Rational Design of a High-Performance KMnO4-Fe(Ⅱ)-Si Coagulant for Dye Removal
2021-06-22WUQianqian吴倩倩TANGLipeng唐立朋XIAOFengWEIQunshan魏群山LIUYanbiao刘艳彪HUANGManhong黄满红CHOWChristopher
WU Qianqian(吴倩倩), TANG Lipeng(唐立朋), XIAO Feng(肖 峰), WEI Qunshan(魏群山)*, LIU Yanbiao(刘艳彪), HUANG Manhong(黄满红), CHOW Christopher W K
1 College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
2 Textile Pollution Controlling Engineering Center of Ministry of Environmental Protection, Donghua University, Shanghai 201620, China
3 School of Water Resources and Hydropower Engineering, North China Electric Power University, Beijing 102206, China
4 Scarce Resources and Circular Economy (ScaRCE), UniSA STEM, University of South Australia, Mawson Lakes, SA 5095, Australia
Abstract: A new ternary coagulant polysilicate ferromanganese (PSFM), composed of Fe, Mn and Si, was designed. Its coagulation performance for dye removal was investigated systematically together with the impact of several key operational parameters. The experimental results indicate that an excellent coagulation efficiency with higher than 95% color removal and higher than 94% total organic carbon (TOC) removal can be obtained at an optimized molar ratio of Si∶Fe∶Mn (5∶5∶1). The coagulation performance of PSFM coagulant was studied comparatively with other conventional coagulants, e.g. poly aluminium chloride (PAC), Al2(SO4)3 and FeCl3. Additionally, various characterization techniques were applied to get detailed morphological and compositional information of PSFM coagulants. Furthermore, the underlying mechanism for improved coagulant performance was proposed based on these results. These results have exemplified quantitatively that PSFM coagulant exhibits a great potential as a promising material for application in various environmental fields.
Key words: ternary coagulant; coagulation mechanism; polysilicate ferromanganese; high-performance
Introduction
The excessive use of textile dyes is causing serious environmental pollution. It is highly desirable to develop effective and affordable technologies to address this issue[1-3]. Various technologies including adsorption, membrane filtration, biological and electrochemical processes have been employed widely for the removal of dye[4-6]. However, with the continuous rising of dyeing wastewater discharge standards and industrial standards, a single treatment process cannot meet the pressing needs for environmental improvement[7]. Recently, the combined coagulation-flocculation technology has attracted much attention as a promising, affordable and effective combined process for dyeing wastewater treatment[8]. However, the performance of such combined processes is coagulant-dependent. Therefore, it is highly desirable to design new coagulants to boost the overall performance of the coagulation-flocculation processes[9].
Conventional coagulants,e.g., AlCl3, Al2(SO4)3, FeCl3and poly aluminium chloride (PAC), were proven to be effective for the removal of suspended solids and colloidal particles from water[10-11], but they only had limited efficiency for dye removal. Alternatively, a molar rational design of the composition of novel coagulation agents would provide a feasible pathway. For example, the surface charge of coagulants can be controlled by changing the coagulant composition[12-13]. In that case, the coagulation performance can be improved significantly possibly due to a synergistic effect[14]. For instance, the coagulation performance of an Al-Fe mixed coagulant was found to be better than Al or Fe coagulant[15]. Similarly, a polyferric aluminium silicate coagulant also performed more efficiently than pure polyaluminium or polyferric sulfate in removing turbidity, organic matter and total phosphate[16]. Therefore, a molar rational design of a high-performance coagulant with mixed compositions may boost significantly the overall treatment of dyeing wastewater.
However, only limited information is available on the development of ternary coagulants. Fe and Mn are abundant in the natural environment, and they play an important role in transport and transformation of pollutants. Moreover, Mn has strong charge variability, large specific surface area and high redox activity, which can enhance adsorption and coagulation-aid of coagulants on pollutants. Polysilicic acid exhibits a long chain structure, which can effectively enhance the bridging polymerization of coagulants. In this work, a new ternary coagulant, polysilicate ferromanganese (PSFM), composed of Mn, Si and Fe, is produced and applied for textile dye removal. The underlying mechanism for improved coagulant performance is proposed based on these results.
1 Materials and Methods
1.1 Materials
All chemicals and materials were available commercially and used without further purification. Hydrochloric acid (HCl) with a mass fraction higher than 37%, sulfuric acid (H2SO4) with a mass fraction higher than 98% and ethanol absolute (C2H6O) with a mass fraction higher than 99.7% were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Disperse blue 56 (DB-56) with a purity of 99%, PAC with an alkalization degree of 86%, FeCl3·6H2O and Al2(SO4)3·18H2O were purchased from Anpel Experimental Polytron Technologies Inc. (China). Table 1 summarizes the chemical structure and the molecular weight of DB-56. Aqueous solutions were prepared with deionized water (DI-H2O) produced from a Milli-Q Direct 8 systems with resistivity of 18.2 MΩ·cm. PAC, FeCl3·6H2O and Al2(SO4)3·18H2O solutions were prepared freshly before the experiments.

Table 1 Chemical structure and molecular weight of DB-56 used in this study
1.2 Prepration of PSFM
A series of polysilicic acid solutions were prepared in the laboratory. Shortly, a certain amount of Na2SiO3solution was acidified by using sulfuric acid until pH=3 and an active polysilicic acid solution was obtained. The FeSO4·7H2O was fixed at 50 mL and 0.12 mmol/L with 20% (volume ratio) sulfuric acid solution. Then, the FeSO4·7H2O solution was added into the polysilicic acid solution to obtain an active Si-Fe solution. Afterwards a certain amount of potassium permanganate was slowly added into the Si-Fe solution with stirring at 100 r/min for 60 min. Finally, the homogeneous solution was aged in the water bath at 30 ℃ for 24 h. Prior to the coagulation tests, the solution was stored in a refrigerator at a temperature of 4 ℃.
1.3 Coagulation tests
Coagulation tests were carried out by using a ZR4-6 stirrer (Wuhan Meiyu Instrument and Equipment Co., Ltd., China). The temperature, pH and DB-56 concentration were 25 ℃, 7.0 and 50 mg/L, respectively. When PSFM coagulant was added into the water sample, the stirring speed was 250 r/min for 30 s followed by 40 r/min for 10 min. After that, the water sample was allowed to settle down for another 30 min[17]. The dosage of PSFM, FeCl3and polysilicate ferric (PSF) was measured by the molar concentration of Fe element, and the dosage of Al2(SO4)3was measured by the molar concentration of Al element.
A laser diffraction instrument (Mastersizer 2000, Malvem, UK) was used to measure thein-situfloc size as the coagulation and flocculation process proceeded. The median equivalent diameter,d50, was selected as the representative floc size. Floc breakage and regrowth experiments were carried out and repeated three times as follows. A regular coagulation process without sedimentation was followed by a breakage phase at 200 r/min for 5 min and a regrowth phase at 40 r/min for 10 min[18]. Floc size, strength factorSfand recovery factorRf, calculated according to Eqs. (1) and (2)[19-20], were comparatively studied to evaluate the performance of different coagulants. Generally, an increase inRfsuggests that flocs have better regrowth properties over high shear forces[21].
(1)
(2)
wheredais the average floc size of the steady phase before breakage or shear phase,dbis the floc size after the floc breakage, anddcis the floc size after re-growth to the new steady phase.
1.4 Analytical methods
All PSFM coagulants samples were dried in a freeze drier (LyQuest-85, Telstar, Spain) at -42 ℃ for 48 h, and they were ground by using a laboratory mortar and a pestle for further characterization studies. The morphology of PSFM was examined by a JEOL scanning electron microscope (SEM) (Quanta 650, FEI Company, USA). X-ray Diffraction (XRD) patterns of different PSFM samples were measured by using X-ray Powder Diffractometer (RINT-UltimaIII, Rigaku Corporation, Japan) in the 2θrange of 10° to 80° at a scanning rate of 2(°)/min. Fourier transform infrared (FTIR) spectroscopy patterns of PSFM were characterized by using a 550Series II infrared spectrometer (Nicolet iS5, BRUKER Company, Switzerland) in the range of 4 000-400 cm-1. An X-ray photoelectron spectroscope (XPS) (ESCALAB 250, Thermo Scientific, USA) was used to test the surface composition of PSFM.
Before SEM characterization, PSFM powder was bonded to the conductive adhesive and then sprayed with gold. Before XPS characterization, PSFM powder was adhered to the double-sided adhesive, and then pressed at 20 MPa for 1 min by a tablet press.
The coagulation efficiencyRcwas calculated by comparing the absorbance value after treatment to the absorbance value for the original solution shown as
(3)
whereC0andCtare dye concentrations in the original and the treated dye solutions, respectively.
2 Results and Discussion
2.1 Characterizations of PSFM
Figure 1(a) shows that PFSM has a blocky structure and a significantly different microstructure. The surface of PSFM with an amplification of 2 000 times appears to be compact and of irregular bulk clusters[shown in Fig. 1(b)]. The surface microstructure of PFSM is obviously a random crystal flake structure with different sizes. The main part of PSFM coagulant shows a long chain (20.0-50.0 μm) shape and small precipitates (0.1-2.0 μm) are mainly attached onto its side faces[shown in Fig. 1(c)]. Such a long chain shape may be the result of the condensation reaction of dehydrated silicic acid. When hetero-atoms (e.g., Fe and Mn) are introduced, these atoms are either adsorbed, chelated or coordinated with the silicic acid. The energy-dispersive spectrum (EDX) of PSFM suggests the presence of O, Fe, Na, Si, S and Mn on the materials surface[shown in Fig. 1(d)]. The atomic molar ratio of Si∶Fe∶Mn was calculated to be 4.67∶4.78∶0.98, in well accordance with the molar ratio of Na2SiO3∶FeSO4∶KMnO4=5∶5∶1 during the preparation process of PSFM.
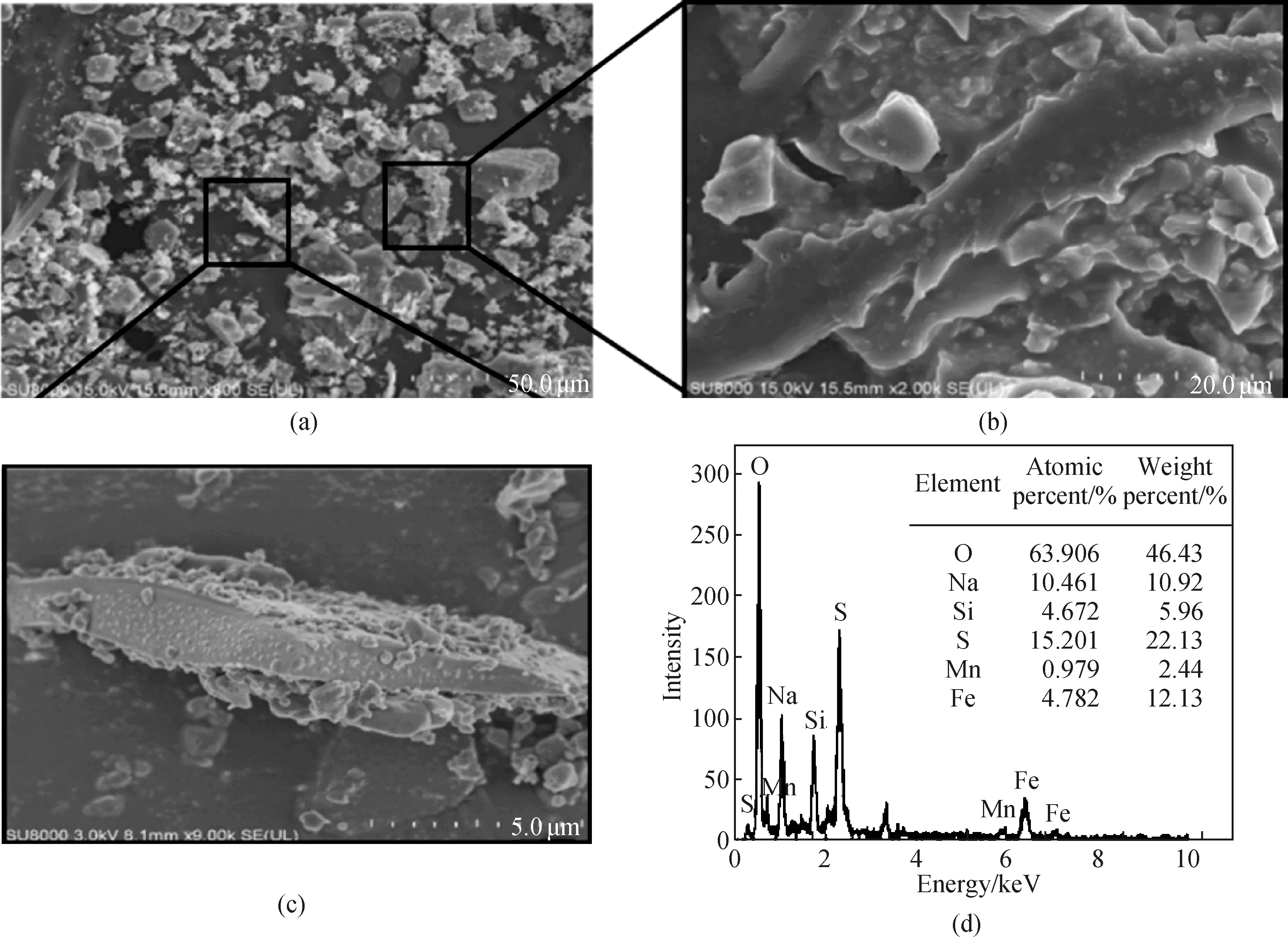
Fig. 1 Characterization of PSFM: (a)-(c) SEM of PSFM; (d) EDX of PSFM
This hypothesis can be confirmed partially by the XRD characterization of PSFM in Fig. 2, which shows different M/Si and Fe/Mn molar ratios namelyRM/SiandRFe/Mn. M represents the total concentration of Fe and Mn. RM/Siis in numerical form andRFe/Siis in scale form to show the increasing ratio of components. The new weak diffraction peaks (2θis 30.9° and 32.8°) appearing at the M/Si molar ratios of 0.5, 0.9, 1.2, 1.6 and 2.0 can be attributed to the increase of Fe and Mn[22-23]compared with the one of the M/Si molar ratio of 0.1. There are also some new weak diffraction peaks (2θis 25.5°, 37.8°, 48.3°, and 52.5°), which could be attributed to silicon oxide at the M/Si molar ratio of 0.1 compared with other M/Si molar ratios[shown in Fig. 2(a)]. The intensity of the diffraction peaks (2θis 22.5°, 23.9°, 30.9°, and 32.7°, respectively) increase to some extent with the increase of the Fe/Mn molar ratio[shown in Fig. 2(b)]. In general, it is suggested that a high proportion of Fe favors the formation of the crystalline phase. Moreover, the spectra of diffractive crystals, such as Fe2(SO4)3, Fe2O3, Fe(OH)3, Fe3O4and SiO2could not be observed. This indicates that Fe, Mn and Si have been polymerized to form a new composite[24]. The new compound PSFM visible in the XRD spectrum cannot be given a standard molecular formula. Obviously, the addition of the silicate to the Fe and Mn solution possibly results in the formation of chemical bonds between Fe—Si, Mn—Si, Fe—O—Si and Mn—O—Si, to some extent, demonstrating that PSFM contains new Fe—Si—Mn species[25]. The presence of these functional groups may contribute positively to the coagulation performance of PSFM materials[26]. The XRD spectra indicate that the major phase of PSFM is similar to the crystal structure of Mn2Fe5Si8O22(OH)2.
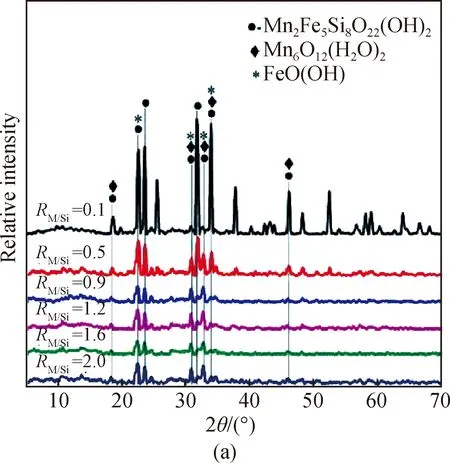
Fig. 2 XRD spectra of PSFM: (a) different M/Si molar ratios; (b) different Fe/Mn molar ratios
Another supporting information for the structure of PSFM was the FTIR analysis (shown in Fig. 3). The FTIR pattern suggests that Fe (Mn) and Si are primarily boundvia—O— or —OH. The strong absorption peaks at 3 432 cm-1originates from —OH stretching vibrations contained in PSFM. The absorption peak at 1 635 cm-1corresponds to the bending vibration caused by the ferric hydroxide contained in the sample[27-28]. The peak centered at 1 172 cm-1belongs to the stretching vibration of Fe—O—Fe/Mn—O—Mn and Fe—O/Mn—O bonds[29]. The bending vibration bands of Si—O—Fe/Si—O—Mn and Si—OH—Mn/Si—OH—Fe bonds are found at 1 039 cm-1and 602 cm-1[30]. The absorption peak of FeO(OH) can be detected at 469 cm-1[31]. In the case of PSFM with an M/Si molar ratio of 0.1 or 0.5 and a Fe/Mn molar ratio of 5∶1[shown in Fig. 3(a)], a peak corresponding to the Si—O—Fe/Si—O—Mn bond vibration is hardly observable, indicating that the Si—O—Fe/Si—O—Mn bond probably disappears under these conditions. Additionally, as the M/Si molar ratio increases[shown in Fig. 3(a)], the intensity of both peaks at 469 cm-1and at 1 635 cm-1also increases, indicating the formation of ferric hydroxide and hydroxyl iron oxide simultaneously. The FTIR spectra of PSFM with different Fe/Mn molar ratios and an M/Si molar ratio of 1.2 are similar, but exhibit a slight shift at 1 039 cm-1[shown in Fig. 3(b)]. Overall, the findings of the FTIR spectra support the statement that new chemical species consisting of Fe, Mn and Si—O have been formed. Furthermore, it is evident that the M/Si and Fe/Mn molar ratios play a significant role in the structure of PSFM coagulant.
The XPS spectra of both survey and high-resolution scans for the key elements, such as Si, Fe, and Mn, on the surface of PSFM were used for the analysis. The spectra of Si, Fe, and Mn[shown in Fig. 4(a)] were detectable. The molar ratio of Fe/Si was 5∶5 and the molar ratio of Fe/Mn was 5∶1. This provides direct evidence that the new composite compound PSFM contains Si, Fe, and Mn. Narrow scan Si 2p spectra revealed a sharp peak at 103.5 eV[shown in Fig. 4(b)]. Mn 2p XPS spectra are shown in Fig. 4(c). The peaks at 642.6 eV and 654.2 eV are assigned to Mn 2p3/2and Mn 2p1/2, respectively, which represent MnO2or MnOOH[32]. MnO2and MnOOH are the primary materials which strongly interact with Si and Fe. The formation of Mn-Si-Fe may also occur, which is in agreement with the experimental results discussed previously. The Fe 2p XPS spectra are shown in Fig. 4(d). The Fe 2p3/2and Fe 2p1/2XPS spectra at 711.8 eV and 726.3 eV are characteristic of Fe(III)[33-34], which represent FeO(OH) or Fe(OH)3respectively. These compounds are the main components of PSFM.
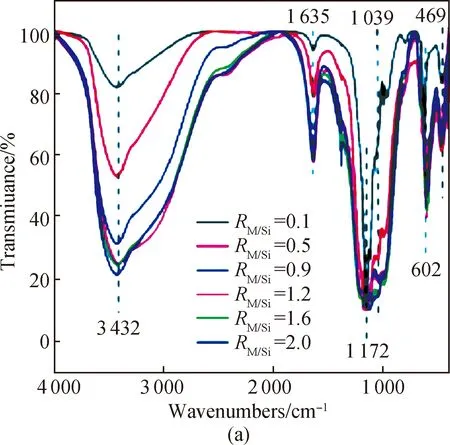
Fig. 3 Infrared spectra of PSFM: (a) different M/Si molar ratios; (b) different Fe/Mn molar ratios
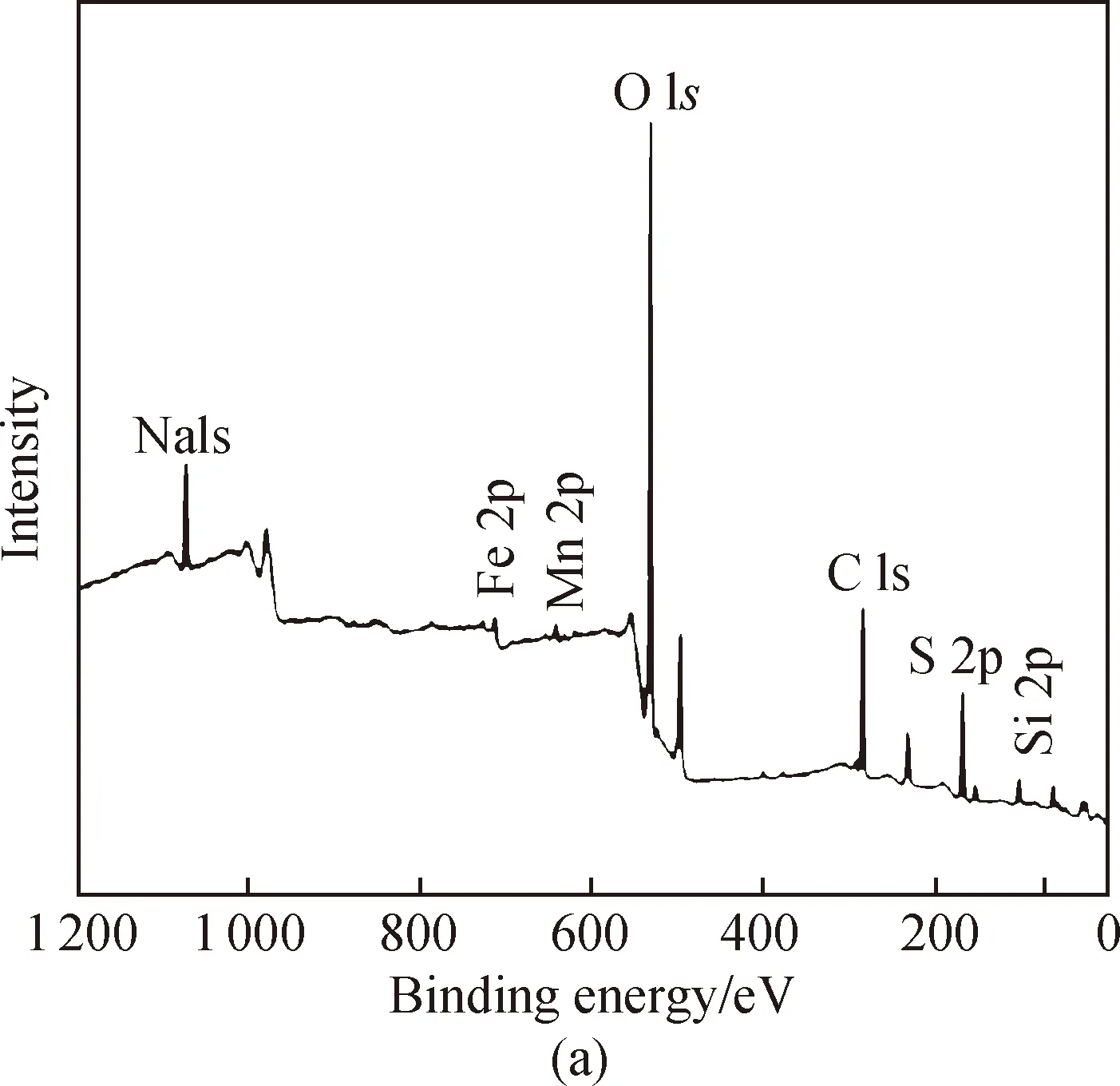
Fig. 4 XPS spectra of PSFM: (a) survey scan; (b) Si 2p; (c) Mn 2p; (d) Fe 2p
Based on the results of the characterization, the as-prepared PSFM material seems to combine the advantages of polysilicic acid, iron and manganese metal ions and their hydrolytic polymers,e.g., bridging polymerization properties of polysilicate acid, and the neutralization caused by polymeric iron and manganese. Thus, a better flocculation performance of PSFM can be envisaged.
2.2 Effect of molar ratios of M/Si and Fe/Mn
As shown in Fig. 5(a), the coagulation efficiency of DB-56 increases with the increase of the M/Si molar ratio from 0.1 to 1.2. Further increase of the M/Si molar ratio contributes negatively to the removal of DB-56. A reduced M/Si molar ratio leads to a limited coagulation effect. In this case, the coagulation performance may have been caused mainly by the bridging polymerization of polysilicate, the van der Waals forces and hydrogen bonding between the anionic groups of dye molecules and PSFM. Furthermore, an increased silicon content will lead to an increased proportion of silicic acid in the agglutinative state, which may cause a rapid gelation of silicic acid[35]. This hypothesis is supported by the almost negligible coagulation observed in the case of an M/Si molar ratio of 0.1. With the increase of the hetero-metal amount, the neutralization ability of PSFM was enhanced significantly. This favors the accumulation of small molecules on PSFM surface. The synergistic effect of the bridging polymerization of polysilicate acid could enhance the coagulation performance of PSFM. If the M/Si molar ratio is too high,e.g., 1.6 or 2.0, the excessive positive charge of the metal ions will repel readily the positively charged organic molecules,e.g., DB-56. The removal of DB-56 increased with the increase of PSFM dosage initially, and then decreased slightly. A proper dosage of PSFM can effectively enhance its coagulation performance. At an optimum M/Si molar ratio of 1.2 and a PSFM dosage of 0.2 mmol/L, the removal of DB-56 could reach 95%.

Fig. 5 Effect of molar ratio on coagulation efficiency of PSFM: (a) different M/Si molar ratios; (b) different Fe/Mn molar ratios
Figure 5(b) shows the effects of the Fe/Mn molar ratio on the removal of DB-56. The coagulation efficiency was found to increase with the Fe/Mn molar ratio from 1∶1 to 5∶1 and it decreased when the Fe content increased further. This finding indicates that PSFM coagulation performance is sensitive to the Fe/Mn molar ratio and the optimal Fe/Mn molar ratio is 5∶1. This also suggests that Fe3+may be the main flocculating contributor in PSFM coagulant, which accelerates the sedimentation speed and improves the compactness of the flocs. Contrarily, if the Fe/Mn molar ratio is low or the content of Fe is low, the contribution of Fe ions to the coagulation is rather limited (e.g.,RFe/Mn=1∶1). At a Fe/Mn molar ratio of 5∶1, the highest removal of DB-56 was found to be 96%. If the Fe/Mn molar ratio increased to 6∶1, the removal of DB-56 decreased slightly. This seems to indicate that Mn ions also contribute to the dye removal.
2.3 Performance comparison with other conventional coagulants
The coagulation efficiency of PSFM was compared with the coagulation efficiencies of PAC, Al2(SO4)3and FeCl3(shown in Fig. 6). At a low PSFM dosage of 0.2 mmol/L, the coagulation efficiency for DB-56 was over 80% and the performance was maintained at this level if PSFM dosage further increased. The coagulation efficiency was 80%, 71%, 5% and 4% respectively for 0.2 mmol/L PSFM, Al2(SO4)3, FeCl3and PAC. The relatively enhanced coagulation performance of PSFM may be due to the synergistic effect of bridging polymerization and neutralization. The contribution from hydrated manganese dioxide, iron hydroxide and other polymers can also not be excluded[36-37]. The above analyses show that the coagulation efficiency of PSFM is higher than that of PAC, Al2(SO4)3or FeCl3.
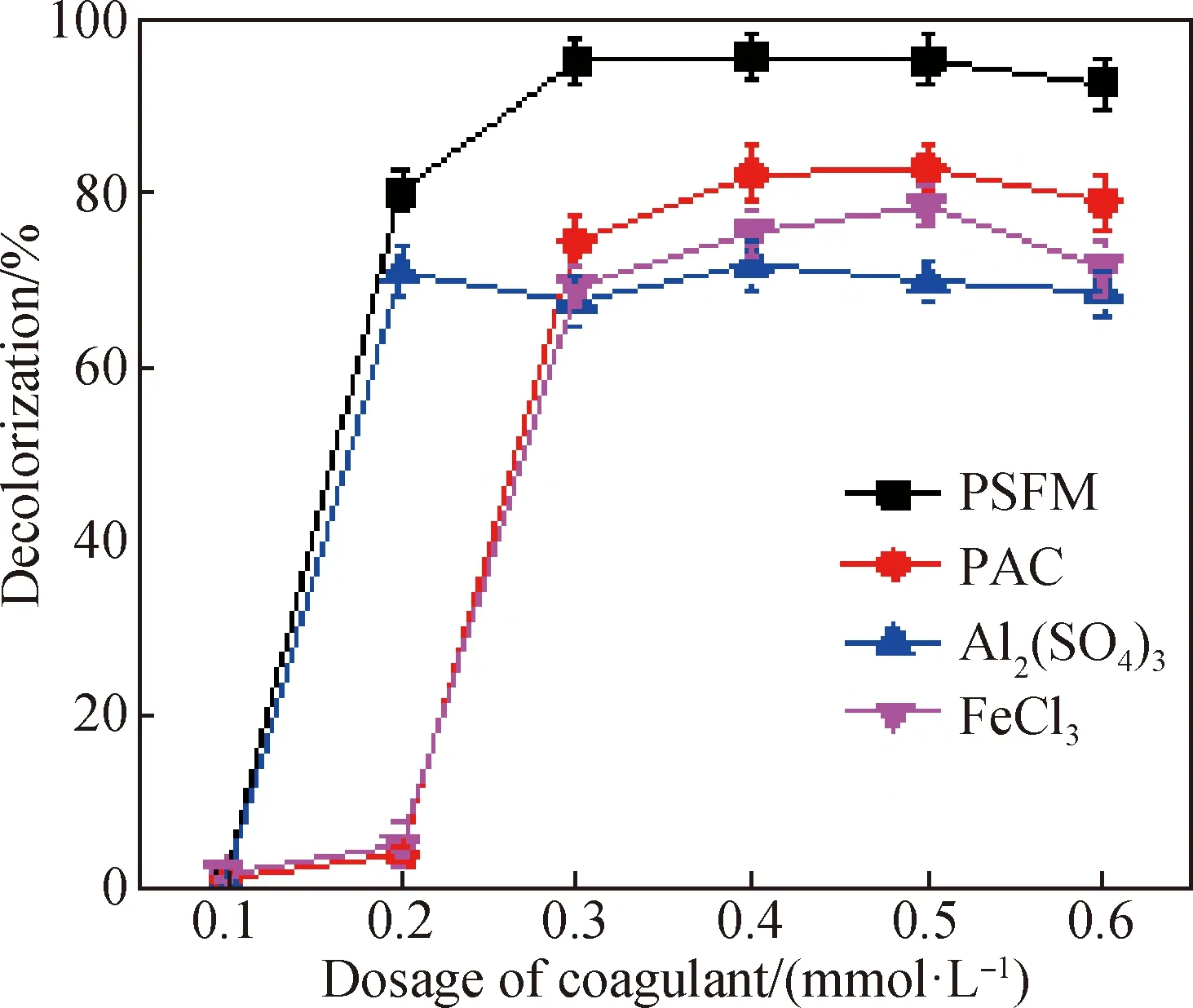
Fig. 6 Coagulation efficiency using different coagulants
In order to further study the coagulation mechanism of PSFM, the particle size, fragmentation and regrowth of flocs were also systematically investigated (shown in Fig. 7). The 50% value in the mass-based size distributiond50was selected as an indicator of the floc size[38]. Compared to the other three coagulants, PSFM flocculation exhibited a faster floc growth rate and a larger floc size than others. The floc size formed by PSFM, FeCl3, PAC and Al2(SO4)3was 245, 200, 185 and 145 μm, respectively.

Fig. 7 Coagulation performances using different coagulants
Figure 7 indicates that the floc sizes after the re-growth period complied with the following order:d50(PSFM)>d50(FeCl3)>d50(PAC)>d50[Al2(SO4)3]. The strength and recovery factors of flocs formed by different coagulants are shown in Table 2. Floc growth, breakage and re-growth process can be interpreted as follows. During the first 2 min, the floc size increases sharply due to charge neutralization between Fe (Mn) and negatively charged particles. As the coagulation process continues, the flocs aggregate to larger flocs (d50≈ 245 μm for PSFM), possibly due to the physical enmeshment of the DB-56 within the coagulant precipitates and chemical sorption. If the slow-mixing continues, the floc size gradually decreases to 130 μm, probably due to a disturbance of the aggregated flocs. The descending order for the recovery factor isd50[Al2(SO4)3]>d50(PSFM)>d50(FeCl3)>d50(PAC). The flocs formed by Al2(SO4)3and PSFM cannot be easily broken and have a better regeneration ability than those formed FeCl3and PAC. Studies show that the flocculation particles formed by electric neutralization are easier to be recovered than those formed by sweep flocs[39-40], which have a poor re-growth after breakage[41]. The neutralization abilities of Al2(SO4)3and PSFM coagulants are more evident compared with those of FeCl3and PAC coagulants. The strength factor has a descending order:d50(FeCl3)>d50(PAC)>d50(PSFM)>d50[Al2(SO4)3]. Although the recovery factor of Al2(SO4)3is higher than that of PSFM and the strength factor of FeCl3and PAC is higher than that of PSFM, the adsorption and the coagulation of the Fe-Mn complex in PSFM improve its performance. Based on the above findings, charge neutralization is not the only mechanism involved in PSFM flocculation process. The coagulation-aid of the Fe-Mn complex in PSFM may also play an important role.

Table 2 Strength factor and recovery factor of flocs formed by different coagulants
2.4 Coagulation mechanism of PSFM
The improved coagulation efficiency of PSFM for organic pollutants is related to its unique composition. According to FTIR and XRD data, the main component of PSFM is a polysilicate Fe-Mn polymer, hydrated manganese dioxide and hydroxyl iron oxide. The bridging polymerization of polysilicic acid and the electric neutralization of Fe and Mn ions play a synergistic role. According to the floc size analysis, the size formed by PSFM is higher than that of the few selected traditional coagulants. Coagulation aid caused by hydrated manganese dioxide and iron hydroxide makes the floc in the coagulation process to grow into large flocs. Studies have shown that the Fe-Mn complex has a high redox activity, and the manganese dioxide has a certain selective oxidation performance. In the coagulation process by PSFM, there may be newly-formed manganese dioxide, which exerts oxidation efficiency on organic pollutants. In the process of coagulation, the oxidation of organic pollutants by manganese dioxide may also support the process of coagulation by PSFM. Therefore, the enhanced coagulation efficiency of PSFM is very excellent, making PSFM to be a new type of multi-functional coagulant. The relevant coagulation mechanism of PSFM is summarized in Fig. 8.

Fig. 8 Mechanism of coagulation process by PSFM
3 Conclusions
A high-performance PSFM coagulant was designed via a copolymerization route. The characterization results reveal that PSFM is composed of a polysilicate-ferromanganese polymer, manganese dioxide, iron oxyhydroxide and other polymers. These contents synergistically strengthen the coagulation performance of PSFM. Its coagulation performance towards the removal of a model compound is better than that of PAC, Al2(SO4)3, FeCl3or other conventional coagulants. Such a new coagulant has a great potential for application with various environmental problems.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Electrochemical Performance of Core-Sheath Dip-Coated Lignin Derived Carbon/Wet-Spun Graphene Electrodes for Fiber-Shaped Supercapacitors
- Preparation and Properties of Polyurethane/Nickel Nanofiber with Core-Shell Structure Using Coaxial Electrospinning Method
- Trajectory Optimization and Motion Simulation of a Flexible Polishing Industrial Robot for Watchcases
- Effect of Supported Bearing Clearance on Load-Sharing Characteristics of a Double-Row Planetary Gear System
- Meta-Analysis on the associations between Prenatal Perfluoroalkyl Substances Exposure and Adverse Birth Outcomes
- Numerical Simulation of Space Fractional Order Schnakenberg Model
