Effects of geosorbent and solution properties on sorption and desorption of PAHs
2021-06-21HuiDongBaohuaXiao
Hui Dong·Baohua Xiao
Abstract Characteristics of phenanthrene and pyrene’s sorption and desorption on two local soils in solutions of simulated groundwater,simulated lung fluid,and simulated saliva were studied with batch equilibrium experiments to understand the fate of PAHs in the karst region of southwestern China and to assess the environmental exposure and the health risk of PAHs.The results showed that the sorption and desorption isotherms of phenanthrene and pyrene on two target soils in the three solution systems could be adequately described by the Freundlich model,while the fitted isotherm parameters for the simulated groundwater solution distinguished notably from those for the simulated body fluid solutions.For the sorption experiments,in the simulated groundwater,the n values were 0.722 and 0.672 for phenanthrene and were 0.724 and 0.663 for pyrene,respectively,on the yellow soil and the limestone soil;The log KF values were 3.118 and 3.323 for phenanthrene and were 3.648 and 3.846 for pyrene,respectively,on the yellow soil and the limestone soil.In the simulated body fluids,the n values for phenanthrene and pyrene ranged from 0.622 to 0.836 and from 0.590 to 0.865,respectively,and the log KF values of phenanthrene and pyrene ranged from 2.845 to 3.327 and from 3.344 to 3.779,respectively.For the desorption experiments,in the simulated groundwater,the n values were 0.662 and 0.744 for phenanthrene and were 0.702 and 0.647 for pyrene,respectively,on the yellow soil and the limestone soil.The log KF values were 3.666 and 3.686 for phenanthrene and were 4.128 and 4.225 for pyrene,respectively,on the yellow soil and the limestone soil.In the simulated body fluids,the n values for phenanthrene and pyrene ranged from 0.612 to 0.668 and from 0.631 to 0.819,respectively,and the log KF values of phenanthrene and pyrene ranged from 3.134 to 3.407 and from 3.533 to 3.839,respectively.The limestone soil had relatively higher log KF values but lower KOC values compared to those of the yellow soil,indicated that the nature of sorbent soils played the dominant role in sorption and desorption behaviors of PAHs.The experimental results showed a remarkable differences in sorption and desorption behaviors of PAHs in simulated body fluids and groundwater.The nonlinearities of measured isotherms and the measured sorption capacities of soils in simulated body fluids were significantly lower than corresponding those in the simulated groundwater,and HI values for simulated body fluids systems were significantly smaller than corresponding those for the simulated groundwater systems.The results underscore cautions in assessing environmental exposure and health risks of PAHs based on their sorption-desorption data in simulated groundwater as this is traditionally done.
Keywords PAHs·Sorption-desorption·Simulated body fluids·Yellow soil·Limestone soil
1 Introduction
Polycyclic aromatic hydrocarbons(PAHs)as a type of anthropogenic pollution are formed mainly during incomplete combustion of fossil fuels and biomass and exist ubiquitously in the natural environment.PAHs are a class of chemicals containing only carbon and hydrogen with structures of multiple aromatic rings.Several PAHs are considered to induce carcinogenic,mutagenic,and teratogenic effects on humans(Barnier et al.2014).Environmental PAHs concentrations have increased in many industrialized and developing countries in decades.The high emissions of PAHs,more than 116,000 tons/year,resulted in heavy contamination of various environmental media(Wang et al.2011a,b;Xia et al.2013).Due to the hydrophobicity,PAHs tend to sorb on soil and sediment particles while their aqueous concentrations are relatively low(Wilcke 2000;Ni et al.2008;Yang et al.2014;Yu et al.2014).Sorption and desorption on soil particles are important processes governing the fate of PAHs in the environment.A comprehensive understanding of sorption and desorption behaviors of PAHs in soils and sediments is a prerequisite for accurately evaluating their environmental risk and human exposure.
Soil,sediment,and aquifer materials are highly heterogeneous and comprise various inorganic and organic components,in which the soil organic matter(SOM)is the primary sorptive component for PAHs.SOM consists of a wide range of organic molecules formed through various oxidation,reduction,condensation,and polymerization processes under different biological and geological environments(Huang 1997).The sorption of hydrophobic compounds from water to SOM is thermodynamically driven primarily by the hydrophobic effect which is caused by water molecules in solution forming an ordered‘cage’around hydrophobic portions of the organic molecule(Pignatella 1998).Previous research had proven that SOM is the dominant component for HOCs sorption,and the SOM heterogeneity has major impacts on the rates and extents of sorption.A conceptual dual-domain model has been proposed which suggested that SOM may consist of two physically and chemically different types of domains.In the hypothesis,a‘soft’rubbery amorphous domain contains fulvic acid and humic acid which are characterized by linear sorption and desorption,while a‘hard’glassy condensed domain may consist of kerogen,black carbon,and humin which are responsible for nonlinear sorption and desorption(Weber and Huang 1996;Xing et al.1997;Xiao et al.2004).
The physical conformation of SOM could be one of the key factors affecting the interaction between SOM and hydrophobic organic chemicals(HOCs)like PAHs.Cornelissen and Gustafsson(2005)summarized the sorption properties for a wide range of carbonaceous geosorbents.The contribution of carbonaceous geosorbents to the overall sorption of a given HOC varied over a wide range,which was led by different physicochemical properties.SOM originated from a different source has quite different structural and physicochemical properties,and this heterogeneous nature should have a strong impact on equilibria and rates of HOC’s sorption and desorption.Quality of SOM significantly influenced the sorption-desorption of HOCs by soils.The value of organic carbon normalized distribution coefficient(K)increased with increasing aromaticity of organic phases while decreased with increasing effective polarity.For example,young,surface soil organic matter had lower sorption capacity than the old organic matter in shale(Xing 1997).The lower the H/C atomic ratio of the SOM is,the more aromatic and hydrophobic the SOM is,and the greater the driving force is for sorption.SOM with a lower H/C ratio coupled with greater cross-linkage of the matrix could exhibit stronger site-limited sorption at external and internal surfaces(Huang and Webber 1997).
Among the various types of SOMs,geological matured humic materials,kerogen,for example,have been shown to play important roles in HOC sorption(Huang 1997).Some studies showed that sorption of HOCs by SOM was controlled by aromatic carbon(Chin et al.1997;Perminova et al.1999;Abelmann et al.2005;Pan et al.2007),while others indicated that the humin fraction of SOM has a significantly higher affinity with HOCs than other components(Ding et al.2002;Gunasekara and Xing 2003;Pan et al.2007).Humic acid aggregates could be considered as an analogy to well-structured organic polymers(LeBoeuf and Weber 1997,2000a,b;Young and LeBoeuf 2000;Huang et al.2003).Humin may consist of complex SOM ranging from unaltered or less-altered biopolymers such as lignin,polysaccharides,mineral-bound lipids,and humic acid-like materials(Huang et al.2003).It is known that resistance to molecular diffusion within the pores whose sizes are slightly larger than sorbate molecules is much greater than within the pores of sizes several times larger than sorbate molecules.Sorptive reactivity exhibited different processes because of different origins and heterogeneous physicochemical properties.The rates and equilibria of sorption/desorption appear to coincide with the types of SOM and physicochemical properties.Humic and fulvic acids are amorphous with the higher O/C and H/C ratios and could be swollen by water molecules,sorption onto this domain would be fast and linear.In contrast,humin is condensed with lower O/C and H/C ratios,its sorption isotherm would be nonlinear,and the rate is relatively slow.
It is often observed that desorption isotherm is not the same as sorption isotherm,suggesting the existence of hysteresis.The hysteresis has been reported for many compounds,including PAHs,chlorinated benzenes,phenols,pesticides,and PCBs(Pignatello 1990;Kan et al.1994),which could be a consequence of slow sorption or desorption kinetics(Wu and Gschwend 1986;Brusseau and Rao 1989;Ball and Roberts 1991).The equilibrium distribution of HOCs was believed to be a result of a partitioning process controlled by soil organic carbon(Chiou et al.1979).For PAHs,sorption has been correlated with the degree of aromaticity in soil humic materials(Carmichael et al.1997).Sorption and desorption of HOCs typically include an initial rapid phase followed by a slower phase to equilibrium.The degree of hysteresis was greater for higher molecular weight PAH on the same soil,and on higher organic soil.Ghosh et al.(2001)had demonstrated for Milwaukee Harbor sediment that PAHs associated with clay/silt particles desorbed faster with low desorption activation energies,while PAHs associated with coal-derived material desorbed at a much slower rate with high desorption activation energies.Several previous studies showed that a fraction of sorbed HOCs desorbed very slowly(Kan et al.1994;Carmicheal et al.1997;Yang et al.2008,2014;Luo et al.2012),which means desorption hysteresis exists in interaction processes between HOCs and soil/sediment particles.Heterogeneous sorption with varied sorption sites has been proposed to explain the persistent release of HOCs,whereby a fraction of HOCs is assumed to sorb on sites with high sorption energy or specific sorption sites on an organic polymer(Kan et al.1998).Prior research had shown that entrapments of sorbed molecules within SOM and inorganic matrices are possibly the major mechanisms for the apparent hysteresis observed for HOCs(Huang and Weber 1998;Huang et al.1998;Weber et al.1998;Farrell et al.1999).
PAHs are important contaminants in the karst area of southwest China since the wide and longtime of uncontrolled coal burning in this area.However,only a few studies had been done for assessing the environmental exposure and health risk of PAHs in this area.Although sorption and desorption behaviors of PAHs on geosorbents like soil and sediment have been extensively explored and underlying mechanisms have been recognized in general,the high variation of sorptive behaviors of PAHs on soils related to the high heterogeneity of soil demands much more specific data to accurately assess environmental exposure and following health risks of PAHs in a specific region.This study aimed to characterize sorption and desorption properties of PAHs on two most common soils of the karst area of southwest China,and to evaluate impacts of physical and chemical differences of soils and different aqueous solutions on sorptive behaviors of PAHs and to discuss their environmental exposure and health risk in the karst area of southwest China.
2 Materials and methods
2.1 Sorbents and sorbates
Two surface soils,the yellow soil(YS)and the lime soil(LS),were selected used as the sorbents.They were selected since they are the most common soils of the study area in southwest China and can be obtained easily.The yellow soil sample was collected at the Duxi forest park in Guiyang,China,while the lime soil sample was collected at the Maolan national forest park in Libo,China.Detailed information about the soil samples was provided elsewhere(Di et al.2018).The soils were collected from the surface(0-12 cm),air-dried,gently ground,and let to pass through a 10 mm mesh sieve.The selected physicochemical properties of soil samples are listed in Tables 1 and 2.Two typical PAHs,phenanthrene and pyrene,were chosen as the sorbates.They were chosen because they are among the most common PAH pollutants in the study area,and their sorption properties have been extensively characterized in prior studies,which may help in comparing and better understanding interactions between PAHs and local soils.Both chemicals in HPLC grade were purchased from Sigma-Aldrich,Inc.
2.2 Solutions
The simulated groundwater solution,which is the commonly deployed aqueous solution,contained 0.005 M CaCland 0.001 M NaHCOto mimic its natural conditions.NaNat a level of 100 mg/L was used as the inhibitor for microorganism growth.The simulated body fluids,lung fluid,and saliva were prepared as suggested in the literature(Stopford et al.2003;Dean and Ma 2007;Colombo et al.2008).The compounds and their contents in simulated lung fluid and saliva are presented in Table 3.The compounds of simulated lung fluid were added exactly as the order presented in Table 3 to avoid salt precipitation,and then the solution was adjusted to pH 7.2 using a HCl solution(0.1 M).Similarly,the compounds of the simulated saliva were also added exactly following the order in Table 4 to avoid salt precipitation,and then was adjusted to pH 6.5 using a NaOH solution(1 M).For both simulated body fluids,NaNat the level of 100 mg/L was added as the inhibitor for microorganism growth.All biochemical reagents in their highest available grade were purchased from Sigma-Aldrich.
2.3 Sorption and desorption experiments
The sorption and desorption experiments were conducted in dark in amber glass vials(40 mL)equipped with Teflon-lined caps(Fisher Scientific)on a temperature-controlled shaker(SY-550B,Taisite Instrument,China)with a rotation speed of 120 rpm.The sorbates,phenanthrene and pyrene,were introduced into the glass vials by spiking predetermined volumes of stock solutions to achieve a series of initial concentrations.The original stock solutions of phenanthrene(1000 ppm)and pyrene(150 ppm)were prepared by dissolving determined amount of each chemical in a 100 mL glass flask with methanol(HPLC grade),and then original stock solutions were diluted sequentially with methanol to make stock solutions of desired concentrations.The operation procedures of the sorption and desorption experiment with simulated groundwater,simulated lung fluid or simulated saliva were identical except the applied solutions.
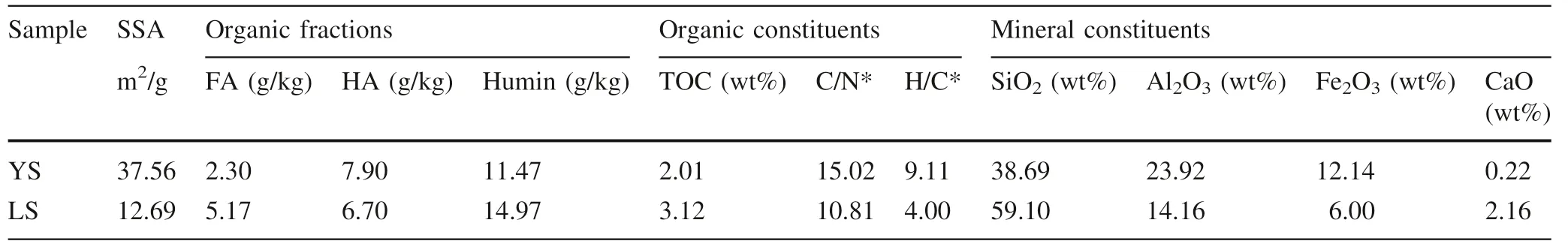
Table 1 Selected physicochemical properties of two target soils
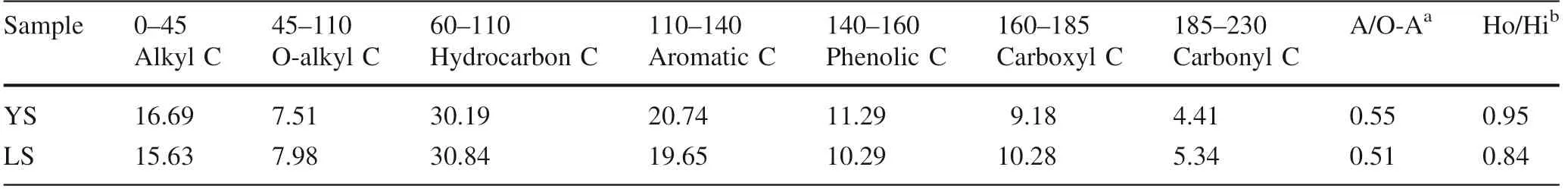
Table 2 Chemical shift of CPMAS 13C-NMR spectra and relative proportions of different carbon types of the sorbents
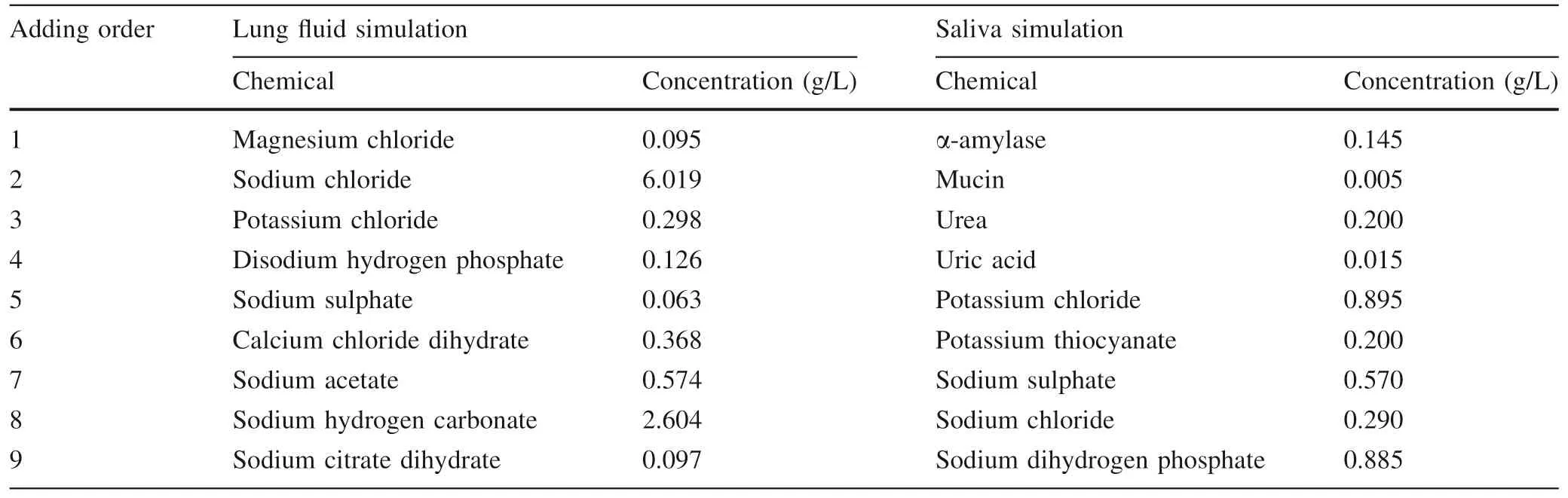
Table 3 Composition lists for simulated lung fluid and simulated saliva

Table 4 Parameters of fitted Freundlich sorption isotherms
Briefly,the sorption experiment was conducted triplicate in batch reactors using amber glass vials(40 mL)with Teflon-lined caps,a certain amount of sorbent was carefully weighted into the amber glass vial,filled with the blank solution,a predetermined volume of stock solution was spiked,and then additional blank solution was added to minimize the head space of the vial(less than 0.1 mL).The vial was set horizontally in the temperature-controlled shaker for shaking at 120 rpm at a temperature of 37 °C.Preliminary tests had showed that 14 d was sufficient to attain of the apparent sorption equilibrium for tested soils,therefore,the duration of sorption and desorption experiments was set as 14 d.After shaking,the vial was set upright stilly for 3 d in dark to settle the suspended sorbent completely.An aliquot of the supernatant was taken from the vial into a prepared 5 mL glass vial with pre-weighted methanol(about 2 mL)for stabilization of phenanthrene and pyrene in the vial,the 5 mL glass vial was then stored at 4 °C in the dark waiting for later measurements.The desorption experiment was conducted immediately after the sorption experiment.Briefly,the supernatant left in the vial was carefully removed as much as possible by siphon,then the corresponding blank solution was filled to minimize the headspace of the vial,the vial was capped,set horizontally in the shaker,and operated the rest steps identically as in the sorption experiment.A control experiment of no sorbent in the vial was conducted accompanying to quantify the loss of solute during experiments and was used as a reference in the data reduction.
2.4 Instrumental analysis and calculations
The initial solution concentrations of phenanthrene and pyrene at the start of the experiment and their equilibrium solution concentrations in supernatants obtained after sorption and desorption experiments were determined by an ultra-high-performance-liquid-chromatography(UPLC,Agilent 1290)equipped with diode array UV detector(G4212A),fluorescence detector(G1321B)and auto-sampler unit(G4226A),using an Eclipse XDB-C18 column(2.1×150 mm,3.5 μm)at the column temperature of 37 °C.The sorbate concentration in the supernatant or initial solution was calculated by manipulating the UPLC measured sorbate concentration with a corresponding dilution factor based on the mass ratio of methanol and water in the mixture.
The sorbent-phase sorbate concentrations were computed based on the mass balance of sorbate in the vial with the following equation:

where qis the equilibrium concentration of sorbate in sorbent(μg/Kg);Cand Care initial and equilibrium concentrations of sorbate in solution(μg/L),respectively;V and M are solution volume(L)and sorbent mass(kg)introduced to the vial,respectively.
3 Results and discussion
The Freundlich isotherm model was commonly applied to describe behaviors of HOC sorption/desorption on soil and sediment.It has the following form:

however,the initial sorbate concentrations in the experiment were designed to distribute evenly at the logarithmic scale,therefore,the logarithmic format Freundlich equation(Eq.3)was applied to achieve a better data fitting

where Kis the sorption capacity-related parameter[(μg/kg)/(μg/L)]and n is the index of the isotherm linearity.The organic carbon content normalized single-point distribution coefficient(K)was also often applied to evaluate the distribution level of sorbate between solution and sorbent and can be calculated by Eq.4:

where qis the calculated sorbate concentration in sorbent at a selected Cby the fitted Freundlich equation,fis the total organic carbon content of the sorbent.
3.1 Sorption experiments
3.1.1 Isotherm nonlinearity

Fig.1 Sorption isotherms of phenanthrene and pyrene on two soils in three different solutions and their Freundlich model fitting results.Note:Phen-YS-W-S and Pyr-YS-W-S:soprion isotherms of phenanthrene and pyrene on yellow soil in the simulated groundwater,repsectively;Phen-YS-L-S and Pyr-YS-L-S:sorption isotherms of phenanthrene and pyrene on yellow soil in the simulated lung fluid,respectively;Phen-YS-S-S and Pyr-YS-S-S:sorption isotherms of phenanthrene and pyrene on yellow soil in the simulated saliva,respectively;Phen-LS-W-S and Pyr-LSW-S:soprion isotherms of phenanthrene and pyrene on limestone soil in the simulated groundwater,respectively;Phen-LS-L-S and Pyr-LS-L-S:soprion isotherms of phenanthrene and pyrene on limestone soil in the simulated lung fluid,respectively;Phen-LS-S-S and Pyr-LS-S-S:soprion isotherms of phenanthrene and pyrene on limestone soil in the simulated saliva,respectively
The data presented in Table 4 and Fig.1 indicated that all measured sorption isotherms are nonlinear,n values ranged from 0.622 to 0.836 for phenanthrene and from 0.590 to 0.865 for pyrene.For phenanthrene sorption,the lowest n value,0.622,showed in the scenario of limestone soil and simulated saliva while the highest,0.836,in the yellow soil in simulated saliva scenario;for pyrene sorption,the lowest n value,0.590,happened in the limestone soil and simulated lung fluid scenario and the highest,0.865,showed in the yellow soil in simulated saliva scenario.The different nonlinearities of sorption isotherms of two sorbates on two soils in three different solutions could be the integration results from different properties of sorbates,sorbents,and solutions.It was suggested that SOM is the dominant fraction of soil to uptake or store HOCs(Chiou et al.1979)while the overall sorption behavior is mainly attributed to SOM compositions,like soft carbon and hard carbon.Linear sorption isotherm for organic matter often results from absorption or partitioning into amorphous organic carbon,which is called‘‘soft carbon’’,such as fulvic acid and humic acid,while nonlinear sorption isotherm is usually related to a slow process of adsorption or diffusion into heterogeneous sorption sites on condensed organic carbon which is called‘‘hard carbon’’,such as humin(Carmo et al.2000;Xiao et al.2004;Duan and Naidu 2013).In general,no matter phenanthrene or pyrene,sorption nonlinearities of the limestone soil were obviously higher than corresponding those of the yellow soil(Table 4),which is consistent with different SOM compositions of the limestone soil and the yellow soil,the limestone soil had a higher content of humin(14.97 wt%)while the yellow soil had the lower(11.47 wt%,Table 1).Nevertheless,the complexity of soil aggregation structure and chemical figures of SOM may make it particularly challenging to predict the overall sorption behavior for soils based on quantitative analyses of different SOM fractions and their respective sorption equilibria(Huang et al.2003;Xiao et al.2004).The sorption nonlinearities of phenanthrene and pyrene on the yellow soil in simulated body fluids are significantly lower than corresponding those in the simulated groundwater(Table 4),which may be attributed to the sorption site competition of organic compounds in body fluids and phenanthrene/pyrene,however,sorption of phenanthrene/pyrene on the limestone soil didn’t give expression to this phenomenon,which may be resulted from the much higher content of calcium ion in the limestone soil and the relative higher content of‘‘soft carbon’’fractions,fulvic acid and humic acid,since the high content of calcium ion may significantly modify physical conformations of fulvic acid and humic acid and such cast shadow on their sorption behaviors(Simpson et al.2003;Gao et al.2006;Chien et al.2010).
3.1.2 Sorption capacities
It is suggested that logK,the fitted parameter of the Freundlich model is related closely to the sorption capacity(Gunasekara and Xing 2003).As shown in Table 4,logKvalues varied from 2.845 to 3.327 for phenanthrene and from 3.344 to 3.779 for pyrene,consistent with the fact that the more hydrophobic pyrene inclines to stay with soil in the aqueous environment more than phenanthrene does.Different geosorbents exhibited different sorption capacities due to their different natures.The limestone soil showed much more sorption capacity,logKranged from 3.021 to 3.327,compared to that of the yellow soil,logKranged from 2.845 to 3.118,which,however,is contradicted to their specific surface area(SSA)data measured by N-BET(Table 1),the SSA of the limestone soil is 12.69 m/g and smaller than that of the yellow soil(37.56 m/g).The higher sorption capacities of the limestone soil must be attributed to its higher TOC content(3.12 wt%).The calculated organic carbon normalized single point distribution coefficient(K)values,on the other hand,indicated that the SOM of the yellow soil has higher sorption capacities of both phenanthrene and pyrene in all circumstances except the simulated saliva(Table 5),which is agreed with the higher A/O-A and Ho/Hi ratios of SOM in the yellow soil(Table 2).The A/O-A and Ho/Hi ratios are suggested as the proxies of SOM’s hydrophobicity(Chung et al.2012),the higher hydrophobicity is,the better the quality of SOM is for sorption HOCs(Huang et al.2003).Interactions among simulated saliva compounds and SOM fractions of the limestone soil may enhance the sorption of phenanthrene/pyrene,especially at their low equilibrium concentration end,where their logKand Kvalues are very closed to those for the simulated groundwater and significantly higher than those for the simulated lung fluid.Nevertheless,the underlying mechanism is largely uncertain.It can be seen easily from Fig.1,sorption capacities varied in different solution systems,highest in the simulated groundwater and lowest in the simulated lung fluid,as showed in Fig.1,the isotherms measured in the simulated lung fluid(red dots)locate below other isotherms and the isotherms measured in the simulated groundwater(blue square)locate above other isotherms.
3.2 Desorption experiments
3.2.1 Desorption isotherm
The desorption experiments were conducted immediately after the equilibrium sorption experiments under the same experimental conditions.All desorption isotherms were plotted in Fig.2.The n values for desorption isotherms were generally lower and the logKvalues for desorption isotherms were significantly higher than corresponding those of sorption isotherms(Tables 4 and 6),indicating that the resistant fraction of phenanthrene/pyrene tended to stay on‘‘high energy sites’’and the desorption is not a reverse process of the sorption.The variation trends of Freundlich parameters for sorption and desorption were complex,they varied for two different sorbents,the yellow soil and the limestone soil,two different sorbates,phenanthrene and pyrene,as well as the three different solutions,simulated groundwater,lung fluid,and saliva.Briefly,for the n value of phenanthrene desorption isotherm in the simulated groundwater,it was 0.662 for the yellow soil,decreased by 0.06 from that of the sorption isotherm(0.722),but was 0.744 for the limestone,increased by 0.072,from that of the sorption isotherm(0.672),the different variation trends may be resulted from the change of the soil conformation under a relatively high temperature of 37 °C during 4 weeks experiment time and/or from the loss of soluble SOM in the supernatant removal at the beginning of the desorption experiment.But the universality of this phenomenon is waiting for more studies.No consistent variation trends were observed for other desorption experiments either,likely indicating the effects of soil conformation changes and the loss of soluble SOM between sorption and desorption experiments.The logKvalues for desorption isotherms increased significantly from corresponding to those of sorption isotherms,suggesting the existence of irreversible sorption.The Kvalues calculated for desorption experiments(Table 7)were also greater than corresponding those for sorption experiments(Table 5).Both logKand Kvalues demonstrated,compared to the simulated groundwater,simulated body fluids,especially the simulated lung fluid,were not favorable for HOCs sorption on the target soils.

Table 5 Calculated Koc values for sorption experiments(×104 L/kg)

Fig.2 Desorption isotherms of phenanthrene and pyrene on two soils in three different solutions and their Freundlich model fitting results.Note:Phen-YS-W-Des and Pyr-YS-W-Des:desorption isotherms of phenanthrene and pyrene on yellow soil in the simulated groundwater,repsectively;Phen-YS-L-Des and Pyr-YS-L-Des:desorption isotherms of phenanthrene and pyrene on yellow soil in the simulated lung fluid,respectively;Phen-YS-S-Des and Pyr-YS-S-Des:desorption isotherms of phenanthrene and pyrene on yellow soil in the simulated saliva,respectively;Phen-LS-W-Des and Pyr-LS-W-Des:desorption isotherms of phenanthrene and pyrene on limestone soil in the simulated groundwater,respectively;Phen-LS-L-Des and Pyr-LS-L-Des:desorption isotherms of phenanthrene and pyrene on limestone soil in the simulated lung fluid,respectively;Phen-LS-S-Des and Pyr-LS-S-Des:desorption isotherms of phenanthrene and pyrene on limestone soil in the simulated saliva,respectively

Table 6 Parameters of fitted Freundlich desorption isotherms

Table 7 Calculated Koc values for desorption experiments(×104 L/kg)
3.2.2 Sorption-desorption hysteresis
Almost all samples performed that the n values of desorption isotherms were lower than corresponding those of sorption isotherms,while the capacities of desorption were higher than corresponding those of sorption,suggesting the non-ideal irreversible processes of sorption and desorption which can be evaluated by the Hysteresis Index(HI)defined by Eq.(5):
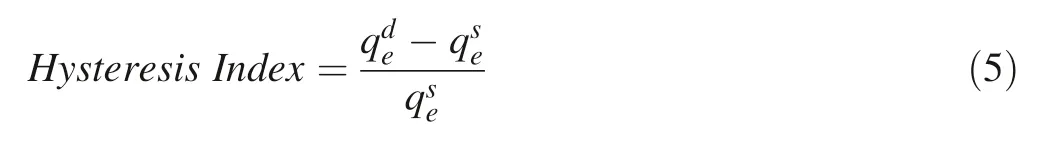
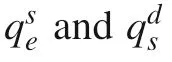

Table 8 Calculated HI values for phenanthrene and pyrene on two soils in three different solution systems

Fig.3 Comparison of sorption-desorption behaviors of phenanthrene and pyrene on two soils in three different solutions.Note:Legends are the same as those in Figs.1 and 2
4 Discussion
Due to the high hydrophobicity of PAHs,once released into the natural environment,PAHs tend to accumulate in soil and sediment and bind primarily with SOM,and their aqueous concentrations are exceptionally low(Wilcke 2000;Ni et al.2008;Yang et al.2014;Yu et al.2014).The contaminated soil and sediment acts as the secondary sources of PAHs and casts durative risks to the surrounding environment(Cornelissen et al.1997;Shor et al.2003).Therefore,the comprehensive understanding of sorptiondesorption behaviors of PAHs on typical soils in a region is of significance to precisely assessing the fate and environmental risks of PAHs in this region.Yellow soil and limestone soil are the two most occurring natural soils of the southwest China karst area,taking up 65% of the surface soil area of Guizhou Province.However,practical data regarding how their natures that affect the sorption and desorption of PAHs are scarce.In this study,we reported the sorption-desorption isotherms and fitted Freundlich parameters for the yellow soil and the limestone soil.The results indicated that,although the TOC contents of the yellow soil and the limestone soil(2.01% and 3.12%,respectively)are remarkable lower than that of the Chelsea soil(5.45%)(Huang,1997),a surface soil frequently used in prior sorption studies,their phenanthrene sorption and desorption capacity parameters(logK),3.118 and 3.648 for the yellow soil and 3.323 and 3.846 for the limestone,are comparable to those of the Chelsea soil(3.644 and 3.658),accordingly their Kvalues are even higher.The possible explanation can attribute to the characteristics of the two soils,they contained relatively high contents of humin(‘‘hard carbon’’),which is consistent with that both two soils were developed from the weathering residuals of carbonate rocks,which may contain old and deeply metamorphic organic matters.The relatively large HI values of the two soils(Table 8)further manifested that PAHs pollutants may undergo natural sequestration through partially irreversible sorption processes.
The different sorption-desorption behaviors of phenanthrene and pyrene on two soils in the three different solution systems demonstrated the influences of inorganic salts,organic acids,and proteins in the simulated body fluids.Generally,sorption-desorption isotherms of phenanthrene and pyrene in the simulated body fluids were relatively more linear and the sorption capacities were lower compared to those in the simulated groundwater experiments.The hydrophobic region of α-amylase and mucin could interact with hydrophobic phenanthrene and pyrene,leading to lower interaction between hydrophobic organic compounds and soil particles(Wang et al.2011a,b).With the change of ionic strength,the DOC content in the solution would be different consequently,resulted in a difference of sorption of phenanthrene and pyrene,because a micelle-like structure of DOC would be formed following the addition of cations(Duan and Naidu 2013).However,once the DOC concentration was high,intra-and interactions could block certain sorption sites,lead to a smaller sorption amount because of the change in surface charge density.The constitutions of simulated lung fluid and saliva were different,in addition to different SOM natures of yellow soil and limestone soil,lead to different associated DOC contents and prosperities,and resulted in different sorption behaviors in consequence.Although the underlying mechanisms of the different sorption-desorption behaviors of PAHs on two soils in the simulated body fluids and in the simulated groundwater were complicated and demand more studies,the lower sorption capacity and small sorption-desorption hysteresis suggested that direct application of sorption-desorption experiment data from the simulated groundwater system may cause erroneous estimations of bioavailability and health risk of PAHs retained in soils.
5 Conclusions
In summary,this study selected phenanthrene and pyrene as the representative PAHs and investigated their sorptiondesorption behaviors on two major soils in the southwest China karst area.Sorption-desorption onto these two types of soils in three different solutions,simulated groundwater,simulated lung fluid,and simulated saliva,were studied.The experimental results showed that the yellow soil and limestone soil have a relatively high ability to retentate PAHs and therefore can serve as a pool of PAHs pollutants in the study region.The sorbent nature and sorbate hydrophobicity dominated the sorption and desorption behaviors of the two PAHs.All measured sorption-desorption isotherms fit well with the Freundlich model.The experimental results further indicated that sorption and desorption behaviors of PAHs in the simulated groundwater were quite different from those in the simulated body fluids.The sorption capacities and the sorption nonlinearities of PAHs on the soils were significantly lower in simulated body fluids than in simulated groundwater,which suggested that the bioavailability and the health risk of PAHs in contaminated soils may be underestimated if the data from simulated sorption-desorption experiments in groundwater were directly applied.
Acknowledgements
This research was supported by the National Natural Science Foundation of China-Guizhou Joint Fund for Karst Science Research Center(U1612441),National Natural Science Foundation of China(41173129,41273149),and the S&T Plan Project of Guizhou(2011-3109).Compliance with Ethical Standards
Conflict of interest
On behalf of all authors,the corresponding author states that there is no conflict of interest.杂志排行
Acta Geochimica的其它文章
- Effect of crustal porosity on lunar magma ocean solidification
- Gold anomaly ranking based on stream sediment geochemistry in the Fariman-Kashmar axis,NE Iran
- Petrographic and geochemical characterization of weathered materials developed on BIF from the Mamelles iron ore deposit in the Nyong unit,South-West Cameroon
- Equilibrium mercury and lead isotope fractionation caused by nuclear volume effects in crystals
- Estimation of evaporation losses based on stable isotopes of stream water in a mountain watershed
- Geochemical significance of tricyclic and tetracyclic terpanes in source rock extracts from the Offshore Niger Delta Basin,Nigeria
