Adsorption experiment of water-soluble rare earth elements in atmospheric depositions and implications for source tracing in South China
2021-06-21XiaojianMiYuanLiYuefengLiuYuXieHanjieLiXiaotaoPengHouyunZhou
Xiaojian Mi·Yuan Li·Yuefeng Liu·Yu Xie·Hanjie Li·Xiaotao Peng·Houyun Zhou
Abstract The distribution patterns of rare earth elements(REEs)in fine-grained materials in various depositions were often found to be similar to those of the aeolian sediments deposited in the Loess Plateau in North China and the fine-grained materials were suggested to be derived from wind-blown dust.However,increasing evidence indicated that the REEs in the water-soluble portion of atmospheric depositions also displayed similar patterns to those of aeolian sediments.In this study,water-soluble REEs in three atmospheric depositions collected from different climatic zones in China were adsorbed with two adsorbents with distinct adsorption capacity,glass powder,and co-precipitated iron hydroxide.The results showed that the REEs adsorbed by the two adsorbents displayed patterns similar to those of the original atmospheric depositions.The typical characteristics of the REE patterns of atmospheric deposition can be well reproduced in the adsorbed REEs.The higher the REE concentrations in the atmospheric depositions,or the higher adsorption efficiency of the adsorbents,the better reproducibility of the REEs patterns.The results suggest that the REEs of the fine-grained materials in various sediments,which have a high adsorption capacity,especially those deposited in South China,may come from the water-soluble REEs in atmospheric deposition,and may not be appropriate tracers of wind-blown dust from North China.
Keywords Atmospheric deposition·Rare earth element·Adsorption experiment·Source tracing·Fine-grained material
1 Introduction
Rare Earth Elements(REEs)are a group of elements with relatively stable geochemical properties and are often used for tracer studies of sediment sources(Kitto et al.1992;Sholkovitz et al.1993;Nozaki et al.2000;Wang et al.2001;Cai et al.2010;Pearce et al.2013;Haley et al.2004;Yang and Li 1999,Yang et al.2002;Singh 2009).Some studies have shown that the REE distribution patterns of the fine-grained portion in surface sediments(including weathered residuals)are mostly similar,and are consistent with the REE distribution patterns of the upper crust(UCC)and the dust deposits in northern China(e.g.the loesspaleosol sequence)(Taylor and McLennan 1985;Yokoo et al.2004;Cheng 2018;Sheng 2011;Ying et al.2013;Zhu et al.2007;Li et al.2006)(Fig.1).

Fig.1 Chondrite-normalized REE patterns of fine-grained materials in various surface sediments
In previous studies,this similarity was often used to support the idea that these fine-grained materials were derived mainly from wind-blown dust as deposited in the depocenter in the Loess Plateau in North China(Zhu et al.2007;Li et al.2006;Ying et al.2013).
With the development of analytical techniques and the increasing attention paid to the atmospheric environment,more and more research on atmospheric chemistry showed that the REE distribution patterns of rainwater or atmospheric deposition were similar to those of wind-blown dust(Suzuki et al.2011;Zhu et al.2016;Peng et al.2019).The fine-grained materials in various surface sediments(e.g.various clay minerals)have strong adsorption ability.Water-soluble REEs in atmospheric deposition may be easily adsorbed by these fine-grained materials,reminding us that the REEs in the fine-grained materials in surface sediments may be derived from atmospheric deposition and may not be an appropriate indicator of wind-blown sediments.However,a key issue related to this association is whether the REE pattern can remain unchanged when the water-soluble REEs in atmospheric deposition are adsorbed by fine-grained materials in various surface sediments.
We attempted to address the issue in this study.Three atmospheric deposition samples were collected from different climatic zones of China.The water-soluble REEs in the three samples were adsorbed respectively by two kinds of adsorbent materials that have distinct adsorption ability.
2 Samples and methods
2.1 The sampling sites
The three atmospheric deposition samples were collected from the following sites:the Shipai Campus of South China Normal University(hereafter referred to as Huashi)in coastal South China,the Baojing Cave in northern Guangdong Province,representing inland South China,and the Loufang Cave in northeast Sichuan Province in central China(Fig.2).The latter two locations were chosen simply due to that the related samples had already been collected in previous works(Chen et al.2017;Pan et al.2020).
The Shipai Campus of South China Normal University(23°0811N,113°2057E)is located on the coast of South China with an annual mean temperature of 22.4 °C and a multi-year average of annual precipitation of 1786 mm.Rainfall concentrates in the summer season.The Baojing Cave(24°0743N,113°2130E)is located in Yingde County in northern Guangdong Province,about 110 km north of Guangzhou.The annual mean temperature at this site is 20.9 °C,and the multi-year average of annual precipitation is 1858 mm.The precipitation falls mainly from April to August.The Loufang cave(32°2446N,107°1045E)is located in Tongjiang County in northeast Sichuan Province,central China.The annual mean temperature is~15 °C,and the annual precipitation is usually between 1000 and 1200 mm.All three locations are in the Asian summer monsoon regime,but the Huashi and Baojing Cave sites are in the south subtropical climate zone while the Loufang Cave is in the middle subtropical climate zone.
2.2 Sample collection and pretreatment

Fig.2 Locations of the three sampling sites,the Loufang Cave,Baojing Cave and Huashi
The Huashi sample was collected during a rainstorm on June 12,2018,representing wet atmospheric deposition precipitated in a heavy convective rainfall event.The rainwater collector was placed about 1.5 m above the ground.A total of 10 L of rainwater was collected.At the Loufang cave,the collector was set on the flat top of a building,about 6 m from the ground.Bulk atmospheric deposition precipitated from September 17 to October 19,2013,was collected.At the Baojing cave,the collector was also set on the flat top of a building,about 6 m from the ground.The sampling time was from October 21 to November 19 in 2012.The sample collection at the Loufang and Baojing caves used polypropylene plastic boxes with a volume of 4 L.The collectors were pre-cleaned with double distilled HNO.The sample collection at Huashi used a big beaker with a volume of 10 L.The beaker was also pre-cleaned with double distilled HNO.Because the Huashi rainstorm sample was expected to have very low trace metals due to dilution effects,it was enriched immediately by~10 times after collection.The enrichment was conducted using a cleaned beaker on a hot plate under a temperature of 90-95 °C,which was placed in a hood.
No particulate materials were observable in the Huashi rainstorm sample.A small amount of particulate materials was visible in the two samples collected at the Loufang Cave and Baojing Cave,respectively.The two samples were allowed to settle for a long time(>72 h)before the upper part of the solution was decanted for trace element analysis.In the laboratory,the solution samples were enriched again by~5(the Huashi sample)to~10 times(the other two samples)in order to get better analytical precision.
2.3 Adsorption experiment
In this work,two kinds of adsorbing materials,glass powder and iron hydroxide colloid,were used to adsorb water-soluble REEs in atmospheric depositions samples.
The glass powder was obtained from glass sands with a diameter of~1 mm which were purchased from the market.The glass sands were ground three times(two mechanically with a grinder and one manually with an agate mortar)due to their grinding-resistance.Afterward,the obtained glass powder was sieved and little was found to be smaller than 0.011 mm(<0.1 g in~50 g).The components 0.038-0.011 mm in size were used for the adsorption experiment.The selected components were cleaned using the following procedures.First of all,antiaqua regia with a concentration of 50% was added to the glass powder,stirring the glass powder evenly and then heating it overnight.Then centrifuged and the upper part of the solution was decanted.The remaining glass powder was cleaned sequentially with Milli-Q water(~18.2 Ω),2% HNO,and Milli-Q water.Finally,the glass powder was cleaned once more with Milli-Q water and was filtered out the water,dried up in an oven,and ready for the adsorption experiment.
For each atmospheric deposition sample,firstly about 20 g of the cleaned and dried glass powder was balanced and transferred into a PFA round bottom beaker.Then about 30 ml of the atmospheric deposition sample was added to the glass powder,stirring the glass powder and settling it down for half an hour,repeating the stirring and settling three times.Afterward,the samples were centrifuged for 5 min and the upper part of the solution was decanted.Then 10 ml of 2% HNOwas added to the remaining glass powder,stirring the glass powder and heating on a hot plate with a temperature of 70 °C.After 1 h of heating,the samples were removed from the hot plate.After the samples were cooled down to room temperature,they were centrifuged for 5 min and the upper part of the solution was transferred with a pipette into a pre-cleaned PFA beaker.Afterward,the solution was evaporated at the temperature of 110 °C until it was nearly dry.Then 10 ml of 2%HNOwas added and heated again at 110 °C overnight.After the samples were cooled down to room temperature the next day,they were tuned to the matrix for ICP-MS analysis.A procedure blank,which uses Milli-Q water instead of atmospheric deposition,was prepared following all the steps of the glass powder adsorption experiment.
The adsorption experiment of ferric hydroxide was carried out as follows.First,7 ml of atmospheric deposition sample was added to a PFA beaker.Then,0.5 ml of a saturated FeClsolution was added to the sample,shaking the sample to make FeCldistributed evenly in the solution.Then,12.5%ammonia water was added to the sample drop by drop until no more reddish-brown Fe(OH)colloid was formed.Shaking the beaker a few times,settling it down for about 1 h.Afterward,the samples were centrifuged for 5 min and the upper part of the solution was decanted.2 ml of 7%HNOwas added to the beaker.After the sample was completely dissolved,it was evaporated to dryness at 110 °C,and then 0.5 ml of 7% HNOwas added.Sealed with lids,the samples were heated at 110 °C overnight.After the samples were cooled down to room temperature the next day,remove the lid and evaporate the samples to dryness at 110 °C.Then,10 ml of 2% HNOwas added to each sample and the samples were sealed with lids and heated at 120 °C for 3.5 h.Later when the samples were cooled down to room temperature,they were also tuned to the matrix for ICP-MS analysis.A procedure blank was also prepared for the co-precipitated iron hydroxide adsorption experiment.
In addition to the REEs adsorbed by glass powder and co-precipitated iron hydroxide,we also determined the REEs in the original atmospheric deposition samples.
2.4 REE determination
The REEs adsorbed by the glass powder and co-precipitated iron hydroxide and in the original atmospheric deposition,samples were determined by ICP-MS.The analysis was carried out at Guiyang Tongwei Testing Co.,Ltd.,using the method improved by Eggins et al.(1997).The detection limit of each REE was listed in Table 1,usually ranging from<10to 10(Lawrence et al.2006).Also listed in Table 1 were the analytical precision(RSDs)of each REE,generally better than 10%.
3 Results and discussion
3.1 Procedure blanks and adsorption efficiency
The REEs adsorbed by glass powder and co-precipitated iron hydroxide as well as those in the original atmospheric deposition samples were listed in Table 1.The REE concentrations of the two procedure blanks were also listed in Table 1.They were similar in the two blanks except that the Ce concentration in the iron hydroxide co-precipitated blank was about 10 times as in the glass adsorption blank,which was currently not clear and might partly be due to much higher particle-reactivity of Ce relative to the other REE(Sholkovitz 1992;Alibo and Nozaki 1999).In the original atmospheric deposition samples,the REEs in the Huashi rainstorm sample have much lower concentrations than in the Baojing Cave and Loufang Cave samples(20 to more than 100 times lower excluding Er,Table 1),which may reflect a strong leaching and dilution effect on atmospheric deposited REEs by heavy rainfall.The original Huashi rainstorm sample showed a significant positive Er anomaly after normalization to chondrite(Fig.3),which was occasionally observed in our speleothem samples(unpublished data).The cause for this is currently not clear.
For the glass powder adsorption experiment,the REEs in the procedure blank are significantly lower than in the other three samples(Table 1),indicating that a significant portion of the REEs from the atmospheric depositions was adsorbed on the glass powder.However,the glass powder adsorption efficiency(the ratio of the REEs adsorbed by glass powder to those in the original samples)of the three samples generally does not exceed~20%(Table 2).For the Huashi rainstorm sample,the REE concentrations are generally less than 2 times of the procedure blank.The REE concentrations of the Loufang cave sample are usually 3 times(heavy REEs)or more(light REEs)of the procedure blank.For the Baojing cave sample which has the highest REE concentrations,the procedure blank is 20 times less in REE concentration(Table 1).These data suggest that the higher the REE concentration,the more reliable the adsorption experiment by glass powder.
Unlike the adsorption experiment by glass powder,the co-precipitated iron hydroxide displayed much higher adsorption efficiency which is usually 80% or higher(Table 2).Higher adsorption efficiency may be expected if more FeCland ammonia are used to co-precipitate iron hydroxide.Even for the Huashi rainstorm sample which has the lowest REE concentration,the REEs adsorbed by the co-precipitated iron hydroxide are 4 times the procedure blank(Table 1).This may be due to that the co-precipitated iron hydroxide has a much higher adsorption capacity than the glass powder,and indicates that the adsorption experiment by co-precipitated iron hydroxide is more reliable.The calculated adsorption efficiency of Lu co-precipitated by iron hydroxide in the three samples was larger than 100%(Table 2).The two values,106%,and 115% could be explained with their analytical errors(Table 1).
3.2 REE distribution pattern
The REEs of the three atmospheric deposition samples and adsorbed by glass powder and co-precipitated iron hydroxide were normalized to chondrite(McLennan 1989)and were shown in Fig.3.The characteristic parameters of REE distribution pattern including fractionation between light and middle REEs(LREE/MREE,calculated by La/Eu,where subscript N indicates normalization to chondrite)and between middle and heavy REEs(MREE/HREE,calculated by Eu/Yb),Ce anomaly(δCe=2Ce/(La+Pr)),Eu anomaly(δEu=2Eu/(Sm+Gd)),etc.were calculated and listed in Table 3.Because most of the REEs had uncertainties(RSDs)less than 5%(Table 1),these ratios usually had errors less than 10%.However,some of the ratios,e.g.the Eu/Ybratio of the procedure blank of the co-precipitated iron hydroxide vehad an error as large as 22%because both Eu and Yb had errors close to 10%(Table 1).
The REEs in the three samples came mainly from two sources,the wet atmospheric deposition,and the hygroscopic part in the dry atmospheric deposition.A combination of Table 3 and Fig.3 indicates that the REEs adsorbed by glass powder and co-precipitated iron hydroxide respectively displayed patterns roughly similar to those of the original atmospheric deposition samples.For example,the original atmospheric deposition samples show significant enrichment of LREE,which was reproduced in the REEs adsorbed by glass powder and co-precipitated iron hydroxide(Fig.3).In particular,for the Baojing cave sample with the highest REE concentrations,all the characteristic parameters such as La/Eu,Eu/Yb,δCe,and δEu are completely consistent in the original atmospheric deposition sample as well as in the adsorbed REEs by glass powder and co-precipitated iron hydroxide.For the Loufang cave sample which has medium REE concentrations,these characteristic parameters are also generally consistent.The REEs adsorbed by coprecipitated iron hydroxide displayed a pattern moresimilar to the original Loufang cave sample.For the Huashi rainstorm samples with the lowest REE concentrations,the characteristic parameters such as La/Eu,Eu/Yb,δCe,and δEu are significantly different between the original and adsorbed samples(Table 3).This may reflect that the interferences from sample pretreatment(reflected by the procedure blank)and REE measurement(reflected by the analytical precision)became more serious for the low REE samples.The adsorption efficiency of 134% for Eu in the Huashi sample,as well as 117%for Lu in the Baojinggong Sample(Table 2),which couldn’t be explained with errors,was possibly related to the two factors.In particular,the remarkable positive anomaly of Ce in the glass powder absorption of the atmospheric deposition at Huashi,which was not observed in the other two Huashi samples(Table 3,Fig.3),might be significantly interfered with sample preparation due to very low REE concentrations.

Table 1 The detection limit,RSD for each REEs and REE concentration of the atmospheric deposition samples as well as adsorbed by glass powder and co-precipitated iron hydroxide,respectively(*10-12)
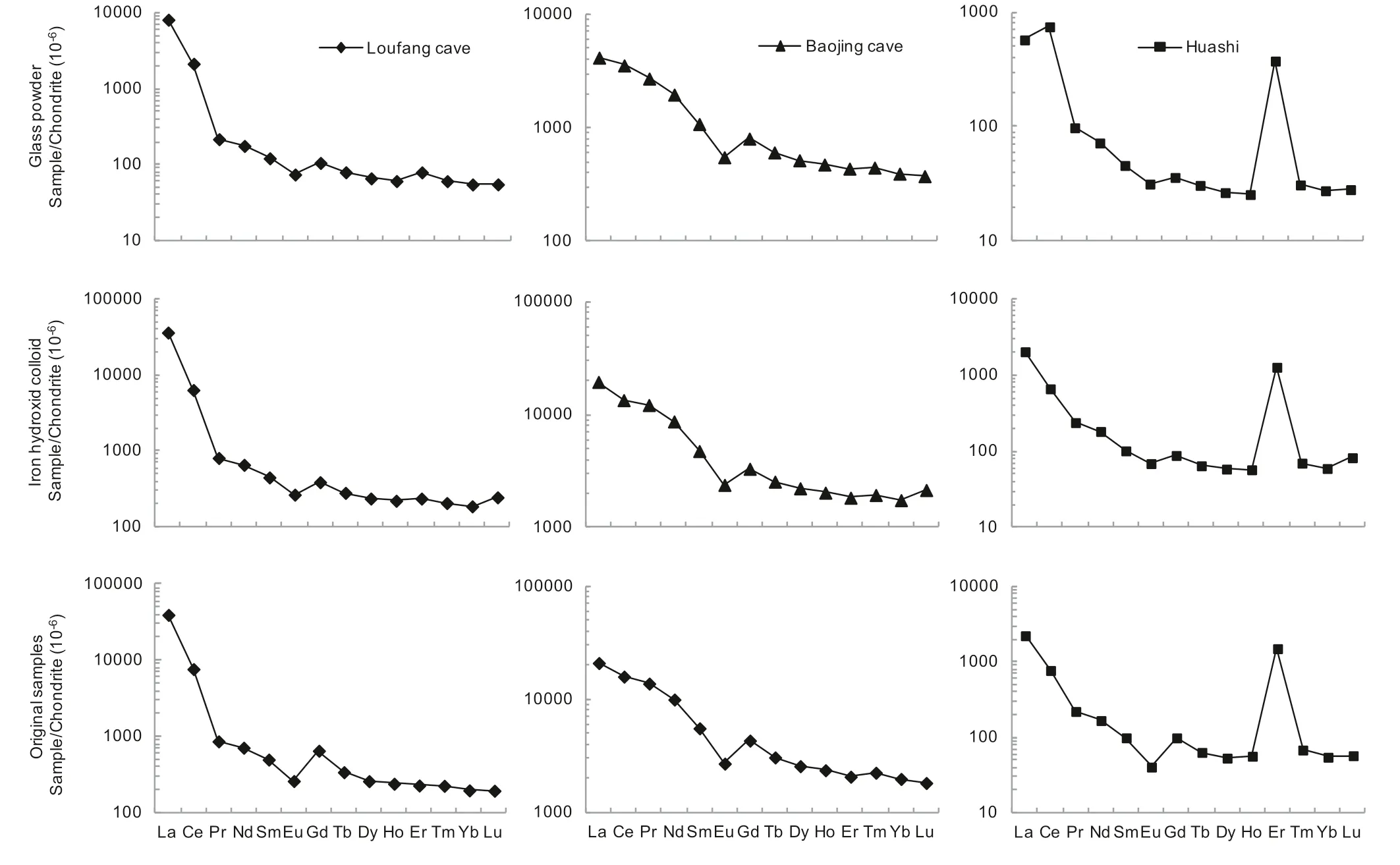
Fig.3 Chondrite-normalized REE patterns adsorbed by glass powder and co-precipitated iron hydroxide as well as in original atmospheric deposition samples

Table 2 Adsorption efficiency(%)by glass powder and co-precipitated iron hydroxide,respectively
However,even for the low REE Huashi sample,the pattern of the REEs adsorbed by co-precipitated iron hydroxide is similar to that of the original sample.This is in accordance with the sample from the Loufang cave.Despite the significant differences in the characteristic parameters for three Huashi samples(Table 3),the basic characteristics of the REE pattern such as the steeperLREEs and flatter HREEs and the significant negative Eu anomaly are uniform.Also,the significant positive Er anomaly of the Huashi sample(although its cause is still unknown and needs further investigations)is presented in all three Huashi samples.
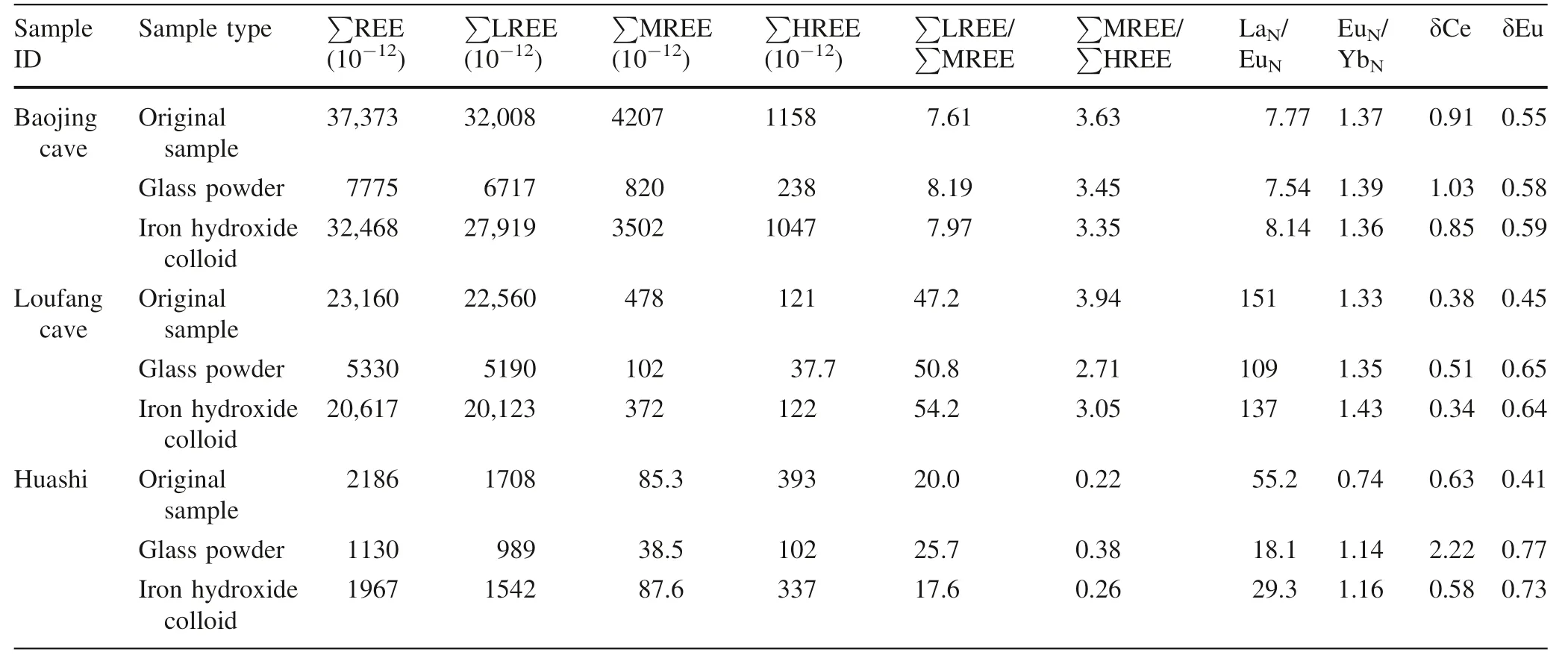
Table 3 The characteristic parameters of REE distribution pattern of the atmospheric deposition samples and adsorbed by glass powder and iron hydroxide respectively
In summary,for atmospheric deposition samples with relatively high REE concentration,whether their REEs are adsorbed by glass powder or co-precipitated iron hydroxide,the patterns of the adsorbed REEs are consistent with the original samples.For atmospheric deposition samples with low REE concentration,the patterns of adsorbed REEs are more affected by sample pretreatment and analytical precisions and may be significantly different from the original samples.However,enhancement of the adsorption capacity(e.g.from glass powder to iron hydroxide)and higher enrichment of the original sample could reduce the difference.
3.3 Implications of the adsorption experiments
An important application of REEs in geology is source tracing.Sediments having similar REE patterns are usually considered to derived from the same source(Henderson 1984,Cullers et al.1988).In particular,because atmospheric dust activity is intensive in North China and hundreds of meters of aeolian sediments deposited in midlatitudes of China(e.g.at the Loess Plateau),the REE distribution patterns in various surface sediments were often used as key evidence to check whether the sediments were derived from wind-blown dust.Sediments having similar REE patterns to those of the wind-blown dust were suggested to come from the same source as the aeolian sediments deposited in mid-latitudes of China(Cheng 2018;Sheng 2011;Zhu et al.2007;Li et al.2006;Ying et al.2013).This was appropriate in mid-to higher latitudes of China.However,increasing investigations showed that the REEs in wet atmospheric deposition(or watersoluble portion of bulk atmospheric deposition)displayed patterns similar to those of wind-blown dust(Suzuki et al.2011;Zhu et al.2016;Peng et al.2019).These REEs could be easily adsorbed by fine-grained materials in various surface sediments that have strong adsorption ability.Therefore,the results of the adsorption experiment suggested that in South China with low-latitudes which was far away from the sources of wind-blown dust,even if the REE patterns are similar to those of the wind-blown dust(Gallet et al.1996),the fine-grained materials in various surface sediments are not necessarily derived from wind-blown dust.The adsorption of REEs from atmospheric deposition could also result in the similarity.
The REE patterns of some weathering rare earth element deposits in China showed typical characteristics of the REE patterns of wind-blown dust,e.g.steeper LREEs and flatter HREEs and significant negative Eu anomaly.This may be explained with adsorption of atmospheric deposited REEs by fine-grained materials during the long-term weathering process.
4 Conclusions
In this study,the water-soluble REEs in different types of atmospheric deposition sample collected from different climatic zones in China were adsorbed by two adsorbents with distinct adsorption capacity,glass powder and coprecipitated iron hydroxide.It was found that the REEs adsorbed displayed patterns similar to those of the original atmospheric deposition samples.The typical characteristics of the REE patterns of the atmospheric deposition can be well reproduced in the adsorbed REEs.The higher the REE concentrations in the atmospheric deposition samples,or the higher adsorption efficiency,the better reproducibility of the REEs patterns.The results suggest that the REEs of various surface sediments,especially of the fine grained materials in the surface sediments which have a high adsorption capacity,may come from the water-soluble REEs in atmospheric deposition.Even if the finegrained materials in sediments display REE patterns similar to those of aeolian sediments deposited in the mid-latitudes of China,they are not necessarily derived from wind-blown dust.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China(Grant Nos.41473093 and 41271212).杂志排行
Acta Geochimica的其它文章
- Effect of crustal porosity on lunar magma ocean solidification
- Gold anomaly ranking based on stream sediment geochemistry in the Fariman-Kashmar axis,NE Iran
- Petrographic and geochemical characterization of weathered materials developed on BIF from the Mamelles iron ore deposit in the Nyong unit,South-West Cameroon
- Equilibrium mercury and lead isotope fractionation caused by nuclear volume effects in crystals
- Estimation of evaporation losses based on stable isotopes of stream water in a mountain watershed
- Geochemical significance of tricyclic and tetracyclic terpanes in source rock extracts from the Offshore Niger Delta Basin,Nigeria
