低硼及高硼胁迫对棉花幼苗生长与脯氨酸代谢的影响
2021-06-09曾紫君姜存仓
曾紫君 曾 钰 闫 磊 程 锦 姜存仓
研究简报
低硼及高硼胁迫对棉花幼苗生长与脯氨酸代谢的影响
曾紫君 曾 钰 闫 磊 程 锦 姜存仓*
华中农业大学资源与环境学院 / 微量元素研究中心, 湖北武汉 430070
以‘鄂抗10号’棉花品种为材料, 采用营养液培养, 设置不加硼(B0, 0 mg L-1)、低硼(B0.002, 0.002 mg L-1)、适硼(CK, B0.2, 0.20 mg L-1)、高硼(B50, 50 mg L-1) 4个硼(Boron, B)水平, 探究低硼和高硼胁迫处理下棉花幼苗生长及脯氨酸代谢的响应。结果表明, B0、B0.002及B50处理较CK显著抑制植株生长, 表现出较低的植株鲜重和干重, 根系伸长受到抑制。在供硼处理下, 随着硼浓度的升高, 棉花幼苗根、茎和叶中硼含量均呈梯度性上升, 其中, B0.2和B50处理下叶片中硼含量均高于根和茎; 而在B0和B0.002处理下, 根中的硼含量高于叶和茎。低硼和高硼处理下叶片中脯氨酸含量明显增加, 而根中脯氨酸含量显著减少。进一步分析叶片和根中脯氨酸合成代谢相关酶活性发现, B0.002和B50处理较CK增加棉花幼苗叶片Δ1-吡咯啉-5-羧酸合成酶(Δ1-pyrroline-5-carboxylate synthetase, P5CS)和鸟氨酸转氨酶(Ornithine-δ-aminotransferase, OAT)活性而降低脯氨酸脱氢酶(Proline dehydrogenase, ProDH)的活性; 叶片中Δ1-吡咯啉-5-羧酸还原酶(Δ1-pyrroline-5-carboxylate reductase, P5CR)活性在B50处理下显著高于CK, 而B0.002处理下该酶活性变化差异不显著。另外, B50处理较CK显著降低棉花幼苗根中OAT和P5CR酶活性, 而B0.002处理显著增加根中P5CS和ProDH的活性。表明低硼和高硼胁迫均抑制棉花幼苗的生长。硼胁迫条件下, 脯氨酸主要积累在棉花幼苗叶片中, 根中脯氨酸含量显著降低。缺硼和硼毒害时, 棉花幼苗叶片中脯氨酸的积累主要是通过调节脯氨酸Glu和Orn途径中的关键酶(OAT、P5CS合成酶和ProDH分解酶)活性, 使得脯氨酸的合成速度高于其降解。而在根中, 缺硼胁迫下主要是促进脯氨酸的降解导致根中脯氨酸含量降低, 高硼胁迫下主要是通过降低OAT和P5CS合成酶以及ProDH分解酶活性来抑制脯氨酸的合成及其分解, 但是对脯氨酸合成的抑制作用远大于其降解, 最终导致根系脯氨酸含量降低。
棉花; 硼; 脯氨酸; 合成和降解
硼(B)是植物必需的微量营养元素[1], 但硼从缺乏到过量的范围过窄, 因此植物易出现缺硼和硼毒害现象[2-3]。棉花是重要的经济作物, 我国是世界上主要产棉国之一, 种植区域主要分布在长江、黄河流域和新疆内陆[4]。受土壤类型和气候条件等的影响, 中国棉区土壤硼含量一般由北向南、由西向东逐渐降低[5-6]。我国棉花主产区普遍处于硼缺乏范围, 土壤有效硼含量为0.47 mg kg-1, 而长江中下游棉区土壤有效硼易流失, 仅为0.33 mg kg-1, 属于严重缺硼土壤, 易导致棉花蕾而不花[6-7]。硼被认为是棉花最易缺乏的微量元素[8], 硼肥的施用可提高棉花的产量和品质, 但近年来, 由于硼肥的不当施用, 造成部分棉区还是面临缺硼状况, 制约棉花产量的提高[9-10]。因此, 深入研究分析棉花对硼胁迫的响应机理对提高其抗逆性、促进农业增效和农民增收具有重要的意义。
研究表明, 植物在胁迫条件下会发生复杂的生理生化反应, 而脯氨酸(proline, Pro)作为一种小分子渗透保护物质, 在植物抵御各种逆境胁迫中发挥重要的作用, 其作用表现为渗透调节剂[11]、活性氧清除剂[12]、氧化还原平衡剂[13]及蛋白质和亚细胞结构稳定剂[14-15]等。有研究发现, 植物在重金属胁迫[16]、碱胁迫[17]、盐胁迫[18]和高温干旱[19]等胁迫下会积累大量脯氨酸。Delauney等[20]认为, 植物体内脯氨酸积累主要是因为胁迫刺激了其从头合成。植物体内合成脯氨酸有2条途径, 分别为谷氨酸途径(glutamate pathway, Glu)和鸟氨酸(ornithine pathway, Orn)途径。参与催化谷氨酸合成脯氨酸的酶主要有Δ1-吡咯啉-5-羧酸合成酶(Δ1-pyrroline-5-carboxylate synthetase, P5CS)和Δ1-吡咯啉-5-羧酸还原酶(Δ1-pyrroline-5- carboxylate reductase, P5CR)[21]。在鸟氨酸途径中, 脯氨酸的合成主要是在鸟氨酸转氨酶(ornithine-δ-aminotransferase, OAT)和Δ1-吡咯啉-5-羧酸还原酶(Δ1-pyrroline-5-carboxylate reductase, P5CR)的催化下进行的[22-23]。其中, P5CS和OAT被认为是脯氨酸合成过程中Glu和Orn途径中的关键酶[24]。植物控制脯氨酸积累的第2个因素是其降解, 该过程是合成途径的逆转, 但涉及的酶不同。脯氨酸在脯氨酸脱氢酶(proline dehydrogenase, ProDH)和吡咯啉-5-羧酸脱氢酶(pyrroline-5-carboxylate dehydrogenase, P5CDH)催化下氧化为谷氨酸, 其中, ProDH被认为是脯氨酸降解途径的限速酶[25-26]。
目前, 关于植物在硼胁迫下的研究较多, 而关于硼胁迫诱导的脯氨酸的合成代谢变化的研究较少。事实上, 脯氨酸的合成与代谢是一个复杂的生理生化过程, 参与脯氨酸累积过程的酶所起的作用程度是不同的。在遭受胁迫时, 植物体内会积累脯氨酸, 但脯氨酸的合成途径究竟是哪一条居于主导地位并不清楚。本研究在前人工作的基础上, 以‘鄂抗10号’为供试材料, 采用营养液培养, 主要研究硼胁迫下棉花幼苗生物量和脯氨酸在不同器官分配积累的不同响应机制, 力求探索其可能的适应机制, 以期进一步揭示硼胁迫如何影响植物体内脯氨酸代谢提高其抗逆性。
1 材料与方法
1.1 试验设计
试验于2019年8月在华中农业大学日光温室进行, 试验材料为‘鄂抗10号’棉花种子。试验设置4个B浓度(B源用H3BO3), 分别为不加硼(B0, 0 mg L-1)、低硼(B0.002, 0.002 mg L-1)、适硼(CK, B0.2, 0.20 mg L-1)和高硼处理(B50, 50 mg L-1), 每个处理设置4个重复。试验所用营养液参考Hoagland和Arnon[27]。各营养物质用量如下: 0.240 g L-1NH4NO3、0.100 g L-1Na2HPO4·12H2O、0.100 g L-1NaH2PO4·2H2O、0.360 g L-1CaCl2·2H2O、0.500 g L-1MgSO4·7H2O、1.81 mg L-1MnCl2·4H2O、0.22 mg L-1ZnSO4·7H2O、0.08 mg L-1CuSO4·5H2O、0.09 mg L-1Na2MoO4·2H2O和32.5 mg L-1Fe-EDTA, 所用试剂均为分析纯。
1.2 试验方法
挑选均匀饱满的种子, 在36℃条件下烘箱处理48 h以破除种子休眠, 然后用60℃的温开水浸泡6 h, 将浸泡好的种子置于湿润的纱布上催芽, 待初生根长至1 cm左右时再将其置于塑料桶上继续培养。子叶展开后转移到5 L黑塑料袋避光的塑料桶中加入营养液培养, 每桶2株。先用1/4浓度营养液培养1周, 再用1/2浓度营养液培养1周, 继而用全量浓度进行后期培养, 总共培养38 d后收获。每4 h通气20 min, 每周更换1次营养液。
1.3 样品采集与测定
1.3.1 样品采集 将采集的样品用超纯水洗净, 分为根、茎和叶3个部位。各部位分别称鲜重后装入信封袋, 于105℃的烘箱中杀青30 min, 后在75℃下烘干至恒重, 称完干物质质量后粉碎过筛用于硼含量的测定。
1.3.2 样品测定 根系形态和生长参数的测定: 采用根系扫描仪(Epson Perfection V700)扫描根系, 并使用WinRHIZO根系分析系统测定总根长(total root length, TRL)、总表面积(total surface area, TSA)、根总体积(total root volume, TRV)、侧根数、根尖数和根平均直径(average diameter of root, RAD)等根系生长指标。硼含量测定: 称取粉碎后的样品在马弗炉中500℃干灰化5 h, 用0.1 mol L-1的HCl溶液浸提, 取滤液用姜黄素比色法测定各部位硼含量[28]。脯氨酸含量的测定: 参照王学奎[29]和吴成龙等[17]的方法。称取根和叶片干样0.02 g, 用3%磺基水杨酸溶液5 mL进行研磨提取, 在沸水浴中提取10 min, 冷却之后吸取2 mL滤液置于25 mL试管中, 加入冰醋酸和酸性茚三酮溶液各2 mL, 沸水浴加热30 min后加入4 mL甲苯显色, 在520 nm处比色。脯氨酸代谢酶的测定: 参照Shan等[30]和Rocha等[31]方法提取P5CS、ProDH、P5CR和OAT四种酶液, 略有修改, 采用Elisa试剂盒(江苏酶标生物有限公司)测定酶活。由于不加硼(B0)处理下植株生长受到严重抑制, 收样时生物量较少, 故没有足够的材料用于脯氨酸含量及脯氨酸代谢酶活性的测定。
1.4 数据处理
采用Microsoft Excel软件作图, 用统计分析软件SPSS 21对各处理试验数据进行统计和分析, 运用Duncan程序进行不同处理之间的差异显著性检验, 显著水平为<0.05。
2 结果与分析
2.1 不同硼浓度处理对棉花幼苗生物量的影响
由图1可以看出, 不加硼(B0)和低硼(B0.002)处理下植株的根系生长明显受到抑制。B0处理下植株老叶叶片变厚变脆, 顶芽受损, 腋芽大量发生而形成多头棉; 新叶较小, 边缘及主脉失绿, 叶片上卷呈杯状; 根短粗, 兼有褐色。高硼(B50)处理下棉花幼苗老叶最先出现硼毒症状, 主要表现在老叶叶尖和叶片边缘灼伤、失绿黄化, 出现黄褐色坏死斑点, 且下部叶的中毒症状和生理变化比上部叶明显。
B0: 0mg L-1硼处理; B0.002: 0.002mg L-1硼处理; B0.2: 对照, 0.2 mg L-1硼处理; B50: 50 mg L-1硼处理。
B0: 0mg L-1of boron treatment; B0.002: 0.002mg L-1of boron treatment; B0.2: CK, 0.2 mg L-1of boron treatment; B50: 50 mg L-1of boron treatment.
与CK相比, 其他3种处理下植株的根、茎、叶以及整株植株的干鲜重明显降低且达到显著差异, 其中B0处理对植株干鲜重影响较大(表1)。B0条件下植株的根、茎、叶鲜重较CK分别降低78.86%、78.24%、66.16%, B0.002处理下植株的根、茎、叶鲜重较CK分别降低49.71%、46.37%、38.51%, B50处理下植株的根、茎、叶鲜重较CK分别降低47.43%、39.64%、39.52%, 表明植株受缺硼影响较其他胁迫处理大, 与其他部位相比, 植株根系对硼胁迫反应更敏感。与低硼处理相比, 高硼处理下植株的根和茎鲜重有增加的趋势而叶片的鲜重降低, 但差异不显著。
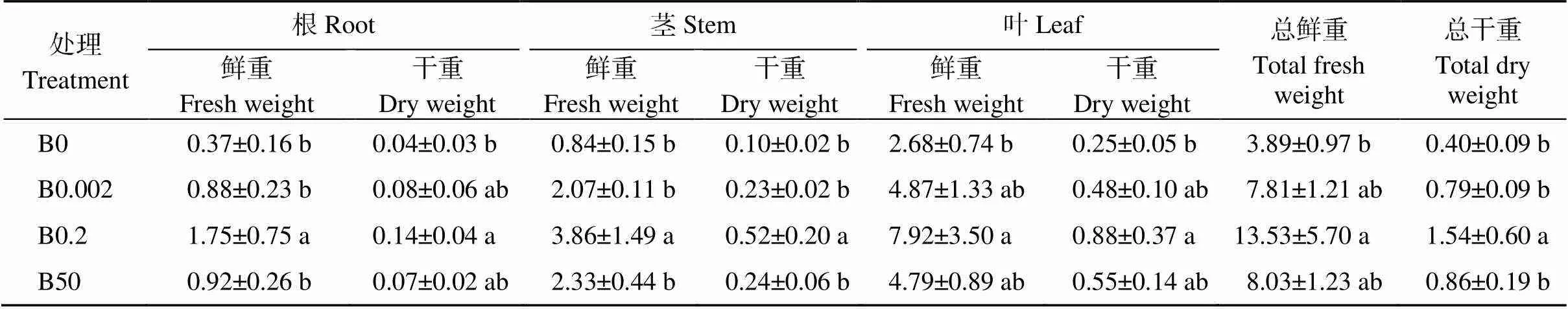
表1 不同硼处理对棉花幼苗生长指标的影响
表中数据代表平均值±标准误差, 含4个独立的重复试验。同列数值后不同小写字母表示处理间在0.05水平差异显著。B0: 0 mg L-1硼处理; B0.002: 0.002 mg L-1硼处理; B0.2: 对照, 0.2 mg L-1硼处理; B50: 50 mg L-1硼处理。
The data in the table represent the mean ± standard error, including four independent repetitions. Values with a column followed by different lowercase letters represent significant differences among the treatments at the 0.05 probability level. B0: 0 mg L-1of boron treatment; B0.002: 0.002 mg L-1of boron treatment; B0.2: CK, 0.2 mg L-1of boron treatment; B50: 50 mg L-1of boron treatment.
2.2 不同硼浓度处理对棉花幼苗根系形态指标的影响
当硼浓度从低水平增加到正常水平时, 植株的总根长(TRL)、总表面积(TSA)、根总体积(TRV)、侧根数、根尖数也相应增加且差异显著(表2)。而当硼处理从正常水平进一步提高到过量水平时, 这些根系生长参数显著降低。不加硼、低硼和高硼处理下的根长较正常硼处理分别减少88.54%、47.06%、40.47%, 侧根数分别减少90.46%、52.13%、45.21%, 总表面积分别减少85.63%、48.95%、42.21%。根平均直径(RAD)随硼浓度的增加呈梯度性下降, 在正常硼处理时有所恢复但到高硼处理时又下降, 不加硼处理时达到了最大值, 不加硼条件下的根较CK平均直径增加24.64%。表明缺硼及高硼处理都会影响植株根系的生长, 导致根系生长能力下降, 影响作物吸收能力。
2.3 不同硼浓度处理对棉花幼苗各部位硼含量及积累量的影响
在供硼处理下, 随着硼浓度的升高, 根、茎和叶中的硼含量均呈上升趋势, 其中叶片中硼含量的差异达到显著水平(表3)。在正常硼和高硼条件下, 硼含量显示为叶片>根>茎; 而在不加硼和低硼条件下, 硼含量显示为根>叶片>茎。B0处理植株叶片中的硼含量较CK显著降低73.05%。
无论何种处理, 植株叶片中的硼积累量均显著高于茎和根中。随着硼浓度的升高, 植株各部位硼积累量均显著增加。叶片中各硼处理间的差异较根和茎中显著, B0、B0.002处理下叶片中硼积累量较CK分别降低92.63%、82.74%, B50处理下升高3.5倍。
2.4 不同硼浓度处理对棉花幼苗叶片和根中脯氨酸含量的影响
由表4可知, 低硼(B0.002)和高硼(B50)处理下叶片中脯氨酸的含量较CK分别增加8.32%和26.87%; 而在根中, 脯氨酸含量分别降低26.84%和34.51%。在所有处理中, 植株不同部位的脯氨酸含量均显示为叶片高于根。
2.5 不同硼浓度处理对棉花幼苗叶片和根中脯氨酸代谢酶活性的影响
由图2可知, B0.002处理下叶片中OAT、P5CS酶活性较CK分别增加19.41%和17.38%; 相同的, B0.002处理下根中OAT、P5CS酶活性增加, 但OAT酶活性未达到显著差异。与CK相比, 低硼条件下叶片和根中ProDH酶活性呈现出相反的趋势, 即根中ProDH酶活性显著增加29.60%, 叶片中ProDH酶活性显著降低。高硼处理下叶片中OAT、P5CR、P5CS酶活性显著增加, 而ProDH酶活性降低。高硼处理下根中OAT、P5CR酶活性较CK显著降低23.40%和63.83%, 而P5CS和ProDH酶活性出现降低趋势, 但未达到显著差异。
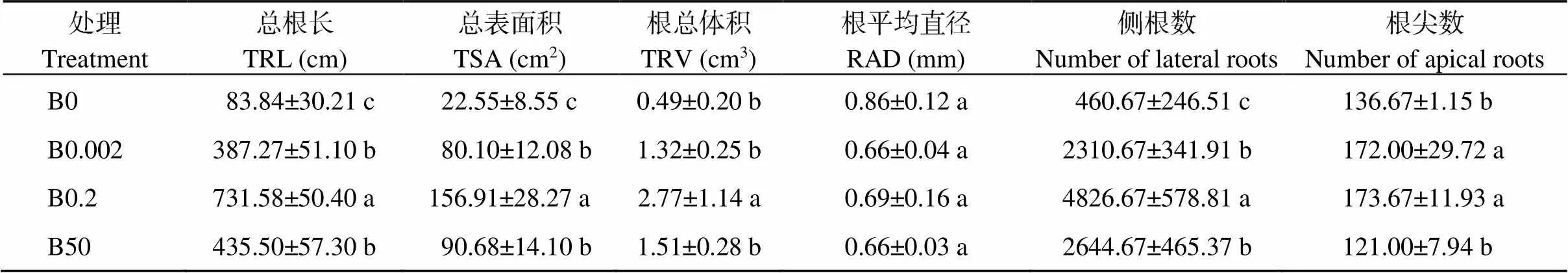
表2 不同硼处理对棉花幼苗根系生长的影响
表中数据代表平均值±标准误差, 含4个独立的重复试验。同列数值后不同小写字母表示处理间在0.05水平差异显著。处理同表1。
The data in the table represent the mean ± standard error, including four independent repetitions. Values with a column followed by different lowercase letters represent significant differences among the treatments at the 0.05 probability level. TRL: total root length; TSA: total surface area; TRV: total root volume; RAD: average diameter of root. Treatments are the same as those given in Table 1.
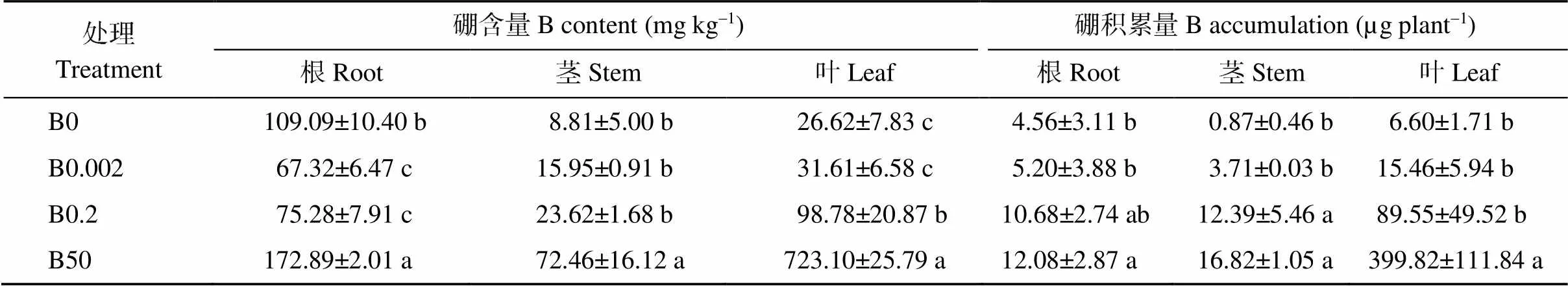
表3 不同硼浓度处理对棉花幼苗各部位硼含量及积累量的影响
表中数据代表平均值±标准误差, 含4个独立的重复试验。同列数值后不同小写字母表示处理间在0.05水平差异显著。处理同表1。
The data in the table represent the mean ± standard error, including four independent repetitions. Values with a column followed by different lowercase letters represent significant differences among the treatments at the 0.05 probability level. Treatments are the same as those given in Table 1.
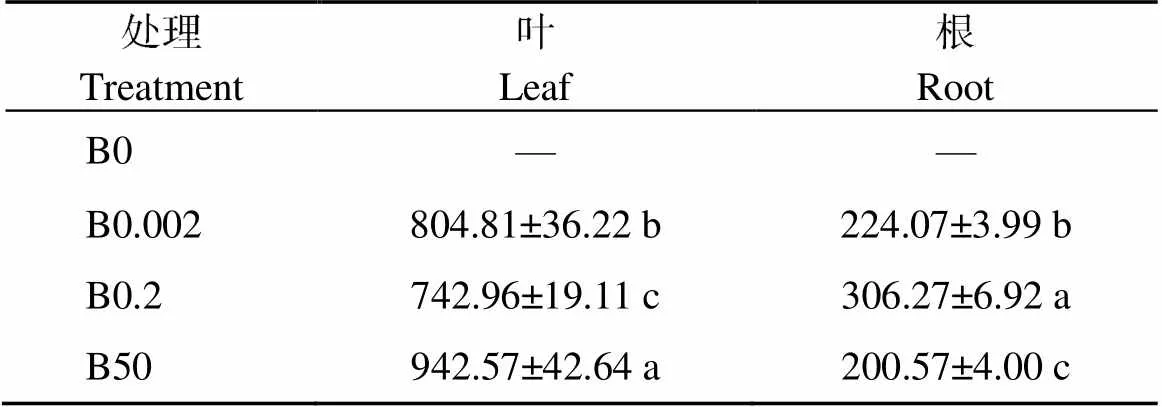
表4 不同硼浓度处理对棉花幼苗叶片和根中脯氨酸含量的影响
表中数据代表平均值±标准误差, 含4个独立的重复试验。同列数值后不同小写字母表示处理间在0.05水平差异显著。处理同表1。
The data in the table represent the mean ± standard error, including four independent repetitions. Values with a column followed by different lowercase letters represent significant differences among the treatments at the 0.05 probability level. Treatments are the same as those given in Table 1.
3 讨论
3.1 低硼及高硼胁迫对棉花幼苗生长的影响
众所周知, 硼是植物必需的微量元素之一[1], 硼缺乏或过量都会对植物的生长发育造成伤害。耿明建等[32]通过对不同硼效率棉花品种根系参数差异研究发现, 缺硼显著抑制棉花根系的伸长, 各品种根体积、根长、总吸收面积等根系生长参数均显著降低。刘磊超等[33]研究发现, 缺硼显著降低枳橙幼苗的干物质积累, 抑制其生长。本试验结果也发现相似的结论, 即缺硼显著抑制棉花幼苗生长, 降低植株的干鲜重。此外, 硼缺乏也会降低棉花幼苗的总根长、根尖数、总根体积和总根表面积, 抑制根的生长和新根的形成。高硼胁迫下, 植物的生长发育同样受到影响。桑雯等[34]通过对硼毒下柑橘根叶蛋白质组学研究发现, 硼毒害严重影响柑橘根、茎、叶的生长, 老叶端和叶边缘变黄并随时间延长而坏死脱落。本试验结果发现, 高硼胁迫下, 首先在叶尖观察到叶黄化, 然后扩展到边缘, 最后整片叶子, 与幼叶相比, 老叶更早的受到硼毒性的影响, 且硼毒症状并未在根系中发现, 分析原因可能是棉花属于硼难以在韧皮部中移动的植物, 所以当硼进入根系后在植物体内主要随着蒸腾流通过木质部向上运输, 从而使老叶最先出现硼毒害症状[35-37]。与叶片相比, 即使处于高硼处理(B50)下, 棉花根系硼的积累量仍然较低, 这也解释了硼毒症状未出现在根系上的原因之一。本研究中, 硼过量时, 棉花幼苗根系总长度、表面积和体积等参数明显降低, 这表明50 mg L-1浓度硼对植株产生毒害作用, 进而抑制根系生长。Asad等[38]在柑橘研究中发现, 硼过量时抑制柑橘幼苗根系的生长、根长和直径降低。
3.2 低硼及高硼胁迫对棉花幼苗叶片和根硼含量的影响
植物具有感知硼水平的变化并调节其在体内重新分配的能力, 这种调节作用对于硼胁迫条件下保证植物生存具有重要的意义[39]。张君等[40]研究发现, 给陆地棉叶缘施硼后, 根系中的硼含量下降。本试验研究发现, 在正常硼和高硼条件下, 棉株叶片中的硼含量高于根和茎, 说明当叶片吸收的硼已经能够满足植物对硼的需求时, 那么根系对硼的吸收量就会减少。Liu等[41]研究发现, 纽荷尔脐橙在低硼胁迫下, 硼由根向地上部的运转受到限制, 导致根中硼含量高于叶中。本试验研究中也发现, 在不加硼和低硼条件下, 棉株根中硼含量高于叶和茎中, 其原因可能是: (1)缺硼导致棉花幼苗显著发育不良, 叶面积小, 从而蒸腾作用大幅下降, 抑制了硼向上运输; (2) 硼缺乏造成植物根尖疏导组织发育不良, 引起导管组织损伤[42-43], 使硼难以向地上部运输, 造成根中硼含量明显高于叶片和茎中。通过对棉花幼苗各部位硼积累量的测定分析发现, 无论何种处理, 植株叶片中硼积累量均显著高于茎和根中。植株各部位硼积累量随着硼浓度的提高均显著增加, 叶片中各硼处理间的差异较根和茎中显著。卢晓佩等[44]研究发现, 在不同硼处理下, 2种砧木叶片中硼积累量均显著高于根和茎中。
柱上不同小写字母分别表示在根、叶片中处理间在0.05水平上差异显著。处理同表1。
Bars labeled with different lowercase letters represent significant differences among the treatments at the 0.05 probability level in roots and leaves, respectively. Treatments are the same as those given in Table 1.
3.3 低硼及高硼胁迫对棉花幼苗叶片和根脯氨酸含量的影响
脯氨酸的积累是植物受到逆境胁迫的一种信号, 大量积累的脯氨酸可调节胞内渗透势, 减轻胁迫对植物造成的伤害。通过研究外源脯氨酸对缺硼下棉花幼苗生长和脯氨酸代谢影响中发现, 在缺硼胁迫时, 棉花幼苗叶片和根中的脯氨酸含量均显著增加[45]。Riaz等[46]在枳壳中发现, 缺硼处理可促进枳壳幼苗叶片中脯氨酸的积累。研究表明, 植物体内游离脯氨酸的含量在不同部位表现不同, 其多集中于光合器官和生殖器官中[47]。本试验发现, 在硼胁迫下, 棉花幼苗叶片中的脯氨酸含量显著增加, 其脯氨酸含量是根的数倍, 说明棉花在硼胁迫下优先保护其光合器官, 以适应逆境胁迫, 提高其抗逆性。脯氨酸生物合成和降解的限速步骤分别由Δ1-吡咯啉-5-羧酸合成酶(P5CS)和脯氨酸脱氢酶(ProDH)催化[16]。本试验结果显示, 棉花在硼胁迫下, 其脯氨酸的积累是由Glu和Orn途径共同起作用的, 其合成和代谢途径见图3。低硼胁迫显著提高叶片和根中Orn途径OAT合成酶和Glu途径P5CS合成酶活性, 降低P5CR合成酶活性。但是由于P5CS合成酶是脯氨酸合成途径中的关键酶, 脯氨酸的积累受P5CS合成酶的影响要高于P5CR合成酶, 且低硼胁迫降低叶片中的ProDH降解酶的活性, 提高了根中ProDH降解酶的活性, 使得叶片中脯氨酸的合成速率高于其降解, 根中脯氨酸的降解高于其合成, 最终棉花幼苗叶片中脯氨酸含量高于根中, 导致叶片中脯氨酸的积累。同样, 在高硼胁迫下, 叶片中Orn途径OAT合成酶和Glu途径P5CS合成酶及P5CR合成酶活性均显著提高, 而ProDH降解酶活性显著降低, 使得叶片中脯氨酸的合成远高于其降解, 最终叶片中积累了大量脯氨酸; 而在根中, 主要是通过降低OAT和P5CS合成酶以及ProDH分解酶活性来抑制脯氨酸的合成及其分解, 但是对脯氨酸合成的抑制作用远大于其降解, 最终导致棉花幼苗在高硼胁迫下根系脯氨酸含量降低。Liu等[48]的研究结果发现, 小麦幼苗在盐胁迫下, 当使用100 mmol L-1NaCl处理时, 小麦幼苗根部脯氨酸的积累最高, 此时P5CS合成酶活性最高, 而ProDH降解酶活性较低, 使得根中积累了大量脯氨酸来缓解盐分胁迫带来的伤害。
Glutamate: 谷氨酸; Ornithine: 鸟氨酸; GSA: 谷氨酰半醛; P5C: 吡咯啉-5-羧酸; Proline: 脯氨酸; P5CS: Δ1-吡咯啉-5-羧酸合成酶; P5CR: Δ1-吡咯啉-5-羧酸还原酶; OAT: 鸟氨酸转氨酶; ProDH: 脯氨酸脱氢酶; P5CDH: 吡咯啉-5-羧酸脱氢酶。
GSA:g-glutamate-semialdehyde; P5C: pyrroline-5-carboxylate; P5CS: Δ1-pyrroline-5-carboxylate synthetase; P5CR: Δ1-pyrroline-5-carboxylate reductase; OAT: ornithine-δ-aminotransferase; ProDH: proline dehydrogenase; P5CDH: pyrroline-5-carboxylate dehydrogenase.
[1] 徐芳森, 王运华. 我国作物硼营养与硼肥施用的研究进展. 植物营养与肥料学报, 2017, 23: 1556–1564.Xu F S, Wang Y H. Advances in studies on crop boron nutrition and application of boron fertilizers in China., 2017, 23: 1556–1564 (in Chinese with English abstract).
[2] Marschner C, Marschner H. Mineral nutrition of higher plants., 1996, 148: 765–765.
[3] 姜存仓, 王运华, 刘桂东, 夏颖, 彭抒昂, 钟八莲, 曾庆銮. 赣南脐橙叶片黄化及施肥效应研究. 植物营养与肥料学报, 2009, 15: 656–661.Jiang C C, Wang Y H, Liu G D, Xia Y, Peng S A, Zhong B L, Zeng Q L. Effect of boron on the leaves etiolation and fruit fallen of Newhall Navel Orange., 2009, 15: 656–661 (in Chinese with English abstract).
[4] 李继福, 何俊峰, 陈佛文. 中国棉花生产格局与施肥研究现状: 基于CNKI数据计量分析. 中国棉花, 2019, 46(4): 17–24.Li J F, He J F, Chen F W. Status of cotton planting and fertilization research in China: based on CNKI data analysis., 2019, 46(4): 17–24 (in Chinese with English abstract).
[5] 张学斌. 施硼对棉花生理生化和产量品质的影响. 西南大学硕士学位论文, 重庆, 2008.Zhang X B. The Physiology Biochemistry and Output the Quality Effect of Cotton Spraying Different Boron. MS Thesis of Southwest University, Chongqing, China, 2008 (in Chinese with English abstract).
[6] 王运华, 刘武定, 皮美美, 王治荣. 我国主要棉区缺硼概况与施硼分区. 华中农业大学学报. 1989, 6(增刊1): 153–157.Wang Y H, Liu W D, Pi M M, Wang Z R. B-deficiency in cotton and division of B-application in important producing cotton area of China., 1989, 6(S1): 153–157 (in Chinese with English abstract).
[7] 李鸣凤. 硼氮互作下棉花生理代谢及叶柄环带形成差异研究. 华中农业大学博士学位论文, 湖北武汉, 2019.Li M F. Study on the Difference of Cotton Physiological Metabolism and Petiole Ring Formation Under Interaction of Boron and Nitrogen. Ph.D. Dissertation of Huazhong Agricultural University, Wuhan, Hubei, China, 2019 (in Chinese with English abstract).
[8] Ahmad S, Akhtar L H, Iqbal N, Nasim M. Short communication cotton (L.) varieties responded differently to foliar applied boron in terms of quality and yield., 2009, 28: 88–92.
[9] 马欣. 硼肥Etibor-48和Colemanite硼释放特性及其对作物产量和品质的影响. 华中农业大学硕士学位论文, 湖北武汉, 2011. Ma X. Evaluation of Boron Release Characterization of Boron Fertilizers Etibor-48 and Colemanite and Effects of Them on Crops Yield and Quality. MS Thesis of Huazhong Agricultural University, Wuhan, Hubei, China, 2011 (in Chinese with English abstract).
[10] 操宇琳, 田绍仁, 杨绍群. 棉花缺硼与硼肥施用技术. 棉花科学, 2011, 33(6): 58–61.Cao Y L, Tian S R, Yang S Q. Boron deficiency of cotton and application technology of boron fertilizer., 2011, 33(6): 58–61 (in Chinese with English abstract).
[11] Yang S L, Chen K, Wang S S, Gong M. Osmoregulation as a key factor in drought hardening-induced drought tolerance in., 2015, 59: 529–536.
[12] Jiang M Y, Guo S C, Zhang X M. Proline accumulation in rice seedlings exposed to hydroxyl radical stress in relation to antioxidation., 1997, 42: 855–859.
[13] Giberti S, Funck D, Forlani G. Δ1-pyrroline-5-carboxylate reductase from: stimulation or inhibition by chloride ions and feedback regulation by proline depend on whether NADPH or NADH acts as co-substrate., 2014, 202: 911–919.
[14] Szabados L, Savouré A. Proline: a multifunctional amino acid., 2010, 15: 89–97.
[15] 王翠平, 严莉, 乔改霞, 李健. 脯氨酸通过活性氧信号抑制植物生长. 植物生理学报, 2017, 53: 1788–1794.Wang C P, Yan L, Qiao G X, Li J. Proline inhibits plant growth by reactive oxygen species signaling., 2017, 53: 1788–1794 (in Chinese with English abstract).
[16] 宋敏, 徐文竞, 彭向永, 孔繁华. 外源脯氨酸对镉胁迫下小麦幼苗生长的影响. 应用生态学报, 2013, 24: 129–134.Song M, Xu W J, Peng X Y, Kong F H. Effects of exogenous proline on the growth of wheat seedlings under cadmium stress., 2013, 24: 129–134 (in Chinese with English abstract).
[17] 吴成龙, 周春霖, 尹金来, 刘兆普, 徐阳春, 沈其荣. 碱胁迫对不同品种菊芋幼苗生物量分配和可溶性渗透物质含量的影响. 中国农业科学, 2008, 41: 901–909. Wu C L, Zhou C L, Yin J L, Liu Z P, Xu Y C, Shen Q R. Effects of alkaline stress on biomass allocation and the contents of soluble osmoticum in different organs of twoL. genotypes., 2008, 41: 901–909 (in Chinese with English abstract).
[18] Wang H Y, Tang X L, Wang H L, Shao H B. Proline accumulation and metabolism-related genes expression profiles inseedlings under salt stress., 2015, 6: 792.
[19] Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y. Effects of free proline accumulation in petunias under drought stress., 2005, 56: 1975–1981.
[20] Delauney A J, Verma D P S. Proline biosynthesis and osmoregulation in plants., 1993, 4: 215–223.
[21] 许祥明, 叶和春, 李国凤. 脯氨酸代谢与植物抗渗透胁迫的研究进展. 植物学通报, 2000, 17: 536–542.Xu X M, Ye H C, Li G F. Progress in synthesis and metabolism of proline and its relationship with osmotolerance of plants., 2000, 17: 536–542 (in Chinese with English abstract).
[22] Liang X W, Zhang L, Natarajan S K, Becker D F. Proline mechanisms of stress survival., 2013, 19: 998–1011.
[23] Iqbal N, Umar S, Khan N A, Khan M I R. A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism., 2014, 100: 34–42.
[24] 余光辉. 水分胁迫下假俭草脯氨酸累积的ABA, Ca2+调节. 华南师范大学硕士学位论文, 广东广州, 2003.Yu G H. The Regulation of ABA, Ca2+on Proline Accumulation inunder Water Stress. MS Thesis of South China Normal University, Guangzhou, Guangdong, China, 2003 (in Chinese with English abstract).
[25] Kishor P B K, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue?, 2014, 37: 300–311.
[26] Yang S L, Lan S S, Gong M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings., 2009, 166: 1694–1699.
[27] Hoagland D R, Arnon D I. The water-culture method for growing plants without soil., 1950, 347: 1–32.
[28] 鲍士旦. 土壤农化分析(第3版). 北京: 中国农业出版社, 2000. pp 123–124.Bao S D. Soil and Agricultural Chemistry Analysis, 3rd edn. Beijing: China Agriculture Press, 2000. pp 123–124 (in Chinese).
[29] 王学奎. 植物生理生化实验原理和技术(第2版). 北京: 高等教育出版社, 2006. pp 278–279.Wang X K. Principles and Techniques of Plant Physiological Biochemical Experiment, 2nd edn. Beijing: Higher Education Press, 2006. pp 278–279 (in Chinese).
[30] Shan T M, Peng J, Zhang Y, Huang Y P, Wang X L, Zheng Y H. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage., 2016, 114: 104–110.
[31] Rocha I M A D, Vitorello V A, Silva J S. Exogenous ornithine is an effective precursor and the ornithine-aminotransferase pathway contributes to proline accumulation under high N recycling in salt-stressed cashew leaves., 2012, 169: 41–49.
[32] 耿明建, 朱建华, 吴礼树, 刘武定. 不同硼效率棉花品种根系参数和伤流液组分的差异. 土壤通报, 2006, 37: 744–747.Geng M J, Zhu J H, Wu L S, Liu W D. Differences in root characters and composition of root bleeding sap between several cotton cultivars with different boron efficiency., 2006, 37: 744–747 (in Chinese with English abstract).
[33] 刘磊超, 姜存仓, 刘桂东, 董肖昌, 吴秀文.低硼胁迫对柑橘枳橙砧木生长及营养生理的影响. 华中农业大学学报, 2015, 34(3): 64–68.Liu L C, Jiang C C, Liu G D, Dong X C , Wu X W. Effects of boron stress on seedling growth and nutrition physiology of navel orange root stock., 2015, 34(3): 64–68 (in Chinese with English abstract).
[34] 桑雯. 硼毒下柑橘根叶蛋白质组学研究. 福建农林大学硕士学位论文, 福建福州, 2013.Sang W. Proteomic Analysis of Citrus Roots and Leaves in Response to Boron Toxicity. MS Thesis of Fujian Agriculture and Forestry University, Fuzhou, Fujian, China, 2013 (in Chinese with English abstract).
[35] Han S, Tang N, Jiang H X, Yang L T, Li Y, Chen L S. CO2assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress., 2008, 176: 143–153.
[36] Ross O N, Gary S B, Jeffrey G P. Boron toxicity., 1997, 193: 181–198.
[37] Sheng O, Song S W, Peng S A, Deng X X. The effects of low boron on growth, gas exchange, boron concentration and distribution of ‘Newhall’ navel orange (Osb.) plants grafted on two rootstocks., 2009, 121: 278–283.
[38] Shah A, Wu X W, Ullah A, Ullah A, Fahad S, Muhammad R, Yan L, Jiang C C. Deficiency and toxicity of boron: alterations in growth, oxidative damage and uptake by citrange orange plants., 2017, 145: 575–582.
[39] Tanaka M, Fujiwara T. Physiological roles and transport mechanisms of boron: perspectives from plants., 2008, 456: 671–677.
[40] 张君, 危常州, 梁远航, 李美宁, 董鹏. 陆地棉对叶面施硼的吸收和分配. 棉花学报, 2012, 24: 331–335.Zhang J, Wei C Z, Liang Y H, Li M N, Dong P. Absorption and distribution of foliar applied boron in upland cotton., 2012, 24: 331–335 (in Chinese with English abstract).
[41] Liu G D, Jiang C C, Wang Y H. Distribution of boron and its forms in young “Newhall” navel orange (Osb.) plants grafted on two rootstocks in response to deficient and excessive boron., 2011, 57: 93–104.
[42] Dell B, Huang L. Physiological response of plants to low boron., 1997, 193: 103–120.
[43] 刘磊超, 姜存仓, 董肖昌, 吴秀文, 刘桂东, 卢晓佩. 硼胁迫对枳橙砧木细根根尖成熟区和幼嫩叶片细胞结构的影响. 中国农业科学, 2015, 48: 4957–4964.Liu L C, Jiang C C, Dong X C, Wu X W, Liu G D, Lu X P. Effects of boron deficiency on cellular structures of maturation zone from root tips and functional leaves from middle and upper plant in trifoliate orange rootstock., 2015, 48: 4957–4964 (in Chinese with English abstract).
[44] 卢晓佩. 不同硼敏感型柑橘砧木对硼胁迫的响应差异及机理. 华中农业大学硕士学位论文, 湖北武汉, 2017.Lu X P. Different Response and Mechanism of Different Citrus Rootstock under Boron Stress. MS Thesis of Huazhong Agricultural University, Wuhan, Hubei, China, 2017 (in Chinese with English abstract).
[45] 曾钰, 闫磊, 刘亚林, 曾紫君, 姜存仓. 外源脯氨酸对缺硼下棉花幼苗生长、生理特性以及脯氨酸代谢的影响. 棉花学报, 2020, 32: 258–268.Zeng Y, Yan L, Liu Y L, Zeng Z J, Jiang C C. Effects of exogenous proline on the growth, physiological characteristics, and proline metabolism of cotton seedlings under boron deficiency stress., 2020, 32: 258–268 (in Chinese with English abstract).
[46] Riaz M, Yan L, Wu X W, Hussain S, Aziz O, Wang Y H, Imran M, Jiang C C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury., 2018, 208: 149–158.
[47] 陈托兄, 张金林, 陆妮, 王锁民. 不同类型抗盐植物整株水平游离脯氨酸的分配. 草业学报, 2006, 15(1): 36–41.Chen T X, Zhang J L, Lu N, Wang S M. The characteristics of free proline distribution in various types of salt-resistant plants., 2006, 15(1): 36–41 (in Chinese with English abstract).
[48] Liu L J, Huang L, Lin X Y, Sun C L. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings., 2020, 39: 567–575.
Effects of boron deficiency/toxicity on the growth and proline metabolism of cotton seedlings
ZENG Zi-Jun, ZENG Yu, YAN Lei, CHENG Jin, and JIANG Cun-Cang*
College of Resources and Environment, Huazhong Agricultural University / Microelement Research Center, Wuhan 430070, Hubei, China
To investigate the response of cotton seedlings growth and proline metabolism to low- and excess-boron stress treatment conditions, the experiment was conducted using ‘E kang 10’ as experimental material using hydroponic method in a greenhouse of Huazhong Agricultural University. Boron (B) were applied at four levels at 0 mg L-1(B0, no boron), 0.002 mg L-1(B0.002, low concentration boron), 0.20 mg L-1(B0.2, CK, sufficient concentration boron), and 50 mg L-1(B50, high concentration boron). The results showed that B0, B0.002, and B50 treatments significantly decreased the fresh and dry weights of plants, and inhibited root elongation relative to sufficient boron (CK, B0.2) treatment. With the increase of B concentration, B content in roots, stems, and leaves of cotton seedlings increased gradiently. Among them, B contents in the leaves under B0.2 and B50 treatments were higher than those in roots and stems, while B content in the roots was increased in leaves and stems under B0 and B0.002 treatments. Under low- and high-boron stress treatments, the content of proline in leaves increased dramatically, while proline in roots decreased. Further analysis of related enzyme activities in proline metabolism, we found that B0.002 and B50 treatments promoted the activities of Δ1-pyrroline-5-carboxylate synthetase (P5CS) and ornithine-δ-aminotransferase (OAT) in leaves, but decreased the activity of proline dehydrogenase (ProDH) compared to CK; and the activity of Δ1-pyrroline-5- carboxylate reductase (P5CR) in leaves under B50 treatment was obviously increased, but there was no significant difference under B0.002 treatment. In addition, compared with CK, B50 treatment reduced the enzyme activities of OAT and P5CR in roots, while B0.002 treatment prominently increased the activities of P5CS and ProDH.The results showed that both low- and excess-boron stress inhibited the growth of cotton seedlings. Under boron stress, proline mainly was accumulated in the leaves of cotton seedlings, and the content of proline in roots decreased significantly. In the case of boron deficiency and boron toxicity, the accumulation of proline in leaves was mainly through regulating the activities of key enzymes (OAT, P5CS synthetase, and ProDH degrading enzyme) in proline Glu and Orn pathways, resulting in the proline synthesis rate higher than its degradation rate. However, in roots, the proline content was decreased mainly by promoting the degradation of proline under boron deficiency stress. Under high boron stress, proline synthesis and decomposition were inhibited mainly by reducing the activities of OAT, P5CS synthetase and ProDH decomposing enzyme, but the inhibitory effect on proline synthesis was much greater than its degradation, which eventually led to the decrease of proline content in roots.
cotton; boron; proline; synthesis and degradation
本研究由国家自然科学基金项目(41271320)和中央高校基本科研业务费专项资金项目(2017PY055)资助。
This study was supported by the National Natural Science Foundation of China (41271320)and the Fundamental Research Funds for the Central Universities (2017PY055).
10.3724/SP.J.1006.2021.04206
姜存仓, E-mail: jcc2000@mail.hzau.edu.cn
E-mail: zjzeng.hzau.edu.cn@webmail.hzau.edu.cn
2020-09-08;
2021-01-13;
2021-02-18.
URL: https://kns.cnki.net/kcms/detail/11.1809.S.20210217.0858.002.html
