COVID-19 in normal, diseased and transplanted liver
2021-06-05AlessandroSignorelloIlariaLenciMartinaMilanaGiuseppeGrassiLeonardoBaiocchi
Alessandro Signorello, Ilaria Lenci, Martina Milana, Giuseppe Grassi, Leonardo Baiocchi
Abstract Starting from December 2019 the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ) has extended in the entire world giving origin to a pandemic.Although the respiratory system is the main apparatus involved by the infection,several other organs may suffer coronavirus disease 2019 (COVID-19 )-related injuries. The human tissues expressing angiotensin-converting enzyme 2 (ACE2 )are all possible targets of viral damage. In fact myocarditis, meningo-encephalitis,acute kidney injury and other complications have been described with regard to SARS-CoV-2 infection. The liver has a central role in the body homeostasis contributing to detoxification, catabolism and also synthesis of important factor such as plasma proteins. ACE2 is significantly expressed just by cholangiocytes within the liver, however transaminases are increased in more than one third of COVID-19 patients, at hospital admission. The reasons for liver impairment in the course of this infection are not completely clear at present and multiple factors such as: Direct viral effect, release of cytokines, ischemic damage, use of hepatotoxic drugs, sepsis, and others, may contribute to damage. While COVID-19 seems to elicit just a transient alteration of liver function tests in subjects with normal hepatic function, of concern, more severe sequelae are frequently observed in patients with a reduced hepatic reserve. In this review we report data regarding SARS-CoV-2 infection in subjects with normal or diseased liver. In addition the risks of COVID-19 in immunosuppressed patients (either transplanted or suffering for autoimmune liver diseases) are also described.
Key Words: COVID-19 ; Liver; Non-alcoholic fatty-liver-disease; Cirrhosis; Liver transplant; Angiotensin-converting enzyme 2
INTRODUCTION
Between December 2019 and January 2020 , first China and then the rest of the world observed the emergence of a new pathogen, responsible for the recent pandemic still plaguing our planet. On January 5 , 2020 , the WHO issued a global alert after 44 patients, hospitalized for pneumonia of unknown origin, were reported by Chinese national authorities[1 ]. On March 11 , 2020 , the WHO declared the new coronavirus,identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 ) and responsible for the so called coronavirus disease 2019 (COVID-19 )[2 ], to be pandemic.To date (January 2021 ), the SARS-CoV-2 infection is approaching 100 million cases worldwide, while 2 million related deaths have been reported globally. SARS-CoV-2 is a virus belonging to the genusBetacoronavirus, familyCoronaviridae, subgenusSarbecovirus, discovered at the end of 2019 . It is a single-stranded, positive-sense RNA virus with a 50 -200 nm diameter. Since it has close genetic similarity with bat coronaviruses, a zoonotic origin has been suggested, based on the transmission from animal to humans. SARS-CoV-2 possesses four structural proteins called the S (spike),E (envelope), M (membrane), and N (nucleocapsid) proteins; the N protein binds the virus genome while the S, E, and M proteins contribute in the assembly of the viral envelope[3 ].
Impairment of respiratory function is the most frequent clinical concern during infection by this new virus; however, other physiological organ systems can be affected. Among these, SARS-CoV-2 -induced hepatic changes are gaining interest for their possible relationship with liver and patient outcome.
The liver plays a fundamental role in metabolism and the synthesis of plasma proteins, as well as in the manipulation and detoxification of diet xenobiotics[4 ].Several conditions may lead to chronic hepatitis and cirrhosis or hepato-carcinoma,including viral agents, alcohol abuse, metabolic derangements, and others[5 ]. The patient affected by liver disease is fragile, and his clinical stability is always in perennial equilibrium. Among the direct causes of hepatic decompensation, the detrimental effect of infections, both viral and bacterial, is well known, and in this particular setting, COVID-19 does not seem less important[6 ]. This review summarizes the effects of SARS-CoV-2 infection in patients with liver disease, as well as in healthy and liver transplanted subjects. Data available on clinical complications and outcomes are also reported.
INTERACTION OF SARS-COV-2 WITH TISSUES
The SARS-CoV-2 molecular pathways employed by the virus to favor host interaction and invasion are of major interest. Several studies have been conducted on this issue,and the majority of evidence converges to identify the angiotensin-converting enzyme 2 (ACE2 ), a surface peptidase responsible for the regulation of blood pressure, as a key factor. In fact, Zhouet al[7 ] demonstrated that ACE2 is the main entry route of SARSCoV-2 within the cell, as also previously observed for the SARS-CoV and HCoV-NL6 viruses[7 ]. During the previous SARS pandemic in 2003 , it was demonstrated that the use of antibodies against ACE2 effectively counteracted viral replication. On the other hand, ACE1 antibodies did not have any effect[8 ]. The interaction between this new virus and ACE2 has also been investigated in a study conducted by Xu et al[9 ], where the strong link between the virus and this receptor was confirmed. ACE2 is present in different organs and tissues, such as alveolar type 2 cells of the lung; epithelial cells of the nasal, nasopharyngeal, and oral mucosa; in the smooth muscles of the gastric and colonic mucosa; in enterocytes of the duodenum, jejunum, ileum, and colon; in myocardiocytes; in the cells of the proximal tubule of the kidney; and in cholangiocytes within the liver[10 ]. From all of the above, it is clear that any tissue expressing ACE2 might be a possible target for SARS-CoV-2 , giving rise to different symptoms with a severity that may be a function of the baseline organ reserve. The main organs expressing ACE2 with the corresponding symptoms/complications COVID-19 -related,are reported in Figure 1 . Although the main clinical expression is represented by a flulike mild syndrome, a severe bilateral interstitial pneumonia resulting in acute respiratory distress syndrome (ARDS) and requiring intensive care unit (ICU)management is not rare[11 ]. Other common clinical manifestations comprise: Nausea,vomiting, anorexia, diarrhea (2 %-10 % of patients), and altered liver function tests[12 ].
COVID-19 AND THE NORMAL LIVER
One large retrospective study, conducted in the city of New York, enrolled approximately 5700 COVID-19 patients. Liver transaminase levels were assessed at hospital admission. Increased aspartate transaminase (AST) or alanine transaminase (ALT) was found in 58 % and 39 % of patients, respectively[13 ]. Disease worsening during inhospital stay was associated with a further increase of these enzymes. However, the pathogenetic mechanism leading to these alterations remained unclear. In fact, several factors may all have contributed to the liver changes in this heterogeneous group of patients, such as: (1 ) a direct viral cytopathic effect; (2 ) the activation of cytokine cascade; (3 ) the onset of multi-organ failure; (4 ) a state of disseminated intravascular coagulation; and (5 ) the use of potentially hepatotoxic drugs (such as remdesivir),among others[12 ]. As demonstrated by other research, in the course of COVID-19 , the increase in liver enzymes is characterized by a preferential AST elevation, thus recalling the picture observed in alcoholic, metabolic, or ischemic liver conditions[14 -16 ]. Abnormal levels of cholestasis markers are seldom observed[17 ], even if an increased gamma-glutamyltransferase (GGT) level was reported in 50 % of patients in one study[18 ].
Even though SARS-CoV-2 viral particles were described in hepatocytes in two severe cases[19 ] a direct cytopathic effect does not seem to explain the liver changes since ACE2 is mainly expressed in the biliary tract, within the liver. On the other hand,despite: (1 ) the presence of the receptor on cholagiocytes; and (2 ) “in vitro” evidences demonstrating tight junction damage and bile acids transporter impairment in these cells[20 ]; cholestasis is seldom observed in COVID-19 clinical setting. With regard to liver damage, however, is to underscore that cardiomyopathy with the resulting myocardial dysfunction, is observed in nearly 33 % of hospitalized COVID-19 patients.In this context, the onset of circulatory impairment could reasonably explain the biochemical pattern of the alteration of AST, ALT, and GGT, as in the more common picture of congestive liver disease[21 -23 ]. Supporting this view, a postmortem analysis of liver tissue coming from COVID-19 patients denoted a necrotic injury associated with hypoperfusion and congestive changes[19 ]. Finally, even if liver involvement is not generally regarded as relevant in subjects without a pre-existing liver impairment,the evaluation of hepatic changes during COVID-19 remains difficult in the clinical setting, due to the lack of a clear understanding of this process[24 ]. In this perspective,bilirubin elevation, even if rarely observed, seems to expose the patient to an increased risk of mechanical ventilation or death in comparison with changes of other liver enzymes[25 ].
COVID-19 AND PATIENTS WITH NON-CIRRHOTIC, CHRONIC LIVER DISEASE
As stated in the previous paragraph, the alterations of liver function tests in the course of COVID-19 could be the result of different causal events that may act simultaneously. When damage occurs in a subject with an impaired hepatic functional reserve,a more difficult resolution of the clinical picture may be expected. Of more concern is the large United States study including more than 60 million electronic medical records, which also demonstrated that subjects affected by chronic liver diseases(CLD) were more exposed to acquiring COVID-19 [26 ]. In this western research, the prevalence of CLD in COVID-19 patients accounted for 5 % of cases, while data coming from China demonstrated a baseline CLD in 2 to 11 % of cases[27 ].
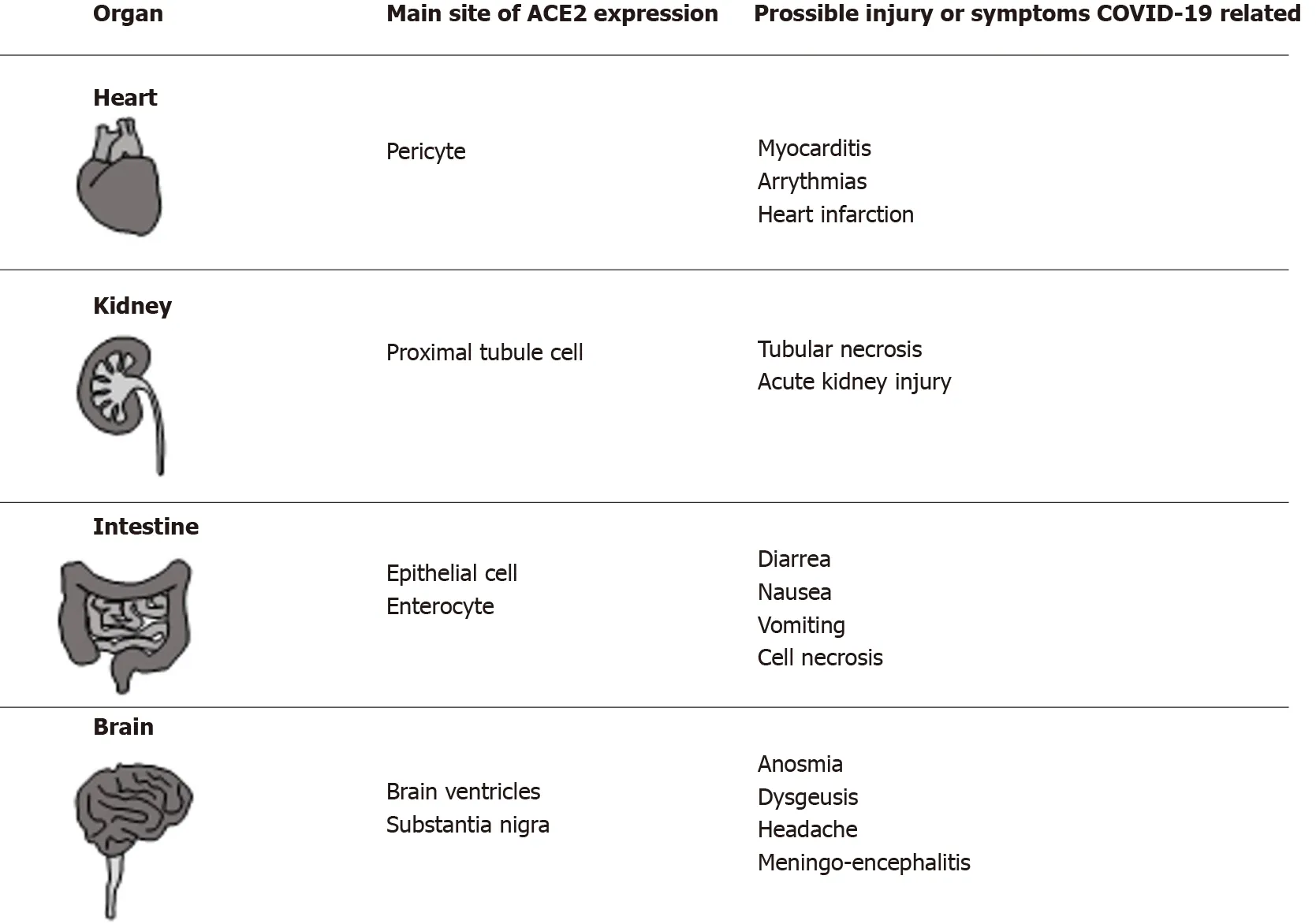
Figure 1 Organs of human body (different from lung and liver) in which angiotensin-converting enzyme 2 was detected, the main site of expression and the possible coronavirus disease 2019 related symptoms/complications are reported in the corresponding columns.COVID-19 : Coronavirus disease 2019 ; ACE2 : Angiotensin-converting enzyme 2 .
Several conditions may determine a CLD in humans, including those of a metabolic,toxic, viral, or autoimmune nature. Non-alcoholic- (or metabolic-associated-) fattyliver-disease (NAFLD), a liver condition ranging from simple steatosis to non-alcoholic steatohepatitis and associated with metabolic syndrome, type II diabetes and obesity,has an estimated prevalence of 25 % worldwide[28 ]. Because of its large burden, the role of NAFLD in determining the degree of liver injury in patients with COVID-19 has aroused much interest.
In China, 70 out of 324 COVID-19 patients (21 .6 %) were diagnosed with hepatic steatosis during radiological investigations by computed tomography. The severity of the clinical picture of COVID-19 was also increased in those with NAFLD[29 ].Moreover, in a retrospective study on 76 patients with COVID-19 , the presence of NAFLD was associated with the progression of lung impairment, with an odds ratio of 6 .4 [30 ]. In another study, NAFLD was again associated with a fourfold increased risk of a severe course of COVID-19 [31 ]. The picture linking NAFLD to a severe outcome of this viral infection was later challenged by a study coming from Qatar[32 ]. This research, including 320 patients with NAFLD, showed that this metabolic condition was only associated with a worse outcome (increased ICU stay and requirement of mechanical ventilation) when univariate analysis was employed. On the other hand,the main predictor for mortality or a worst outcome at multivariate analysis were age> 50 years and diabetes, respectively. On the basis of the above studies, it is clear why the possible link between NAFLD and severity of COVID-19 remains controversial.Heterogeneity among studies, related to retrospective patient inclusion and classification, do not contribute to a clear picture. A further analysis possibly shed some light on this issue, linking the grade of liver fibrosis in NAFLD, rather than NAFLD by itself, to a worse COVID-19 outcome[33 ]. In this perspective, it seems that the extent of liver damage might have a more relevant role in comparison with the metabolic derangement observed in these patients.
Alcoholic liver disease (ALD) represents an important cause of hepatic morbidity and mortality worldwide[34 ]. Despite the fact that clinical data are not available on COVID-19 ALD patients, concern exists for the increased frailty of these subjects during the pandemic. On the other hand, psychological stress and social distancing seem to have increased individual alcoholic beverage consumption in general.
Alcohol abuse disorders frequently facilitate several comorbidities such as viral infections, diabetes or renal failure, all factors that may contribute to an increased risk of a severe course once SARS-CoV-2 infection occurs[35 ]. Moreover, increased consumption of alcoholic beverages is considered to be a risk factor for the development of respiratory tract infections that may be complicated by the onset of ARDS, one of the main features of severe COVID-19 [17 ]. Also, in the recovery phase after COVID-19 , previous alcohol abuse is considered a predisposing factor for the development of pulmonary fibrosis[36 ]. However, evidence-based indications in the management of ALD COVID-19 -infected patients are lacking at present, and further studies are needed to improve our understanding on this issue. For the time being,expert opinion suggests, in general, to avoid the use of steroids in alcoholic hepatitis while concomitant uncontrolled infections are ongoing, because of the lowering effect on patient's immune defenses[37 ]. In clinical practice, on the other hand, the use of these drugs might be beneficial in the treatment of COVID-19 despite ALD,challenging our before-SARS-CoV-2 beliefs.
Another important challenge concerns the management of patients with autoimmune hepatitis on immunosuppressive therapy. The lack of data from scientific evidence has encouraged an empirical approach based on dose reduction of immunomodulatory therapy in order to prevent the most deleterious effects of COVID-19 [38 ]. However, preliminary data from the city of Bergamo, in Italy, did not support this view since an increased risk was not observed in immunosuppressed patients during the SARS-CoV-2 epidemic. On the other hand, it must be considered that a possible reactivation of autoimmune hepatitis, after immunosuppressive therapy tapering, would probably require high-dose corticosteroids, greatly increasing the risk of infectious complications in these patients[39 ]. Therefore, current guidelines do not recommend the reduction of immunomodulatory therapy in the absence of SARS-CoV-2 infection; in the case of overt infection, dose adjustment may be pursued in order to increase the white blood cell count[40 ,41 ].
Few data are available on other forms of CLD. With regard to HBV, in a Chinese series represented by 105 hepatitis B surface antigen positive patients hospitalized for COVID-19 (1 .9 % with cirrhosis, 12 .4 % on antiviral therapy), 14 developed significant liver injury[12 ]. In these subjects, liver damage was associated with a severe course of infection in nearly 80 % of cases. Since the possible prevention or attenuation of COVID-19 should be postulated with the use of antiviral drugs employed for hepatitis B virus (HBV) or hepatitis C virus (HCV), a Spanish study focused on subjects with chronic viral hepatitis following their specific antiviral regimen[42 ], with 1 out of 341 HCV patients and 8 out 1764 HBV patients developing COVID-19 . Although the majority of them (nearly 80 %) were hospitalized, no cases of fatal outcome were observed in this group. Finally, is to underscore that extensive use of intravenous steroid therapy to reduce the inflammatory state can lead to significant HBV reactivation. Routine testing for this virus would be wise in COVID-19 severe patients in order to adopt a timely prophylactic treatment[12 ].
No data are available at present on the impact of COVID-19 in patients with cholestatic CLD, such as primary biliary cholangitis or primary sclerosing cholangitis.
COVID-19 IN PATIENTS WITH CIRRHOSIS OF THE LIVER
Several factors may lead a cirrhotic patient from a compensated to a decompensated clinical condition. Among these, infections play a relevant role, also promoting the occurrence of acute-on-chronic-liver-failure (ACLF)[43 ,44 ]. In this perspective, SARSCoV-2 infection, characterized by an important activation of the cytokine cascade,sepsis, and altered hepatic perfusion, may determine a significant impairment of hepatic reserve in cirrhosis[6 ]. Some studies evaluated the clinical outcome of COVID-19 in patients with liver cirrhosis. Preliminary data coming from two international registries and including 103 cirrhotic COVID-19 positive patients demonstrated an increased mortality, in comparison with the general population[45 ]. Moreover,increased fatality was strictly related to the degree of pre-existing liver impairment; in fact, more than 60 % of Child-Pugh C patients died in this study. In the APCOLIS study, a worst COVID-19 outcome in cirrhotic patients was again confirmed[46 ]. In this research, 43 cirrhotic patients exhibited: (1 ) increased need of ICU care; (2 ) more liver-related complication; and (3 ) enhanced mortality in comparison with noncirrhotic CLD patients. COVID-19 determined the onset of liver decompensation or ACLF in one out of five patients with cirrhosis. A Child Pugh score > 9 was a significant predictor of mortality.
A study conducted in Italy compared the 30 -d in-hospital mortality in a group of patients admitted for COVID-19 infection with or without liver cirrhosis. The all-cause mortality rate was found to be significantly higher in cirrhotic patients with a model for end-stage liver disease score ≥ 15 . In addition, this research also evidenced a deterioration of liver function in patients with a long-standing stable liver disease. In fact, a worsening of the Child-Pugh score was observed, from grade A at admission to status B/C during the hospital stay[47 ]. The mortality rate induced by COVID-19 in patients with decompensated cirrhosis was increased in comparison with other infective causes. In Figure 2 , the main postulated mechanisms of liver injury, during COVID-19 ,are reported, together with the possible outcome as a function of previous liver condition. Finally, cirrhotic patients with hepatocarcinoma (HCC) deserve a separate consideration since neoplastic patients, in general, may have an additional immunologic impairment related to cancer and/or chemotherapy. A national study performed in China on 1590 patients in 575 hospitals showed that patients with an underlying malignancy had a higher risk of SARS-CoV-2 infection than the general population. Obviously, many patients with HCC had an underlying liver impairment that placed them in this higher risk group, with a decidedly more inauspicious clinical outcome[48 ]. Considering this increased risk, the America Association for Study on Liver Disease (AASLD) and the European Association for the Study of Liver (EASL)recommend a prolongation of the time of clinical and ultrasound surveillance of cirrhotic patients so as not to expose them to possible contagion in the places of treatment, deferring locoregional treatment for HCC where possible in patients with a relatively stable disease, and reserving interventions for patients in which the benefit outweighs the risk of acquiring SARS-CoV-2 infection[40 ,41 ]. However, the relationship between an impairment of the immune system (for cancer, immunological diseases, or transplant) and a worse COVID-19 outcome is not demonstrated as yet, as will be reported in details in the following paragraph.
COVID-19 IN LIVER TRANSPLANTED PATIENTS
As there is an assumption that the integrity of the immune-system is an important factor for preventing the most severe sequelae of COVID-19 , since the pandemic began, concern has been raised about the possible outcome in liver transplant (LT)patients under continuous immunosuppressive regimen. However, current data do not confirm this worrisome view. An early pediatric analysis coming from the North of Italy (an area badly beaten by the pandemic) demonstrated just three children infected by COVID-19 , and with an uneventful outcome, among those liver transplanted or under chemotherapy[39 ]. Data on adult LT recipients coming from the same geographic area gave a different picture[49 ]. Three patients having a LT more than ten years before, and with COVID-19 , died from complications. All had metabolic impairment (diabetes, hypertension) and were > 65 years. Paradoxically, their immunosuppressive regimen was minimal in comparison with patients with a shorter transplant history, suggesting a possible protective rather detrimental effect of immunosuppression. A large series was published, gathering the data from two international registries (COVID-Hep and SECURE-Cirrhosis)[50 ]. In this study, data coming from 151 liver transplanted patients were compared with those of 627 controls.The main factors associated with death at multivariate analysis were: Age, creatinine levels, and non-liver malignancy. LT and immunosuppressive regimen were not associated to an increased risk of fatality. Finally, patients with LT required invasive ventilation more frequently (20 % LT vs 5 % control; P < 0 .001 ), while the overall mortality was increased in the non LT control group (19 % LT vs 27 % control; P = 0 .05 ).A Spanish prospective study reassessed this issue in 111 LT patients with COVID-19 [51 ]. Although the fatality rate was slightly lower in LT patients, mycophenolate use(especially at > 1 g/daily dose) was associated with an increased risk of severe COVID-19 disease. From these findings, the authors suggested a possible benefit in mycophenolate tapering in COVID-19 LT patients, while a complete drug withdrawal was discouraged. Taken together, the available data do not suggest an increased fatality in LT patients with COVID-19 ; however, enhanced surveillance would be wise in these subjects because of the increased morbidity reported by some studies. While a possible detrimental effect has been suggested for mycophenolate, a clear picture of the relationship between immunosuppression and COVID-19 severity is still lacking.
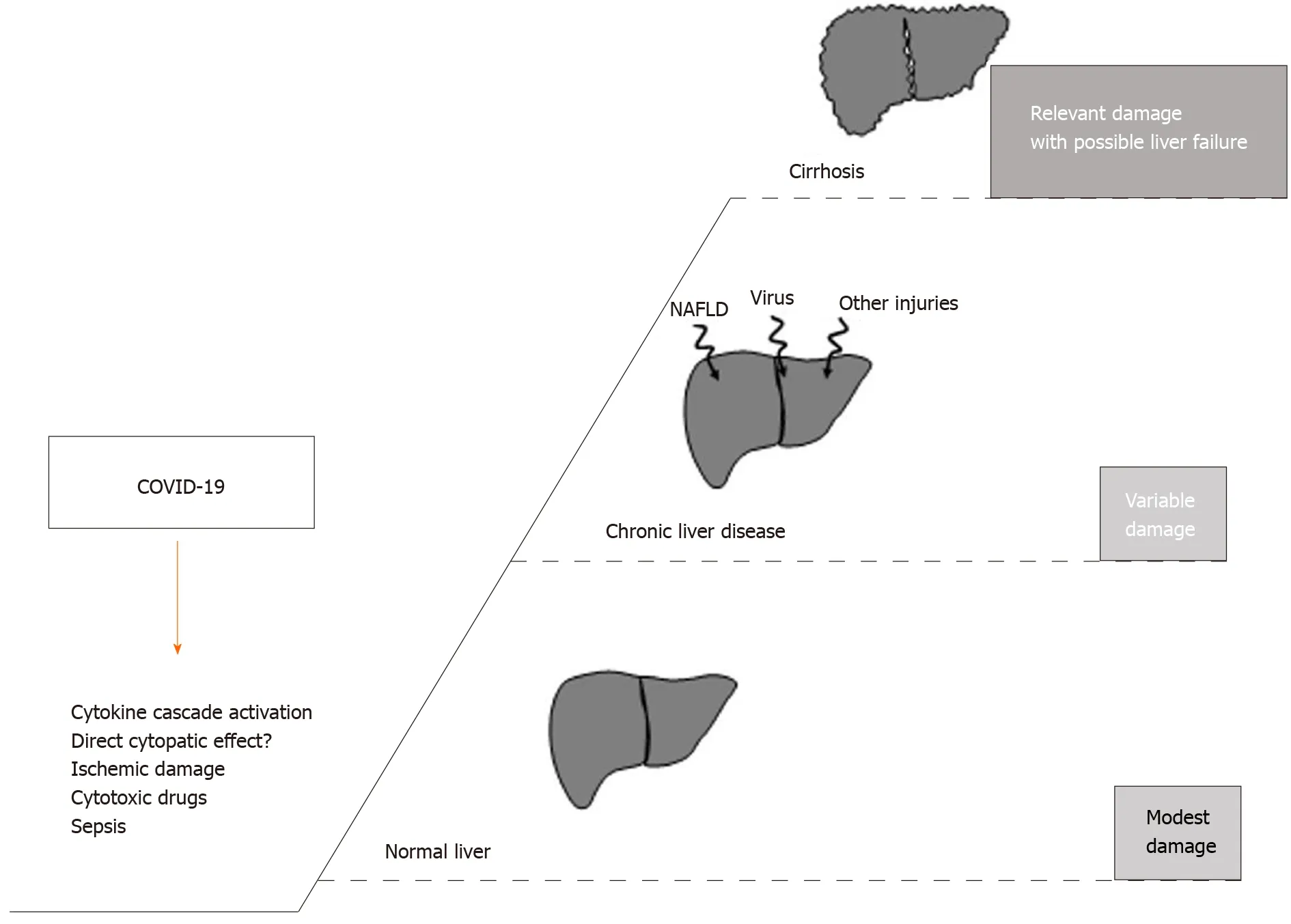
Figure 2 The putative mechanisms inducing liver injury in the course of coronavirus disease 2019 are reported in the left side of the figure (? = controversial data on the specific mechanism). The right part describes the possible outcome according to patient baseline liver condition.NAFLD: Non-alcoholic fatty-liver-disease; COVID-19 : Coronavirus disease 2019 .
CONCLUSION
The effects of COVID-19 on liver health are complex and largely dependent on the underling functional hepatic reserve. The reasons for the elevation of liver enzymes in a significant number of COVID-19 patients, is not clear at present. As a general rule,the effect of COVID-19 seems negligible on the normal liver, but concern exists for patients with impaired liver function. Expert societies, such as AASLD or EASL, are making a great effort to give indications on issues such as LT and the clinical management of liver disease patients during the SARS-CoV-2 pandemic, also in the lack of definitive evidence. While this scenario is quickly evolving, also for the possible emergence of some variant of concern[52 ] ,other issues are rising for patients with liver disease. Among the pandemic-associated and non-COVID-19 -related adverse effects,the reduction of health resources for patients with liver disease may significantly impact their morbidity and mortality. A study in Italy evidenced that there was already a 25 % reduction of transplant activity related to an increased ICUs saturation in the first 4 wk of the national pandemic[53 ]. Abrupt interruption of HCC surveillance in the majority of patients with liver cirrhosis will also have a cost in the future. Finally, a recent study modeling a one year delay in HCV cure since the pandemic estimated an increase of more than 100 thousand in liver related deaths and cancers in the population affected by this virus in the coming years[54 ]. In conclusion,while hepatologists are presently working in an emergency environment full of uncertainties and with limited resources, an “in search of lost time” attitude will probably be pursued by the same physicians in the future, when the pandemic ends.
杂志排行
World Journal of Gastroenterology的其它文章
- Deep learning for diagnosis of precancerous lesions in upper gastrointestinal endoscopy: A review
- State of machine and deep learning in histopathological applications in digestive diseases
- Upregulation of long noncoding RNA W42 promotes tumor development by binding with DBN1 in hepatocellular carcinoma
- Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study
- Inflammatory bowel disease in Tuzla Canton, Bosnia-Herzegovina: A prospective 10 -year follow-up
- Open reading frame 3 protein of hepatitis E virus: Multi-function protein with endless potentia
