. All the results of the meta-analysis were displayed in forest plots[52].
Potential publication bias was evaluated by Egger’s test and Begg’s test in Stata 14 with metabias mode[53-54]. It could also be presented in the funnel plots with the natural logarithm of the estimate of RR (lg RR) versus the inverse of standard error (1/SE). We also performed a series of sensitivity analysis based on “leave one out” principles, robustness of the summary OR was investigated by excluding one study at a time. These omitted studies were included: the study with the highest and the lowest weight percentages, the study with the highest and the lowest quality scores and the study with the highest and the lowest OR or RR .
2 Results
2.1 Study characteristics and maternal-neonatal exposure of PFASs
The entire retrieval scheme of articles is shown in Fig. 1. According to the selection criteria of the search strategy, a total of 1 169 articles were identified through the literature search. Our exclusion criteria were as follows. (1) A study was not an epidemiological research. (2) PFASs were not target compounds. (3) Environmental medium samples were measured. (4) Birth outcomes were irrelevant to the birth weight or gestational age or there was an absence of dichotomous variables (OR/RR and 95% CI). After that, a total of 17 articles including 148 269 participants were included in the meta-analysis[25-26, 28-33, 40, 55-62]. Information regarding the study design, PFASs exposure levels, OR/RR (95% CI) were all included in Table S1. The prospective birth cohort was the most prevalent study type, accounting for 70.6% in total. Additionally, cross-sectional, case-control and cohorts were found in target studies, and the score for all the included studies were above 7, which indicated medium to high quality levels.

Fig. 1 Flow chart of study selection in the meta-analysis
Results of these 17 studies demonstrated the ubiquitous PFASs in maternal-neonatal cohorts. PFOS and PFOA were quantifiable in 100% of the cohorts, with the median range of 6.05-30.10 ng/mL and 5.38-16.40 ng/mL. Highest PFOS level was observed from two Danish national birth cohorts[29, 40]. In the U.S., median plasma concentrations of prebirth cohort from eastern Massachusetts (25.7 ng/mL) was much higher than two birth cohorts in Mid-Ohio Valley (12.8 ng/mL and 13.9 ng/mL)[57, 61-62]. Compared with the European and U.S. countries, Chinese studies showed lower exposure levels[26, 33, 55, 60]. Highest PFOA concentration was reported in Denmark with mean maternal plasma levels of 35.3 ng/mL[40]. The second highest was given to population from Mid-Ohio Valley, U.S. (median: 21.2 ng/mL)[61]followed by Norway (median: 13.0 ng/mL)[31]. The lowest concentration was found in maternal serum from Guangzhou, China (median: 1.54 ng/mL)[55]. Besides, perfluorohexanesulfonic acid (PFHxS), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDeA), perfluoroundecanoic acid (PFUnDA), and perfluorododecanoic acid (PFDoDA) had been detected in the maternal-neonatal populations.
Factors including maternal age, pregnancy body mass index (BMI), parity, birth gender, smoking habits might also affect the PFASs burden. Levels of PFOA decreased substantially as parity increased in multiple birth cohorts[40, 43, 62]. Xuetal.[26]pointed out primiparous mothers had higher levels of PFOS, PFOA and PFUnDA, which might be related to fetal intake during pregnancy and excretion during lactation. PFASs showed significantly higher levels in girls from 223 mothers and their term infants from China[60]. However, in the Spanish study cohort, higher PFOS exposure was associated with boys who were lighter at birth[30]. Additionally, Darrowetal.[62]reported that women with normal BMI, no previous births, or higher education at enrollment had higher PFOA and PFOS serum levels than other women. Feietal.[40]found that overweight or obese (BMI>22.5 kg·m-2) mothers had higher plasma levels of PFOS and PFOA. After analyzing seven European birth cohorts, Govartsetal.[59]pointed out that smoking mothers were more closely related to PFOS and SGA, while non-smokers had a significantly lower incidence of SGA in newborns.
2.2 Associations between PFOS exposure and adverse birth outcomes
The 13 studies generating a total of 25 OR or RR estimators met the inclusion criteria and were taken into consideration of PFOS exposure and birth outcomes (Fig. 2). A total of six articles related to the association between maternal PFASs exposure and LBW. Based on the fixed-effect model, the exposure to PFOS showed positive association with the prevalence of the risk of LBW. In Fig. 2(a), the combined OR was 1.17 (95% CI: 1.01, 1.36), with low heterogeneity (P=0.30,I2=17%).
A total of 10 articles related to the association between maternal PFASs exposure and SGA. In Fig. 2(b), the combined results of 10 studies indicated consistently null effect of PFOS, the OR point estimates were close to 1 in all different cohorts performed (OR=1.06; 95% CI: 0.82, 1.37;P<0.000 01;I2=76%).
In Fig. 2(c), with regard to preterm birth, the forest plot results also demonstrated positive association between prenatal exposure to PFOS and the risk of preterm birth (OR=1.19; 95% CI: 1.01, 1.39;P=0.007;I2=62%).
In Fig.2, IV refers Inverse Variance method,Tau2refers to Tau-square test.
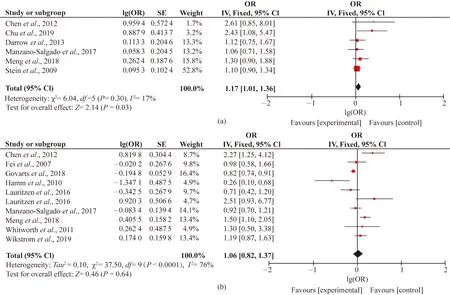
Fig. 2 Forest plots of studies on adverse birth outcomes from PFOS exposure: (a) LBW; (b) SGA; (c) preterm birth
2.3 Associations between PFOA exposure and adverse birth outcomes
A total of nine articles including 10 cohorts related to the associations between PFOA exposure and birth weight (Fig. 3). However, no significant increase in LBW risks had occurred when exposed to elevated PFOA exposure. In Fig. 3(a), the combined OR equaled to 1.02 (95% CI: 0.95, 1.11;P=0.48;I2=0%).
The nine articles focused on the relationships between prenatal PFOA exposure and SGA. In Fig. 3(b), risk estimates for SGA did not increase monotonically across quartiles of PFOA exposure (OR=1.15; 95% CI: 0.90, 1.46;P=0.05;I2=48%). No overall association was apparent in the present results because the direction of SGA and PFOA was inconsistent throughout different cohort cases and some of them were close to null.
The 10 articles focused on the associations between prenatal PFOA exposure and preterm birth. In Fig. 3(c), the combined results of OR showed no statistical significance and with moderate heterogeneity (OR=0.93; 95% CI: 0.77, 1.11;P=0.004;I2=62%). This concurred with some other cases that no evidence of an association between log2(PFASs) and fetal growth for the whole population in Spanish INMA-Project was observed[63].
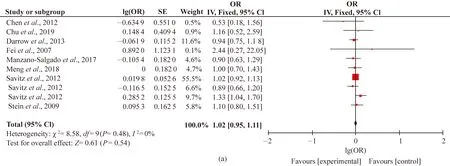
Fig. 3 Forest plots of studies on adverse birth outcomes from PFOA exposure: (a) LBW; (b) SGA; (c) preterm birth
2.4 Associations between other PFASs exposure and adverse birth outcomes
To date, no review paper has been published regarding the other PFASs exposure and birth outcomes. By now, prenatal PFHxS/PFNA exposure and SGA/preterm birth had been reported in only four articles, and their directions seemed variable. As displayed in Figs. S1 and S2, the results did not suggest an overall association between prenatal PFHxS/PFNA and birth outcomes after combining the four estimators. Evaluations of other PFASs and birth outcomes were hampered by insufficient cases (less than three articles), which yield a less evidence of an association.
2.5 Subgroup analysis, publication bias and sensitivity analysis
Study characteristics including the sampling period (first, second, third trimesters and at birth), birth gender (girls and boys), biologic specimens (serum or plasma) and study design (cross-sectional, case-control, prospective, cohort, study) which were chosen as potential sources of heterogeneity. Since high level of heterogeneity (I2>75%) was only found in the associations between PFOS and SGA, we conducted subgroup analysis for this association. When studies were stratified by the sampling periods, heterogeneity and inconsistency among the studies were still very high, withI2values of 31.0%, 72% and 81% for LBW, SGA and preterm birth, respectively (Figs. S3-S5). We only found stronger positive associations between PFOS and preterm birth in first (OR=1.14; 95% CI: 1.04, 1.24), second (OR=1.14; 95% CI: 1.04, 1.24) and third trimesters (OR=2.03; 95% CI: 1.24, 3.32). Therefore, the different sampling periods might not be the source of heterogeneity in this meta-analysis. When the studies were divided by the birth gender, the consistency was observed by the subgroup differences (I2= 0). However, the subgroup analysis also did not show the sex-specific effects of PFOS on the SGA (Fig. 4). When the studies were further stratified by biologic specimen, the high heterogeneity in both the serum (I2=89%) and plasma (I2= 86%) existed (Fig. S6). Because there was only one cross-sectional and case-control study with remaining all cohort studies, subgroup analysis stratified by study design had no significance. In general, the findings from each subgroup analysis did not substantially alter the results of the overall pooled estimated ORs.
Funnel plots of SE versus ln(OR) suggested that risk estimates stemmed mostly from large and precise studies, which were distributed in the superior part of Fig. 5 and Fig. S7). Additionally, publication bias was further assessed by Begg’s and Egger’s tests[53-54]. No evidence of publication bias was observed in Begg’s test for any association cohort, thepvalues for significance, values ranged from 0.089 to 1.000 (pvalues for significance, Table S2). However, the Egger’s test found the publication bias of PFOS-LBW and PFOA-SGA. Meanwhile, other associations did not show an evidence of substantial publication bias withpvalues between 0.057 and 0.997 (Table S3). In the sensitivity analysis, the “leave-one-out” results demonstrated that the estimates were stable. None of the included studies could affect the robustness of current conclusions.
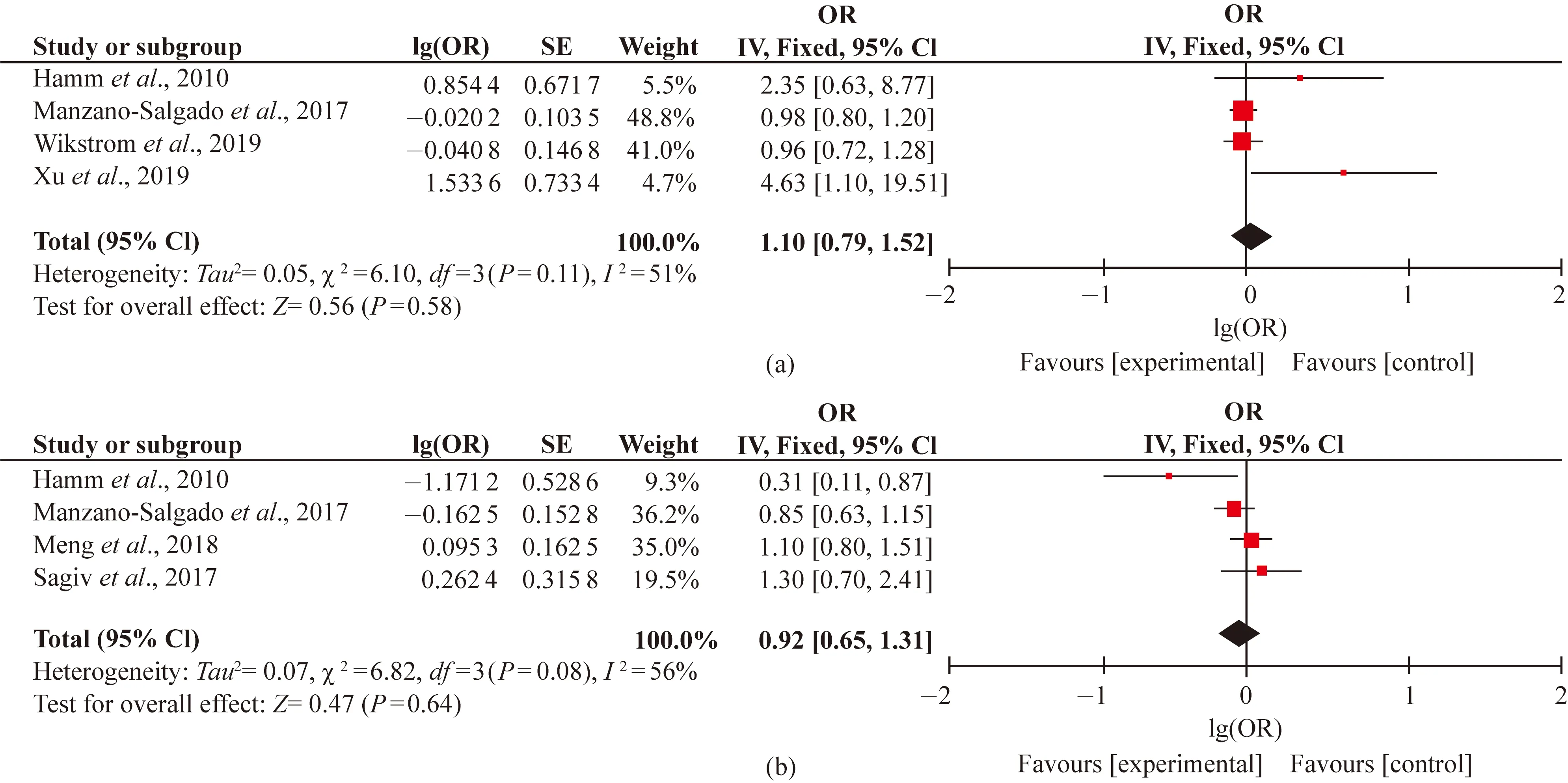
Fig. S1 Forest plots of studies on adverse birth outcomes from PFHxS exposure: (a) SGA; (b) preterm birth

Fig. S2 Forest plots of studies on adverse birth outcomes from PFNA exposure: (a) SGA; (b) preterm birth
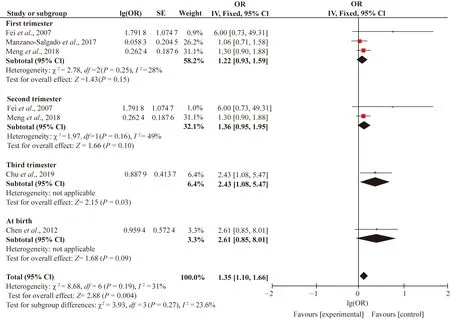
Fig. S3 Subgroup analysis of sampling period of PFOS and LBW

Fig. S4 Subgroup analysis of sampling period of PFOS and SGA
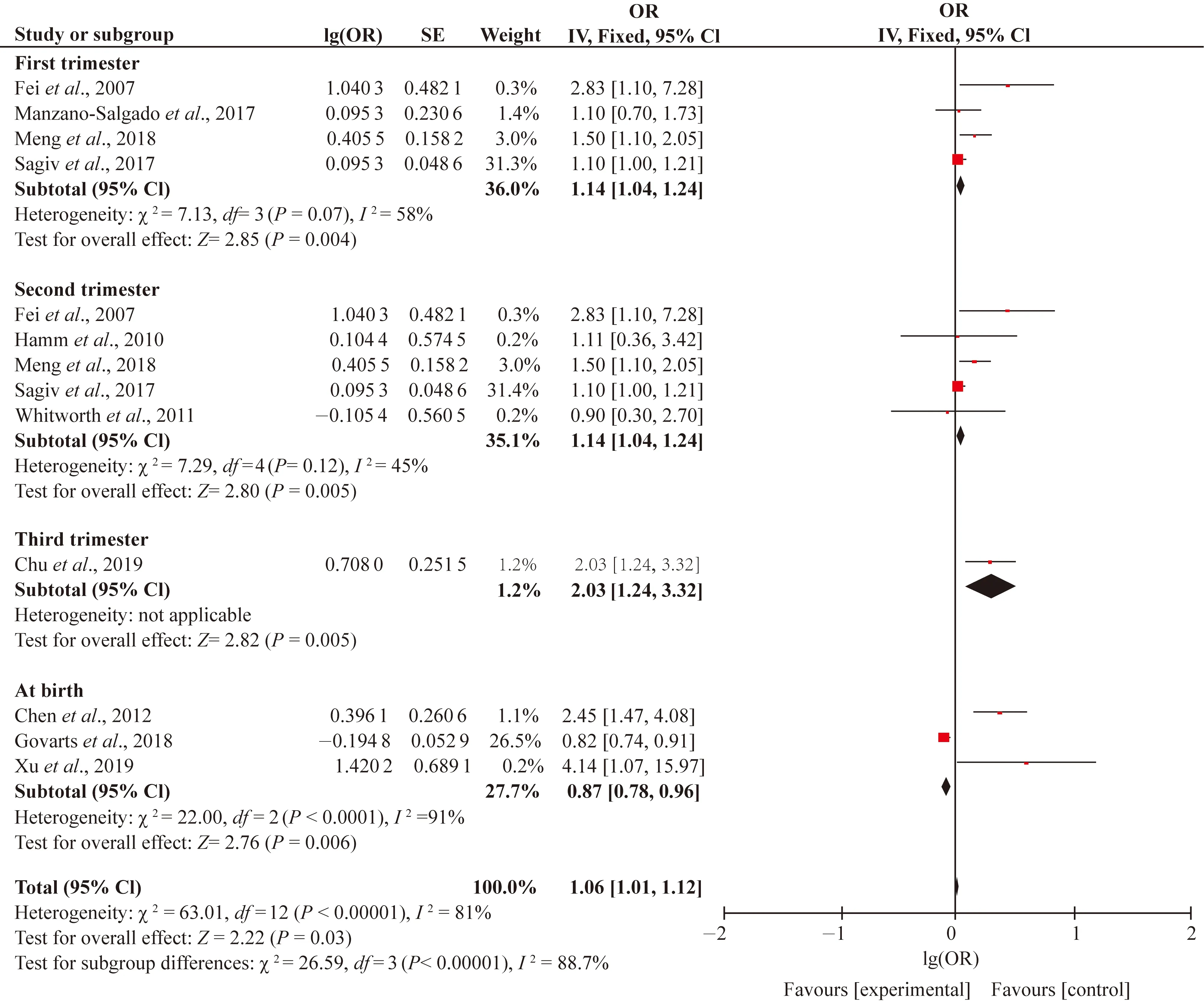
Fig. S5 Subgroup analysis of sampling period of PFOS and preterm birth
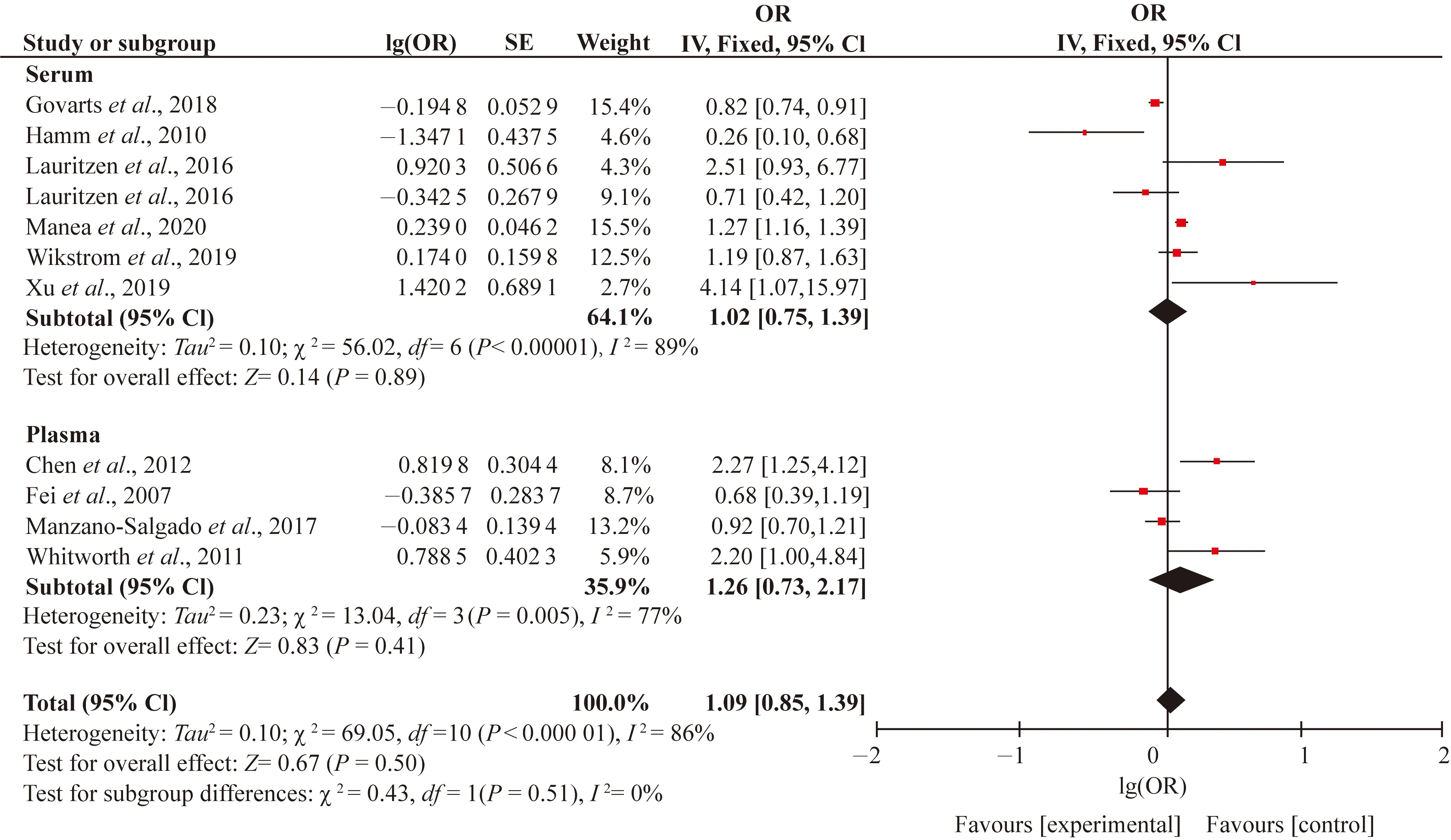
Fig. S6 Subgroup analysis of blood species of PFOS and SGA

Fig. S7 Funnel plots of SE versus lg(OR) for the meta-analyses: (a) funnel plot for the meta-analysis on SGA from PFHxS exposure; (b) funnel plot for the meta-analysis on preterm birth from PFHxS exposure; (c) funnel plot for the meta-analysis on SGA from PFNA exposure; (d) funnel plot for the meta-analysis on preterm birth from PFNA exposure
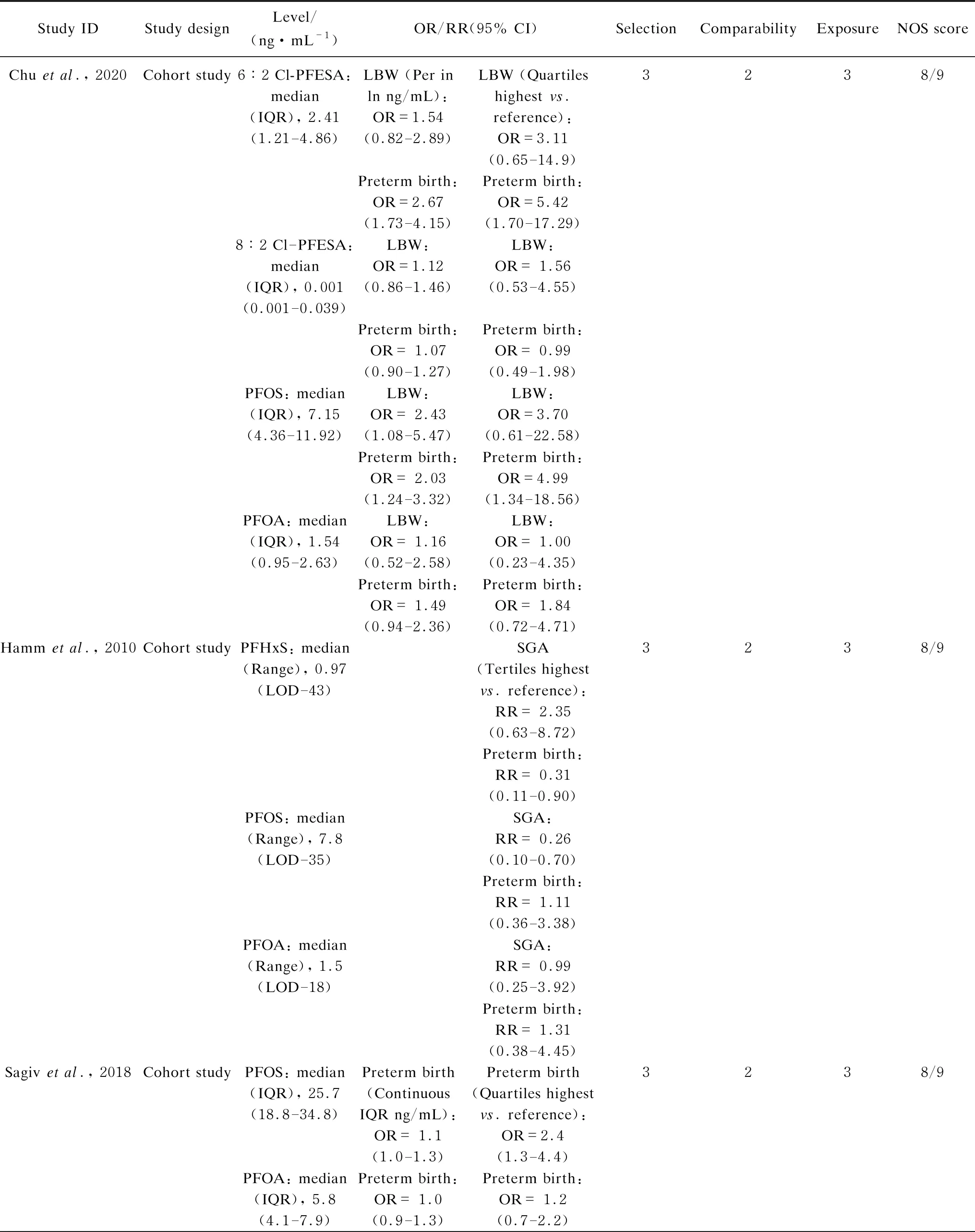
Table S1 Characteristics and quality scores of studies included in the meta-analysis

(Table S1 continued)
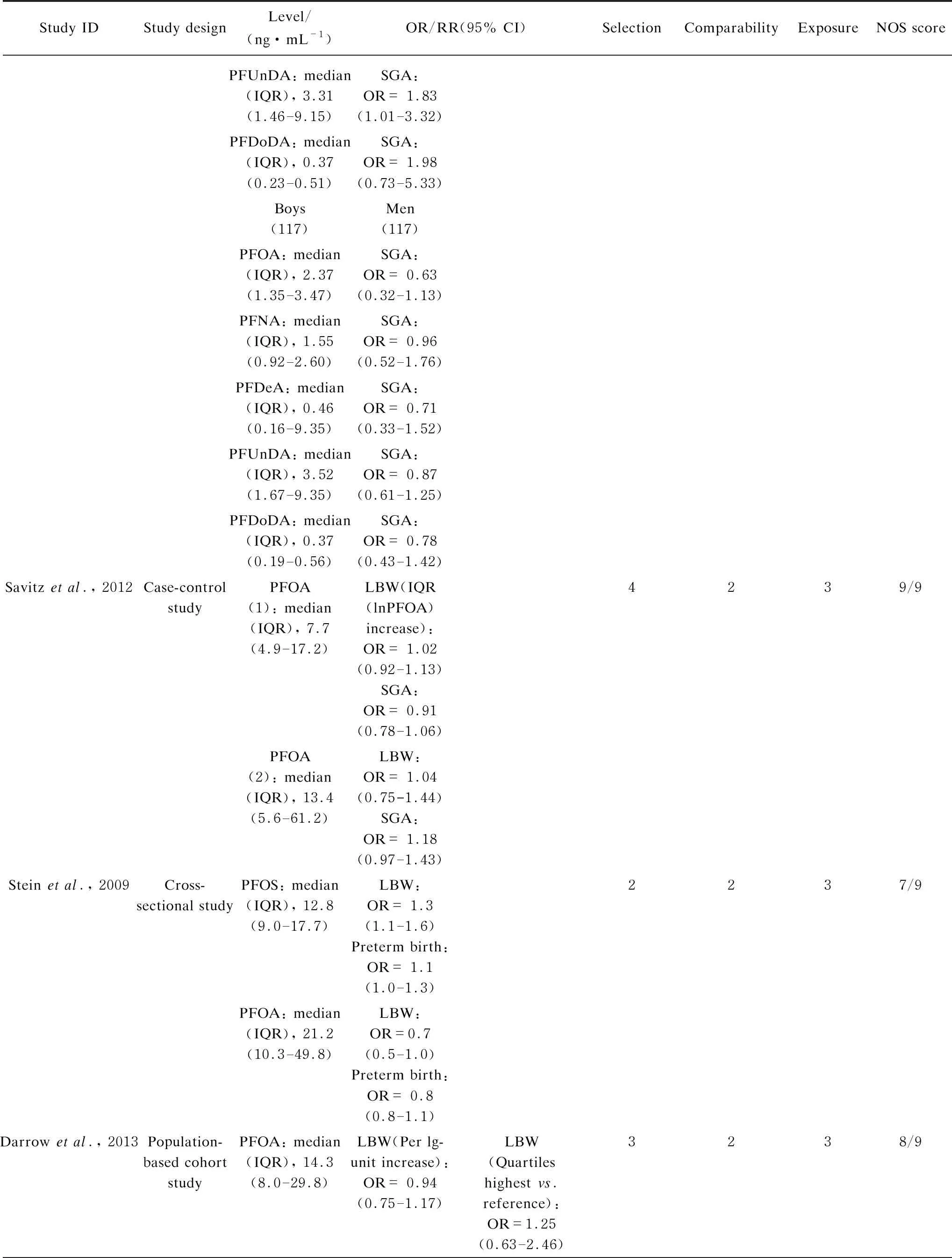
(Table S1 continued)

(Table S1 continued)
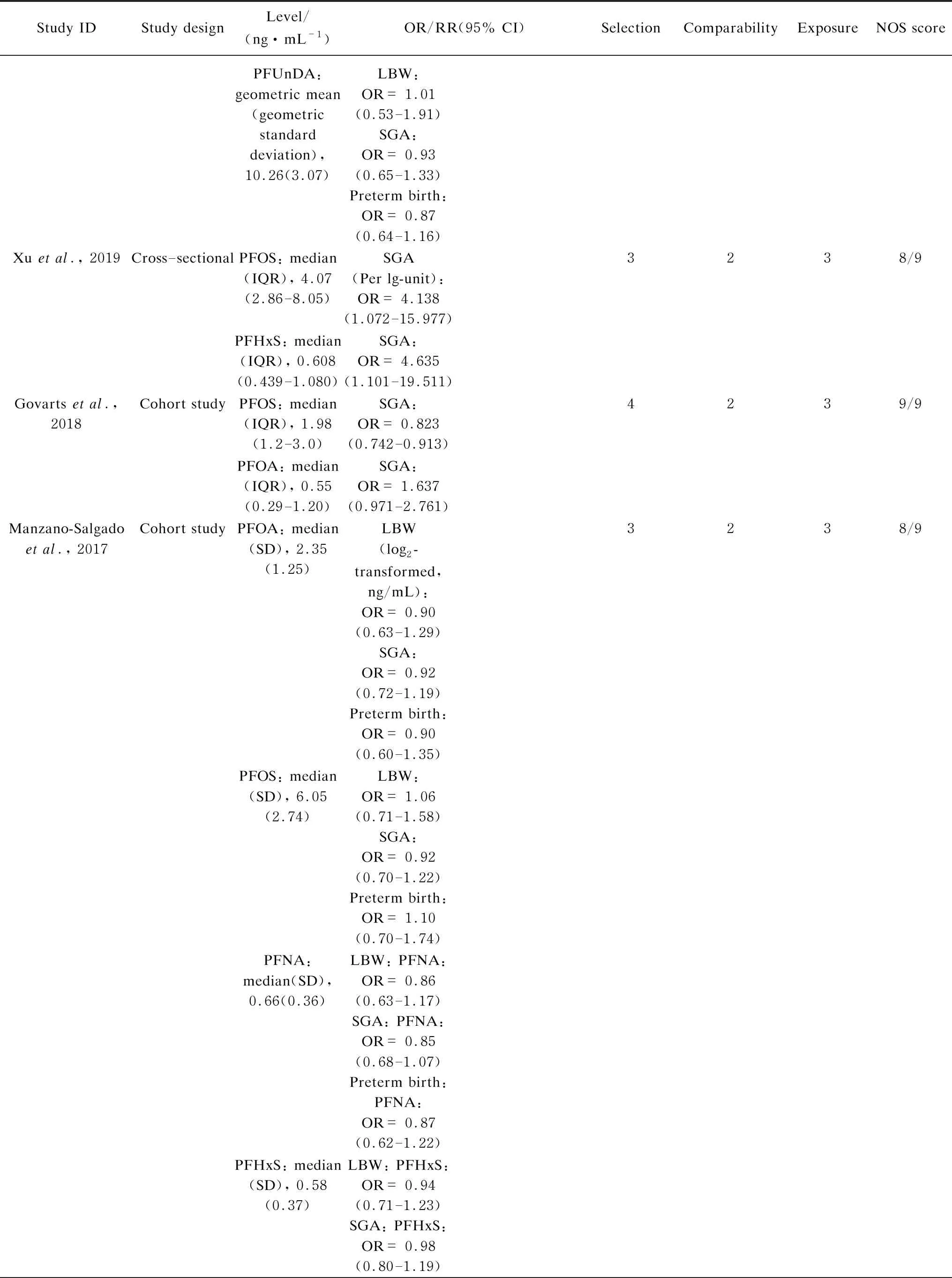
(Table S1 continued)

(Table S1 continued)
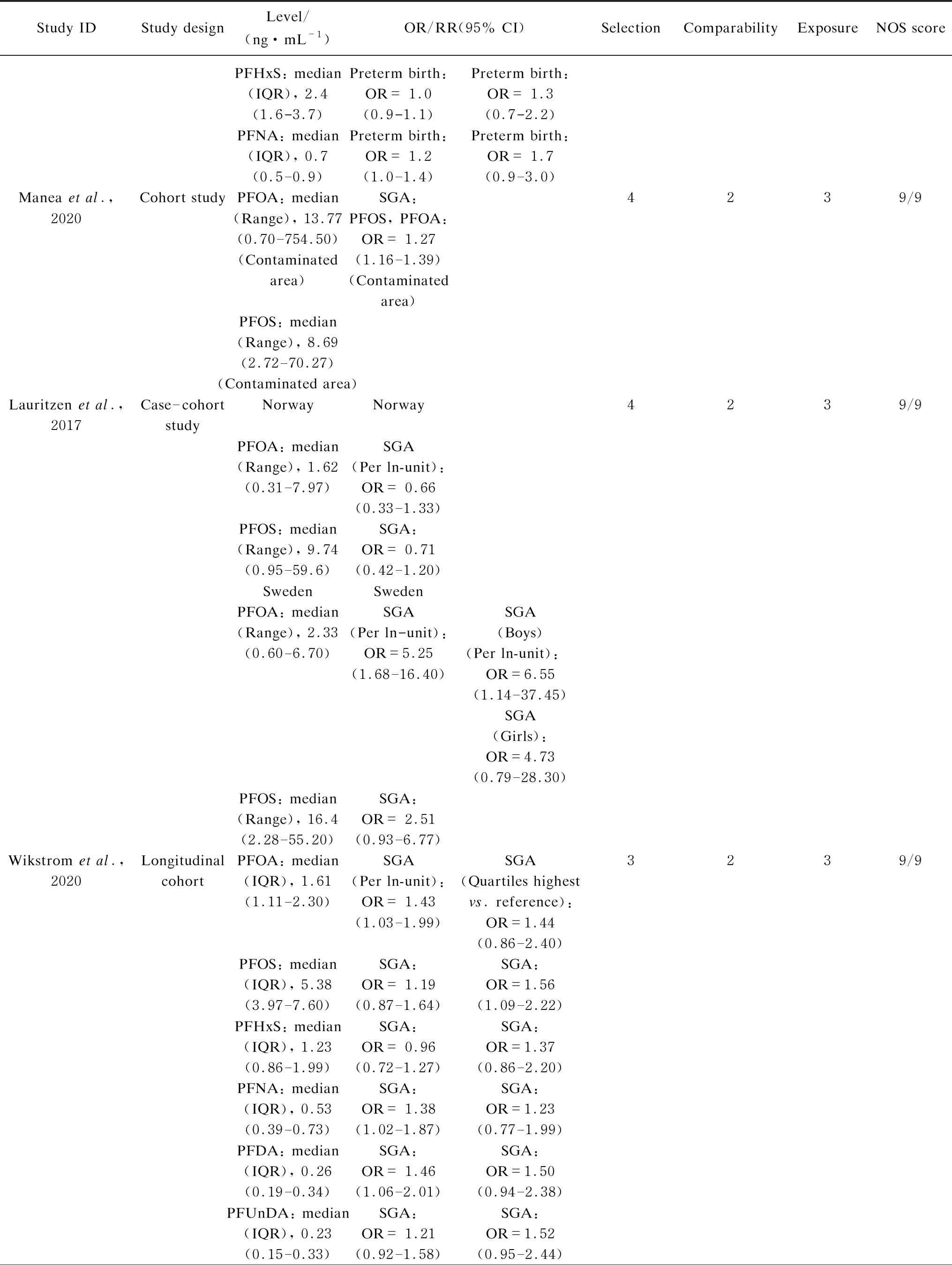
(Table S1 continued)

Table S2 Begg’s test for publication bias

Table S3 Egger’s test for publication bias

Fig. 4 Subgroup analysis of sex-specific effects of PFOA on the SGA

Fig. 5 Funnel plots of SE versus lg(OR) for the meta-analyses: (a) funnel plot for the meta-analysis on LBW from PFOS exposure; (b) funnel plot for the meta-analysis on SGA from PFOS exposure; (c) funnel plot for the meta-analysis on preterm birth from PFOS exposure; (d) funnel plot for the meta-analysis on LBW from PFOA exposure; (e) funnel plot for the meta-analysis on SGA from PFOA exposure; (f) funnel plot for the meta-analysis on preterm birth from PFOA exposure
3 Discussion
This study was the first meta-analysis focusing on the relationship of prenatal PFASs exposure with the LBW, SGA and preterm birth. The quantitative results demonstrated that elevated prenatal PFOS exposure was associated with a higher risk of LBW and preterm birth. There was a paucity of evidence regarding the negative effects of PFOA, PFHxS and PFNA on pregnancy outcomes. No significant sex or smoking difference was observed in the association of the combined meta-analysis in this study. The findings provided some evidence for the associations between maternal PFASs exposure and birth outcomes.
The meta-analysis suggested that elevated prenatal PFOS exposure was associated with higher risks of LBW. The LBW induced short and long-term morbidities and chronic diseases in later life. Recent evidence raised the possibility that the glomerular filtration rate (GFR) may confound selected epidemiologic associations[57]. Verneretal.[64]found a reduced birth weight of 2.72 g (95% CI: -3.40, -2.04) for each 1 ng/mL increase in prenatal PFOS levels. There were sensitive to variations in PFOS distribution and strength of the GFR-birth weight associations[64]. GFR is proportional to fetal size[65], and GFR itself could disrupt the urinary excretion of xenobiotics like PFOS, it was plausible that birth weight reduction resulted from the changes of GFR[64]. Another birth cohort study from Japan suggested an inverse association between PFOS exposure and polyunsaturated fatty acids (FAs) levels in pregnant women[66]. The FAs were regarded as fetuses’ sources of energy, the FAs deficiency resulted from PFOS disruption which might cause metabolic and energy problems and ultimately interfered with fetal growth[67].
The pooled OR for the effect of maternal PFOS exposure during gestation on preterm birth revealed a significant role (OR=1.19; 95% CI: 1.01; 1.39,P=0.007;I2=62%). The most apparent positive associations were observed in cohorts from the Taiwan Birth Panel Study, China and Guangzhou, China. The two studies reported over 2-fold odds of preterm birth with per ln-unit increase in PFOS exposure[33, 55]. Since the neonates with LBW and preterm birth arose the growth restriction and higher risks of death, such as neurodevelopmental delays and cardiovascular disorders[55, 68-69], we had made such conclusion that transplacental exposure of PFOS needs further attention for maternal-neonatal populations.
In this study, we found no significant associations between PFOA, PFHxS, PFNA and pregnancy outcomes. With regard to PFOA, this was in line with another meta-analysis of 24 studies that blood sampled early in pregnancy, which showed little to no associations between PFOA and birthweight. A unit increase in PFOA was associated with a 3.3 g decline in birth weight (β=-3.3 g,βrefers to beta value; 95% CI: -9.6, 3.0)[46], but the association was not significant. In the concluded studies in this meta-analysis, only birth record to the C8 Health Project from Mid-Ohio Valley yields modest supported for the positive associations between LBW risk and per ln PFOA increase[32]. Previous analysis of decreased birth weight largely focused on the continuous birth weight rather than on the LBW or SGA (dichotomous variables). Two previous meta-analyses concluded the reductions in birth weight with per 1 ng/mL increase in serum, a reduced birth weight of 18.9 g (β=-18.9 g; 95% CI: -29.8, -7.9) for per 1 ng/mL increase in serum PFOA and plasma PFOA (β=-14.7 g; 95% CI: -21.76, -7.80) exposure[47, 64]. It was obvious that we could not equate the decreased birth weight (continuous parameter) with LBW, but we could conclude that the PFOA was not consistently related to the risk of LBW[40]. Confounding and reverse causality would be of less concern in these studies. In term of PFHxS and PFNA, it was found that the four included articles reported the relevant information, and the combined OR results showed no correlation with the birth outcome. The results required a further comprehensive review to support.
To our knowledge, no systematic analysis examined that the maternal smoking habits, birth gender and blood sample collection time may change the associations between PFASs and pregnancy outcomes. No significant smoking difference was observed in our study, but relevant information was reported in the related cohort studies[59]. Smoking during pregnancy itself might also have an impact on the birth outcome[70-71]. Therefore, to explore whether the smoking habits of pregnant women would alter the relationship between PFASs and pregnancy outcome, more literature might be needed. Our subgroup analysis did not show the sex-specific effects of PFOA on the SGA. This was in line with a recent meta-analysis that sex was only considered as a confounder in PFOA effects on fetal growth without sex differences[72]. Additionally, in another birth cohort from Taiwan, China, significant associations between PFDeA and PFUnDA and the odds of SGA were only found among girls, with ORs (95% CI) of 3.14 (1.07, 9.19) and 1.83 (1.01, 3.32)[60].
There are several limitations to this study that need to be taken into consideration for the best interpretation of our results. Firstly, our research did not fully analyze the heterogeneous sources. The included studies had differences in population characteristics, exposure times and adjustments to potential confounding factors. This meta-analysis was tracking the heterogeneous source for sampling period (first, second, third trimesters and at birth), birth gender (girls and boys) and biologic specimens (serum or plasma). However, if the clinical, method, and statistics were further subdivided and then analyzed the articles number will be too small to meet the statistical requirements, therefore, we did not carry out the one-by-one analysis. Then, most of the included studies have used the cohort study design, and furthermore the cohort studies were pooled for the combined effects. However, there was only one cross-sectional and case-control subgroup analysis stratified by study design, therefore, no significance was observed. This study was unable to perform heterogeneous source analysis on the study design because some study designs had insufficient samples. Finally, it was difficult to assess the possible effects of multiple PFASs, because there were currently no relevant models for prediction, which warranted further studies.
4 Conclusions
This meta-analysis demonstrated that the risk of LBW increased significantly with the increase of prenatal PFOS exposure. A positive correlation was observed between PFOS and the risk of preterm birth. There was a lack of evidence on the negative effects of PFOA, PFHxS and PFNA on pregnancy outcomes. Subgroup analysis did not show the gender-specific or smoking-specific effects of the PFASs on adverse birth outcomes. However, normal development is highly dependent on thyroid and sex steroid hormones and maternal living habits. These findings extended our understanding of the adverse effects of PFASs exposure on the fetal health and further emphasized that it was crucial to reduce the environmental PFASs pollution and the prenatal PFASs exposure to improve birth outcomes.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Rational Design of a High-Performance KMnO4-Fe(Ⅱ)-Si Coagulant for Dye Removal
- Advances in Electrospun Nanofiber Yarns
- Thermal Insulation Properties of Microfibrillated Cellulose Aerogel
- Mechanical and Thermal Properties of Apocynum and Ramie Fiber Mat Reinforced Polylactic Acid Composites
- Symmetry Classification of Partial Differential Equations Based on Wu’s Method
- Numerical Simulation of Space Fractional Order Schnakenberg Model
