Understanding celiac disease monitoring patterns and outcomes after diagnosis: A multinational,retrospective chart review study
2021-06-05KnutEALundinCiaranKellyDavidSandersKristinaChenSheenaKayaniyilSisiWangRajviWaniCaitlinBarrettShakiraYoosufEllenPettersenRobertSambrookDanielLeffler
Knut EA Lundin, Ciaran P Kelly, David S Sanders, Kristina Chen, Sheena Kayaniyil, Sisi Wang, Rajvi J Wani,Caitlin Barrett, Shakira Yoosuf, Ellen S Pettersen, Robert Sambrook, Daniel A Leffler
Abstract
Key Words: Celiac disease; Outcomes research; Endoscopy; Real-world; General practice;Villous atrophy
INTRODUCTION
Celiac disease is a chronic, immune-mediated disorder that affects genetically susceptible individuals. The only accepted current standard of care for celiac disease is a life-long gluten-free diet (GFD). Previous studies have reported that adherence rates to a GFD range between 42 % and 91 %[1 ,2 ]. Inadequately managed celiac disease can lead to health complications such as malnutrition, osteoporosis, neurologic complaints,and lymphoma[2 ]. It has been hypothesized that long-term management and regular follow-up of patients with celiac disease will improve adherence to a GFD, and improve disease outcomes including mucosal healing and symptom resolution. For this reason, long-term management and regular follow-up of patients with celiac disease are advocated by current practice guidelines[3 ,4 ], yet it is unclear how these are actually implemented in practice. It is understood, however, that practice patterns vary widely both between countries and between practices.
Given that celiac disease is a chronic disorder, it is important to understand realworld, long-term outcomes and routine monitoring practices; however, there are few published data in these areas. Therefore, the aims of this multinational study were twofold. First, to understand, in real-world clinical practice, patterns of patient followup and management and how these practices vary by country. The second aim was to characterize patient outcomes, specifically related to ongoing symptoms and ongoing villous atrophy after diagnosis. Together, these data may be helpful in informing clinical practice, studies, and interventions aimed at improving celiac disease outcomes, and for quality improvement initiatives.
MATERIALS AND METHODS
A retrospective chart review study was conducted using medical chart data of patients diagnosed with celiac disease. Three large gastroenterology centers with substantial expertise in celiac disease participated, capturing patients in the United Kingdom, the United States, and Norway. Each site contributed 100 patients. Ethics approval was obtained before data collection commenced.
Patients were eligible if they had biopsy-confirmed celiac disease[3 ,5 ,6 ], were diagnosed between 2008 and 2012 , and had at least one follow-up visit before 31 December 2017 . This study period was selected to allow for at least five years of follow-up after diagnosis. Patients were excluded if they had initiated a GFD before receiving a diagnosis of celiac disease.
Using the database of patients at each site, the assigned staff at each center identified eligible patients by first looking at the date of diagnosis. The data abstractor reviewed and identified eligible patients who were diagnosed in December 2012 , and then continued review of eligibility for patients consecutively backwards from that date (back to a diagnosis date in 2008 ). After examining the date of diagnosis, other inclusion/exclusion criteria were assessed to verify patient eligibility for the study. All three sites were explicitly asked to follow the same approach regarding selection of consecutive patients to avoid selection bias. The assigned staff at each site responsible for data abstraction then entered de-identified data for eligible patients into a custom electronic case report form. All data collected were based on the patient’s pre-existing medical record. No direct personal identifiers were attached to the abstracted data.Data describing patient demographic and clinical characteristics, biopsy/serology tests and results, symptoms, and comorbidities were captured at diagnosis and for each clinic visit occurring within the study period (i.e.,before the study end date of December 31 , 2017 ).
In terms of diagnostic testing, available serology results were collected, including tissue transglutaminase-immunoglobulin (Ig) A, IgA endomysial antibody, total serum IgA, deamidated gliadin peptide (DGP) IgA, DGP IgG, and DGP IgA-IgG. As not all pathology reports across sites utilized Marsh-Oberhuber classification, a descriptive assessment of biopsy results was recorded as follows: normal, increased intraepithelial lymphocytes only, mild/partial villous atrophy, subtotal villous atrophy, total/complete atrophy, and other.
Analysis
Data are summarized by descriptive statistics [mean, standard deviation (SD), median,and interquartile range for continuous variables, and number and percentage for categorical variables]. Gastrointestinal symptoms and extraintestinal comorbidities/complications (termed extraintestinal manifestations) are described at diagnosis and during study follow-up.
The presence of symptoms during the follow-up period was characterized specifically for patients who had a symptom at diagnosis and a record of symptoms at least once during follow-up. For each patient, the duration of the follow-up period was calculated as the time from diagnosis to the last follow-up visit within the study period. The mean number of visitsperpatient and the number of follow-up visitsperpatient with biopsy data were summarized overall and by country.
Following the classification proposed by Kurienet al[2 ], subsets of study patients with available symptom (defined as diarrhea, abdominal pain, abdominal distention,poor appetite, weight loss, tiredness/lethargy, brain fog, malabsorption and/or bloating) and biopsy data were grouped into four main disease states at diagnosis and at each follow-up visit: Class 1 (no symptoms and normal duodenal histology); class 2 (no symptoms and abnormal duodenal histology); class 3 (symptoms and normal duodenal histology); class 4 (symptoms and abnormal duodenal histology). This classification provides an intuitive framework for assessing celiac disease outcomes and may help to identify patients with the highest risk of complications. In addition,biopsy results reported as mild/partial/subtotal/total/complete villous atrophy were considered as abnormal histology; all other findings were considered normal for this classification. Those with ‘other’ biopsy findings were excluded in the classification.
Analyses were based on available data. Descriptive statistics were restricted to the subset of patients for whom data were available, with relevant denominator information provided in the results. All analyses were conducted in SAS 9 .4 .
RESULTS
A total of 300 patients with celiac disease were included in this study, comprising 100 patients from each of the three participating gastroenterology referral centers in the United Kingdom, the United States, and Norway. Table 1 presents the demographic and clinical characteristics of included patients at diagnosis.
Patients were, on average, 39 years of age at diagnosis, with 24 patients (8 %) less than 18 years of age; there were 216 females in the study (72 .0 %). The study populations across the three sites were quite similar with respect to age, gender, and ethnicity distributions (Table 1 ). Gastrointestinal symptoms were the most common reason leading to diagnosis. There was a significantly greater proportion of patients in the United Kingdom (57 .0 %, n = 57 ) who presented with extraintestinal manifestations at diagnosis compared with patients in the United States (17 .0 %) and Norway (17 .0 %)(P< 0 .0001 ). Nutritional deficiency was the most commonly reported extraintestinal manifestation in the United States and Norway, whereas in the United Kingdom anemia was most frequently documented at diagnosis (Table 2 ). Almost all (n = 299 ,99 .7 %) patients had an esophagogastroduodenoscopy (EGD) conducted at diagnosis,and two patients (0 .7 %) had an enteroscopy. Overall, 90 .7 % (n = 272 ) of patients had serologic testing concurrently with biopsy, and these findings were similar across patients at the three sites. Biopsy results are presented in Table 1 . Serology results at diagnosis and during the follow-up period are presented in Supplemental Table 1 .
The types of gastrointestinal and extraintestinal manifestations and associated conditions at diagnosis and during follow-up were similar across sites and are presented in Table 2 . At diagnosis, 256 patients (85 .3 %) and 228 patients (76 .0 %) had at least one gastrointestinal or extraintestinal manifestation, respectively. The most common symptoms across all sites were diarrhea, abdominal pain and bloating and the most common laboratory findings included nutrient deficiencies, anemia and low bone mineral density. Interestingly, both weight loss and weight gain were more commonly reported in the United States compared to the United Kingdom and Norway. There were 147 patients (49 .0 %) who presented with diarrhea, 124 (41 .3 %)who presented with abdominal pain, and 90 (30 .0 %) who presented with bloating. In addition, 104 patients (34 .7 %) had documentation of a nutritional deficiency, and 34 patients (11 .3 %) presented with another autoimmune disease, in addition to celiac disease, at diagnosis. During follow-up, diarrhea [n= 100 (33 .3 %)], abdominal pain[n= 93 (31 .0 %)], and bloating [n = 76 (25 .3 %)] continued to be the most frequently reported gastrointestinal symptoms. Of the 256 patients who had gastrointestinal symptoms at diagnosis, 175 (68 .4 %) had at least one visit reporting gastrointestinal symptoms during the follow-up period.
The duration of follow-up and average number of follow-up visits for the overall study population and by country are presented in Table 3 . Patients were followed-up for a mean of 29 .9 mo (SD: 22 .1 ) and there were, on average, three follow-up visitsperpatient during the study period. Patients from the United States site had the longest follow-up duration during the study period (mean: 38 .7 mo), compared with the United Kingdom and Norway sites (mean: 26 .5 and 24 .5 mo, respectively; P < 0 .0001 ).Overall referral patterns to other specialists were captured, indicating that approximately 80 % of patients were referred to a dietician at least once during the follow-up period. Details on the last-recorded follow-up with the patient indicated that almost half (48 %) of all patients had a follow-up appointment scheduled. Some were discharged (approximately 10 %) or were referred to another specialist (approximately 19 %), otherwise, the last follow-up decision was recorded as ‘unknown’ or ‘other’.
After EGD, bone densitometry was the next most frequently reported procedure during follow-up, performed in 89 patients (29 .7 %) from the overall study population.Bone densitometry was performed at least once in 45 United States patients (45 .0 %)during the follow-up period, compared with patients who received this procedure in
the United Kingdom and Norway [n= 22 (22 .0 %) for both United Kingdom and Norway patients;P< 0 .001 ]. As this procedure is not performed in the gastroenterology unit, the results of these tests were not routinely available.
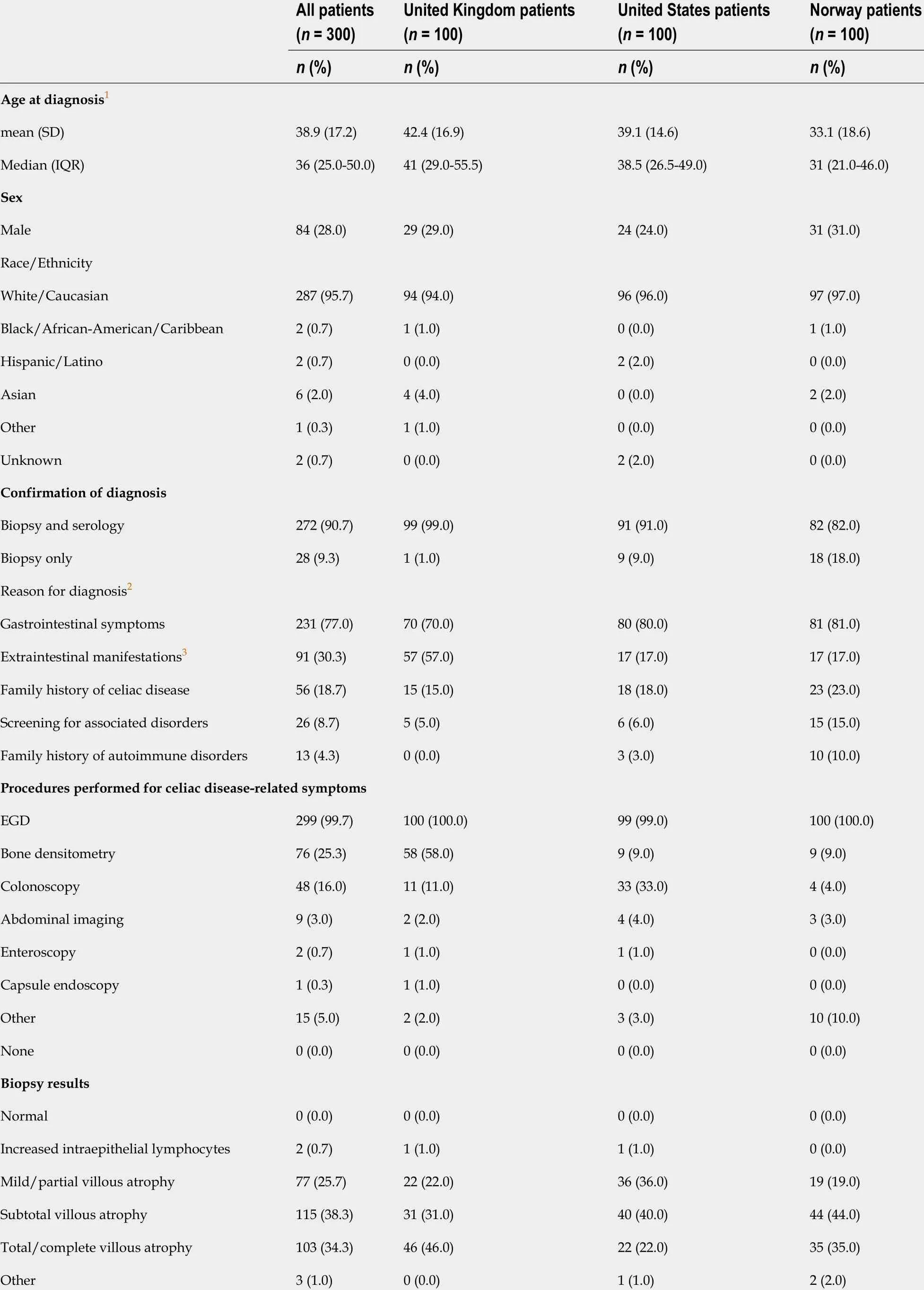
Table 1 Demographic and clinical characteristics of patients at diagnosis, by country
A summary of endoscopies with duodenal biopsy performed during the follow-up period, overall and by country, is also presented in Table 3 . Of the 300 patients included in this study, 150 (50 .0 %) had at least one endoscopy with duodenal biopsy during the follow-up period. Of these 150 patients, 116 (77 .3 %) had a single follow-up endoscopy with biopsy during the follow-up period and most (14 .7 %, n = 22 /150 ) of the remaining 34 patients had two follow-up endoscopies. A significantly higher proportion of Norway patients received a follow-up biopsy (82 .0 %, n = 82 ) compared with patients in the United Kingdom (42 .0 %, n = 42 ) and United States (26 .0 %, n = 26 )(P< 0 .0001 ).
The proportion of patients in the four disease state classes at diagnosis and at last follow-up with available data within the study period are presented in Figure 1 . Of patients in classes 2 or 4 at diagnosis (n = 295 ) and who had a follow-up biopsy(n= 150 ), 53 (36 .6 %) continued to have villous atrophy (classes 2 or 4 ) at their last follow-up visit with biopsy data. The proportions were similar for the United Kingdom, United States, and Norway sites, where 39 .0 % (n = 16 ), 40 .0 % (n = 10 ), and 34 .6 % (n = 27 ) of patients, respectively, remained in classes 2 or 4 based on the last available biopsy data within the study period.
Overall, there were 54 patients who were in class 1 (no symptoms and normal duodenal histology) by the last follow-up visit with biopsy data. Of the patients with data available for the classification at diagnosis and at the last follow-up, the proportion of patients in class 1 during the follow-up period was slightly higher in Norwegian patients [n= 34 (43 .6 %)] compared with patients from the United Kingdom[n= 12 (29 .3 %)] and the United States [n = 8 (32 .0 %)].
DISCUSSION
This real-world study characterizes patients with celiac disease over time, and provides insight into routine monitoring practices from three large referral centers in the United Kingdom, the United States, and Norway. The majority of patients were female, which is consistent with other reports on the demographics of the celiac disease patient population[7 ,8 ]. Patients were followed for a mean of 29 .9 mo (median 25 mo) and there were, on average, three follow-up visitsperpatient. Over two thirds of patients had a documentation of gastrointestinal symptoms during the follow-up period, which may indicate inadequate control of celiac disease despite patients being on a GFD. In addition, the fact that a higher proportion of patients from the United Kingdom site presented with extraintestinal manifestations at diagnosis, compared with patients from the United States and Norway sites, indicates that differences may exist in diagnostic or referral practices between different countries. This is particularly true for the United Kingdom site, which was known to see a greater number of patients with neurological manifestations of celiac disease. It is therefore likely that the differences in extraintestinal manifestations at diagnosis between the countries are due to a combination of referral bias and ascertainment bias at the individual sites, such that some manifestations may be evaluated more frequently at some sites than others.
While the study did collect information on extraintestinal manifestations, including liver abnormalities, it did not specifically assess metabolic disorders of patients with celiac disease. Given that an increased risk of non-alcoholic fatty liver disease in patients with celiac disease on a GFD has been reported[9 ], it would be valuable for future long-term studies to examine such metabolic disorders in this patient population. Country/site-specific differences were also evident in the routine monitoring of patients after diagnosis. While the United States patients had the longest follow-up duration within the study period, compared with Norwegian and United Kingdom patients, a higher proportion of Norwegian patients received a follow-up biopsy, indicating differences in diagnostic or referral practices across the different sites/countries that may not necessarily be reflective of differences in national guidelines.
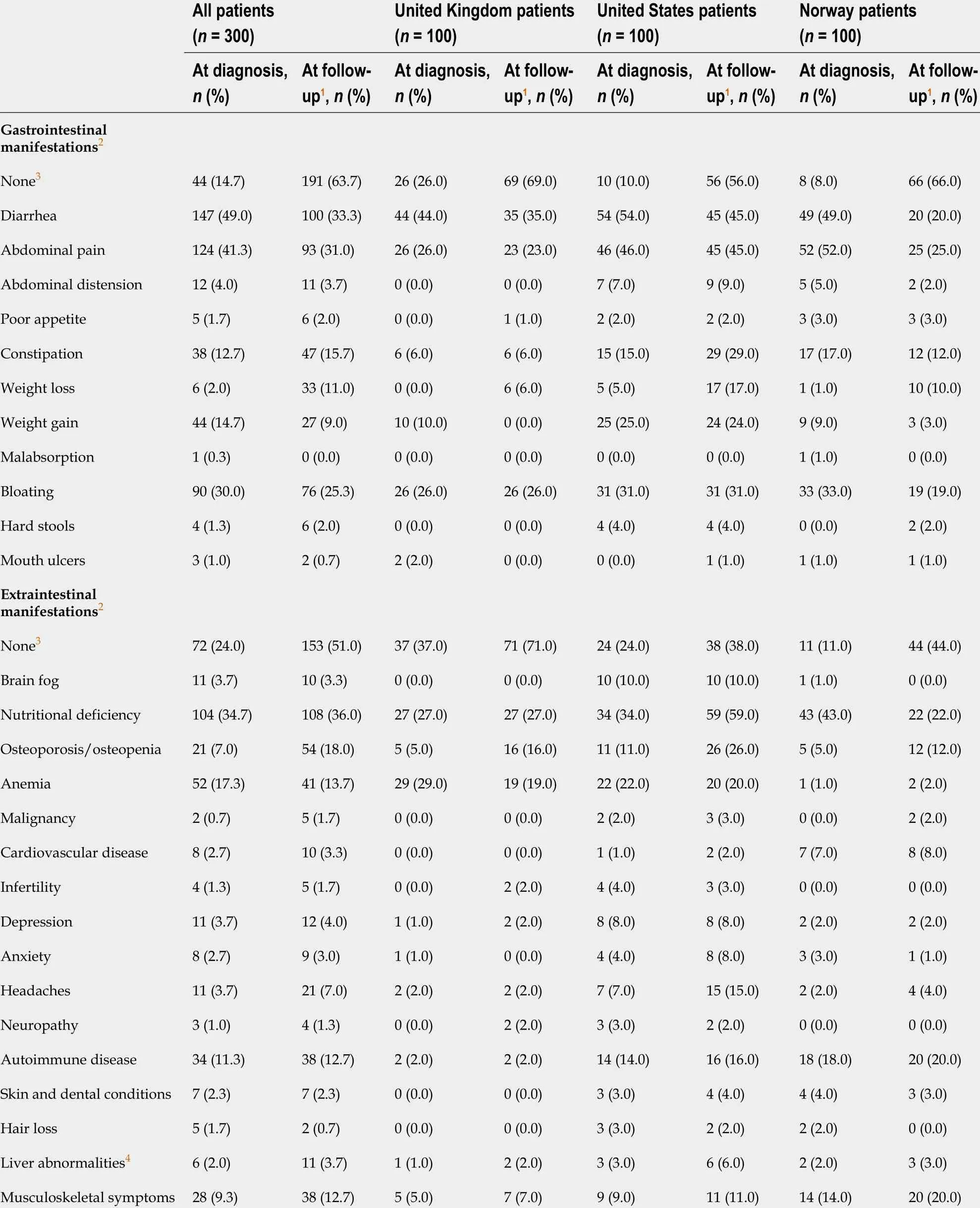
Table 2 Presentation of gastrointestinal and extraintestinal manifestations at diagnosis and at follow-up visits, by country
In this study, half of patients received at least one follow-up biopsy after diagnosis within the study period, with significant variability between sites. While there is currently no consistent recommendation to perform routine follow-up biopsy on all patients, recent European guidelines suggest a follow-up biopsy in adults one to twoyears after diagnosis and after starting a GFD to assess mucosal healing, as treatment of ongoing mucosal injury is less well defined and depends on likely etiology[3 ].

Table 3 Descriptive characteristics of follow-up visits and endoscopies during the follow-up period, overall and by country
The grouping of patients into four disease state classes in this study allows for examination of the persistence of celiac disease symptoms as well as mucosal recovery/healing. Patients in this study with ongoing mucosal injury likely represent a combination of ongoing gluten exposure, slow recovery post diagnosis, and refractory celiac disease. Analysis of specific etiologies of ongoing villous atrophy, however, is outside the scope of this manuscript. Study results indicated that 36 .6 % of patients overall had presence of villous atrophy (classes 2 or 4 ) at the last follow-up visit with available biopsy data, with similar findings across sites. While it is unclear how many of these patients would progress to histologic remission given longer follow-up, these data suggest that a substantial proportion of patients may not be achieving therapeutic goals, even at specialized celiac disease centers. Furthermore, it is important to note that among those with at least one follow-up visit only half of patients had a follow-up biopsy to examine mucosal recovery. While the proportion of patients with persistent villous atrophy may be partially related to referral bias, the inclusion of patients diagnosed only at tertiary centers should have mitigated this. Conversely, patients who are not followed-up or who receive care at less well-equipped centers may have even higher rates of inadequate disease control.
The reasons for the variability in follow-up, both within and between centers, are unclear. However, it seems that many of the patients in this study were either not continuing to see their gastroenterologist or not having a follow-up biopsy, which would limit the ability to assess continued presence of symptoms and villous atrophy.Yet, previous studies reported that having a follow-up biopsy did not impact longterm outcomes when compared with those who did not have a follow-up biopsy,possibly due to lack of effective interventions to address ongoing villous atrophy[10 ,11 ].

Figure 1 Number of patients grouped into the four disease state classes at diagnosis and at last follow-up with available biopsy data.Class 1 : no symptoms and normal duodenal histology; Class 2 : No symptoms and abnormal duodenal histology; Class 3 : Symptoms and normal duodenal histology;Class 4 : Symptoms and abnormal duodenal histology. 1 Patients with biopsy result indicated as ‘other’ in the data collection form were excluded from this classification.
Potential country differences in healthcare policies may also be at play here. Indeed one previous study conducted in Norway reported that only 6 % of patients had prevalence of villous atrophy after a median follow-up of 8 .1 years[10 ]. The authors of this Norwegian study indicated that this may be partially driven by the fact that, in Norway, patients diagnosed with celiac disease automatically qualify for a reimbursement to cover the extra costs associated with following a GFD. In another study,from Australia, rates of mucosal remission and response were 50 % and 85 % at one and five years, respectively[12 ]. In addition, Pekki et al[11 ] reported that 42 % (n = 200 ) of the 476 patients examined in Finland, who had a repeat biopsy, continued to have atrophy after one year of follow-up[11 ]. In yet another study from Finland, the authors reported that 96 % (n = 177 ) of patients had villous recovery after a mean of 11 years of follow-up while adhering to a GFD[13 ]. The present study, however, did not find a large difference by country for the proportion of patients with continued presence of villous atrophy during follow-up.
Strengths of this study are the inclusion of patients with biopsy-proven celiac disease, the multinational sample, and the use of consecutively diagnosed patients,which should have reduced selection bias. However, future research may be warranted to examine whether patterns of care are different in community-based compared with tertiary centers, and whether there are potential differences in outcomes for patients diagnosed by serology alone and followed-up in general practice. Given that the sites in this study were large gastroenterology referral centers,it is anticipated that they should be reflective of practice patterns in similar centers within the countries studied, and where there were commonalities between the centers, these are likely generalizable. However, as this cannot be tested, it is also likely that the selected sites may not be truly representative of the country, and these findings would need to be confirmed by further research within each country. In addition, patterns of care are reflective of those in gastroenterology referral centers,and may be more rigorous than patterns of care in general practice.
Limitations of this study include the lack of information regarding adherence to a GFD, as this information is often not readily available in patient charts, although most patients (approximately 80 %) were referred to a dietician at least once during the follow-up period. Future studies may be able to assess GFD adherence objectively through the presence of gluten immunogenic peptides in the urine[14 ]. There is also the possibility that variation in pathology assessment and reporting may influence inter center results; although, good interobserver agreement for the detection of villous atrophy has been reported[15 ]. In addition, the majority of patients included in this study were diagnosed on the basis of symptoms, with approximately 12 % diagnosed by screening alone. While asymptomatic patients may have different outcomes, related in part to GFD adherence, the current study was not designed to address this.However, it would be valuable for future studies to consider and compare outcomes based on whether diagnosis was based on asymptomaticvssymptomatic disease.
Further, it is unclear what proportion of patients in this study were diagnosed elsewhere and referred to one of the participating gastroenterology centers owing to lack of healing. This may have resulted in a higher proportion of patients with villous atrophy compared with a community setting. In addition, this study captured patient visits to the gastroenterologist only, and any continued management with another healthcare provider (e.g.,general practitioner, dietician) was not captured. Therefore,the results of this study are reflective of follow-up and outcomes for patients with celiac disease aspertheir management by the gastroenterologist. While it is expected that most patients will continue to be managed by a gastroenterologist, particularly if they continue to experience symptoms and have no evidence of mucosal healing,management by a general practitioner or other specialist (e.g.,dietician) may occur in parallel. In addition, given that the inclusion criteria required selection of patients with at least one follow-up visit within the study period, to report on follow-up patterns and outcomes, the study is unable to provide information on patients who did not return to the gastroenterologist for a follow-up visit during the study period. Further,comparisons made between sites/countries relied on standard parameters assessed across sites including celiac serologies (but heterogeneous in the frequency of retesting), symptoms assessment, GFD adherence and nutritional values. However,differences across the sites and the standard of practice would largely be the driver of follow-up endoscopy/biopsy, and the authors recognize this limitation in adequately comparing outcomes across patients.
There is a lack of clarity in guidelines on types of clinicians who are most appropriate to administer follow-up care and management for patients with celiac disease, and this may be especially important given increasing activity of nontraditional practitioners. Results from a patient survey indicated that 27 % of patients had not visited a healthcare provider about celiac disease over the past five years, with almost half of these patients reporting that they felt that they were managing their celiac disease effectively on their own[16 ]. Therefore, despite the present study focusing specifically on management by gastroenterologists, it may be that some patients choose to manage celiac disease on their own and do not return for regularly scheduled visits.
This study provides valuable insight into the monitoring patterns and outcomes of patients with celiac disease managed at large referral centers in real-world practice.Overall, the monitoring of patients, including the rate of follow-up biopsy, varied across the participating sites, with a higher proportion of Norwegian patients receiving a follow-up biopsy compared with patients in the United Kingdom and United States. Differences were also observed in the presentation of extraintestinal manifestations at diagnosis across the sites. In addition, the study results indicate that a large proportion of patients continue to have villous atrophy and continue to experience symptoms after diagnosis; a finding that was consistent across sites.Pharmacological management may be required for patients who are adherent to a GFD but who still experience symptoms and mucosal injury.
CONCLUSION
In general, patients are not routinely monitored for the outcome of a GFD on symptoms, which may have an impact on intestinal health and can be a burden to patients. Overall, these data suggest that more routine follow-up assessment of celiac disease activity is needed. The inconsistent rates of mucosal assessment may be of concern, especially as many patients do not achieve histological remission. Novel, less invasive measures for assessment of ongoing villous atrophy, in combination with adjunctive pharmacologic therapy, may be needed to improve outcomes in patients with celiac disease.
ARTICLE HIGHLIGHTS

杂志排行
World Journal of Gastroenterology的其它文章
- Deep learning for diagnosis of precancerous lesions in upper gastrointestinal endoscopy: A review
- State of machine and deep learning in histopathological applications in digestive diseases
- COVID-19 in normal, diseased and transplanted liver
- Upregulation of long noncoding RNA W42 promotes tumor development by binding with DBN1 in hepatocellular carcinoma
- Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study
- Inflammatory bowel disease in Tuzla Canton, Bosnia-Herzegovina: A prospective 10 -year follow-up
