Cardiac exercise rehabilitation for heart failure: a review of the literature
2021-06-05YuLiuXianLiangWangYingFeiBiYaWenCaoShuaiWangYaZhuHouZhiQiangZhaoShanShanLinZheWangJingYuanMao
Yu Liu, Xian-Liang Wang*, Ying-Fei Bi, Ya-Wen Cao, Shuai Wang, Ya-Zhu Hou, Zhi-Qiang Zhao, Shan-Shan Lin, Zhe Wang, Jing-Yuan Mao*
Cardiac exercise rehabilitation for heart failure: a review of the literature
Yu Liu1, Xian-Liang Wang1*, Ying-Fei Bi1, Ya-Wen Cao1, Shuai Wang1, Ya-Zhu Hou1, Zhi-Qiang Zhao1, Shan-Shan Lin1, Zhe Wang1, Jing-Yuan Mao1*
1Cardiovascular Department, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China.
The most common symptom of patients with heart failure is reduced exercise tolerance, as indicated by decreased peak oxygen uptake (VO2peak), which is associated with both reduced quality of life and survival. Cardiac rehabilitation is a safe and effective treatment for clinically stable patients with heart failure, and is associated with improvements in cardiopulmonary function, muscle strength, physical functional performance, and quality of life. Further, cardiac rehabilitation is associated with a reduction in heart failure hospitalization and mortality. Despite evidence of these benefits, cardiac rehabilitation referral and compliance among patients with heart failure remains low. In this review, we discuss exercise and training program selection for patients with heart failure, including optimal exercise training intensity, and a summary of recent literature on the use of cardiac rehabilitation for patients with heart failure.
Cardiac rehabilitation, Heart failure, Exercise training, Quality of life, Cardiopulmonary function
Background
Heart failure (HF) is a common and complicated disease that can lead to death. It is associated with a decline in exercise tolerance, decreased peak oxygen uptake (VO2peak), and a diminished quality of life (QoL). Indeed, HF is the end stage of cardiovascular disease. The prevalence of HF is on the rise, and has been measured as high as 10% among persons aged 70 years and older, with clear socio-economic implications for our aging society [1]. Cardiac rehabilitation (CR) is a comprehensive program that combines exercise with family and social support. CR is currently highly recommended by international guidelines and has been shown to be an effective treatment for patients with HF, including reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), improving functional capacity and relieving symptoms [2, 3].
As a result of abnormal heart structure or function, HF is often associated with respiratory syndromes, fatigue, lung sounds, peripheral edema, and a variety of other clinical syndromes, affecting about 26 million people worldwide [4]. In recent decades, CR has evolved from simple monitoring of the safety of physical activity to a multidisciplinary program including patient education, individualized exercise training (ET), controlling risk factors, and addressing the overall health of patients with heart disease. CR is associated with significant improvements in functional capacity and health-related QoL in patients with chronic heart failure (CHF), acting by controlling cardiovascular risk factors (blood pressure, heart rate, BMI, waist circumference, smoking preference, and metabolic syndrome) [5]. Whereas traditional CR programs are often unsatisfactory due to low participation rates, high costs, and dependence upon on-site exercise sessions, family-based remote rehabilitation models have some advantage. Patients with HF who participated in CR programs improved their cardiovascular health, which was associated with reduced mortality and risk of cardiac hospitalization [6−9]. This review aims to describe recent developments in our understanding of CR for HF.
Global state of cardiac rehabilitation for HF
Among the 1 billion people in the 47 countries of Europe, approximately 5% of that population has CHF. In the United States, there are very few patients with HF who participate in CR, which is especially infrequent among elderly non-white women with a history of depression or other chronic diseases [10]. Patients with HF have a lower participation rate in the UK and have limited access to either CR or clinical guidance [11]. In a survey of the European HF Association, 67 (40%) centers responsible for 36,385 (48%) patients did not implement a sports training program due to lack of resources, especially due to lack of personnel or financial allocation. After implementation, ET plans were presented to all HF patients in only 55% of centers and was limited. Despite evidence that the availability of ET has increased for HF patients in Europe, there is still dramatically less availability than is needed for the treatment of all patients with HF, primarily due to lacks of resources and logistical difficulties [12]. Indeed, exercise-centric CR is strongly recommended by international guidelines. According to the "China Cardiovascular Disease Report 2018" [13], at present, cardiovascular disease has a high incidence and mortality rate in China. The total number of discharged patients with cardiovascular and cerebrovascular diseases in 2015 was 18.8872 million, of which cardiovascular disease accounted for 6.61% [14]. In China, CR for patients with HF is still under development. Telehealth training is an alternative to CR in China, and significantly improved QoL, 6MWD, and resting heart rate at a 4-month post-test [15]. Additionally, studies have suggested that traditional Chinese medicines can reduce cardiovascular events, improve clinical symptoms, and promote the rehabilitation of coronary heart disease pathology through multiple channels and multiple targets. Non-pharmacological treatments such as Tai Chi、Sitting Tai Chi、Baduanjin traditional Chinese Qigong exercise, when combined with a routine CR program, leads to improvement in cardiopulmonary function and exercise tolerance in patients with ischemic HF on phase-II CR, for example 6MWD、depressive symptoms、social domain of quality of life and QoL [16, 17] . Additionally, it helps regulated the balance and tone of the cardiac sympathetic and vagal nerve. Despite the large amount of scientific and cultural evidence that generally supports the potential benefits of traditional Chinese herbs and exercises, high quality randomized controlled trials are required to confirm the extent of those benefits for patients with cardiovascular disease.
Exercise training and HF
Exercise training model: in or outside of the hospital
ET is an effective intervention for patients with HF. One study found that physical capacity (i.e., METs) done during CR is independently associated with a decreased risk for both all-cause mortality and HF-specific hospitalization [18]. One of the core goals of ET is to improve physical ability. In this work, high BMI index, female gender, being retired and being married/in a relationship were all significant predictors of a negative change in physical capacity [19]. CR for HF is primarily based upon training, and the 12-month "Resistance ET program" was associated with improved physical fitness at one year of follow-up. Further, improved survival was observed during long-term follow-up (= 0.063) [20].
Although ET has been shown to improve functional capabilities and clinical outcomes in many clinical trials, hospital-based ET was associated with a greater improvement in VO2peakand QoL than family-based ET for patients with HFrEF (= 0.014) [21]. ET significantly improves exercise duration and cardiopulmonary functional capacity in elderly and middle-aged patients with acute decompensated HF [22]. For HFrEF patients, outpatient programs have a higher CR participation rate than inpatient programs, but both are associated with improvements in aerobic capacity, muscle strength, and depressive symptoms (< 0.05) [23]. For example, the Rehabilitation Enablement in CHF home-based intervention for heart failure with reduced ejection fraction (HFrEF) was associated with significantly improved QoL (per Minnesota Living with Heart Failure questionnaire (MLHFQ) at 12 months [24]. Further, it improves 6-minute walking distance (6MWD) in patients with CHF by 8 weeks [25] and only costs an average of £418 per participant [26]. In addition, REACH-HF improves caregivers’ confidence in self-management and is perceived as helpful in supporting their caregiver role [27]. Recent evidence suggests that ET also plays a supporting role in improving cardiovascular outcomes [28]. Another study found that right ventricular strain during leg-positive pressure may be a good echocardiographic parameter for assessing the beneficial effects of CR [29]. As part of comprehensive CR, ET is recommended for patients with HFrEF to improve exercise tolerance. Studying the addition of low-frequency myoelectric stimulation in ET can improve exercise performance and QoL in patients with HFrEF, but, compared with the control group, there was no additional advantage [30].
Exercise training program
Recently, high-intensity interval training (HIIT) has been strongly advocated for CR of patients with HF due to data suggesting larger improvements in exercise capacity, symptomatic relief, and QoL. Therefore, this training mode has been a topic of increasing attention and preference among rehabilitation teams. Research has shown that HIIT can lead to increases in VO2peakand decreases in left ventricular end systolic diameter (= 0.0198), compared to non-exercise participants, which are associated with improved survival (= 0.044) in patients with HF [31]. Exercise-induced oscillatory ventilation is a factor associated with risk of cardiac events, worse prognoses, and increased mortality in patients with CHF. HIIT rehabilitation programs improve exercise oscillatory ventilation in CHF patients and improve cardiopulmonary function [32]. Compared with daily activities, home-based intermittent resistance training,improved aerobic capacity, endurance, ventilation threshold, and QoL in patients with HF [33]. Training improves the maximum workload, VO2peak, oxygen uptake at the anaerobic threshold, and maximum oxygen pulse. The post-training QoL (SF-36, MLHFQ) showed improved body function scores, but the SF-36 total score and MLHFQ did not change after training. The use of HIIT, supplemented with peripheral and inspiratory resistance training, led to larger improvements in peripheral and inspiratory muscles strength, compared with standard exercise-based CR programs [34]. Further, HIIT was associated with significantly better improvements in VO2peakand left ventricular ejection fraction than classical moderate-intensity continuous training programs after a short period of rehabilitation [35]. Although there is no evidence that HIIT is superior to continuous training, HIIT training results in significant improvements in physical function parameters in HFrEF patients NYHA class II-III [36]. One study found that an ET program based on traditional Greek dance design safely and effectively improves jumping ability, muscle strength, and lower limb endurance, especially as measured by extended walking distance of patients with HFrEF [37]. Nordic walking, a walking technique that mimics cross-country skiing, requires more muscle groups and thus increases the intensity of aerobic training, but does not significantly exacerbate difficulties with breathing [38], and improves the parasympathetic-sympathetic balance in patients with HFrEF. This is associated with improved VO2peakand significant improvements for patients with heart rate disorder [39]. Conveniently, a moderate, self-regulated 500 m treadmill-walking test can be used to assess the VO2peakof HFrEF patients. This finding may facilitate the transition from clinically based programs to programs for HFrEF patients that are self-guided or take place in fitness facilities [40].
Mechanism of benefits from exercise training
ET for patients with CHF has been shown to reduce muscle sympathetic nerve activity (MSNA) at rest, desensitize the sympathoexcitatory metaboreflex, and diminish MSNA elicited by mild exercise [41]. ET can balance angiotensin converting enzyme and angiotensin converting enzyme inhibitors levels in patients with CHF, thereby inhibiting sympathetic outflow [42]. Reduced exercise capacity leads to decreased skeletal muscle mass and accumulation of body fat, but CR can reduce visceral adipose tissue and improve exercise capacity in patients with CHF during hospitalization [43]. HFrEF patients have reduced fibrinogen concentration after CR [44], and neurohormone spectroscopy results are improved with lowering of plasma heart biomarkers [45]. Other studies have shown that overtraining induces inflammation in the serum, muscle, hypothalamus, and liver, which can lead to left ventricular (LV) hypertrophy and cardiac fibrosis [46]. Low-intensity physical exercise increases functional capacity and is associated with a reduction in the duration of the Timing Rise and Ascent Test and helps regulate neuroendocrine and NT-proBNP concentrations in patients with CHF [47]. Studies in a spontaneously hypertensive rat model of HF have suggested that long-term, low-intensity aerobic exercise improves myocardial function, cardiac remodeling, and motor function. Further, the same study found that long-term low-intensity exercise reduces decompensated HF in aging spontaneously hypertensive rat and improves functional status in order to improve LV function [48, 49].
About half of patients with HF are diagnosed with HFpEF. Kitzman and several studies have shown a decrease in aerobic capacity in VO2peakin patients with HFpEF. ET programs can restore physiological function to allow for increased aerobic exercise [50, 51]. Participation in a 6-month exercise program resulted in significant improvements in exercise performance in patients with HFpEF and HFrEF [52]. Of interest, Training related improvement in VO2peakwas higher in HFpEF vs. HFrEF patients [53].
HF benefits from cardiac rehabilitation
Effects of cardiac rehabilitation on quality of life
CR improves the symptoms, exercise capacity, and QoL of patients with CHF. Family-based rehabilitation services significantly improve activities of daily living (ADL) function and gait performance. Greater ADL improvement is significantly associated with younger age, faster baseline gait speed, and higher ADL composite score [54]. CR programs significantly improve QoL in patients with Chagas HF. Using the SF-36 questionnaire to assess QoL, CR was found to promote physical functional improvement, body pain scores, and to improve physical health scores [55]. Inpatient CR leads to improvements in disabilities among patients with acute decompensated heart failure (ADHF). Greater improvements in Barthel Index (BI) among those treated with inpatient CR were significantly related to better outcomes in patients with impaired baseline BI [56]. The transition from hospital to home is critical for reducing the rate of preventable adverse post-discharge results. After 12 weeks of CR, MLHFQ, 6MWD, timing, and GO score were significantly improved in the intervention group compared with the control group. Low-intensity exercise during the transition is an effective way to improve health-related QoL and physical functioning in the elderly [57]. In a study of an 8-week home exercise training program for patients with CHF, 6MW work was improved, and the correlation between 6MWD and QoL was similar [58]. Home-based CR not only significantly improves exercise tolerance, but also significantly increases VO2peakand QoL for patients with HF. Patients who received home CR increased VO2peakby 14.2%, with a 37% increase in QoL score and an increase of 41 meters in the 6MWD. The 90-day readmission rate for patients who received CR was reduced from 14% to 5%. Home-based CR is associated with the greatest improvement in functional capacity, QoL, and reduced readmission rates within 90 days of discharge [59]. Of note, 8 months of CR improves QoL in patients with CHF, indicated by responses to the MLHFQ [60]. A 2-month cardiac rehabilitation program (including prescribed exercise training, 2 courses/week) improves 6MWD and MLHFQ scores, cardiovascular risk factors are also significantly improved [61]. In summary, CR is associated with significant improvements in QoL after treatment and assessment by multiple endpoints.
Effects of cardiac rehabilitation on cardiopulmonary function
CR is effective, safe, and provides important clinical benefits for patients with CHF, especially in terms of cardiopulmonary function. LVEF at the end of CR is an independent predictor of hospitalization and mortality from cardiovascular causes in patients who receive coronary revascularization [62]. LVEF and respiratory intensity were found to be improved after 8 months of physical exercise intervention. Patients with right ventricular dysfunction showed improvements in functional ability after 4 months and improved LV diastolic blood pressure [63]. Patients with advanced HF with LVEF less than 30% completed a supervised CR procedure that showed significant improvements in VO2peakand LVEF without any serious cardiovascular events [64]. Treatment of patients with both coronary and non-coronary HF was associated with improved LVEF, confirming the benefits of initial hospital-based rehabilitation for patients with HF and encouraging long-term outpatient monitoring [65].
The success of CR is usually assessed by an objective improvement in the VO2peak, measured by the Cardiopulmonary Exercise Test. The main chronic symptom in patients with HF is reduced exercise tolerance, measured by a reduced VO2peakand associated with reduced QoL and survival [66]. The addition of HIIT the CR programs has been proposed as a method to improve VO2peak. After HIIT, VO2peakis significantly improved in patients with HF. The recovery interval should be active and between 40% and 60% of the VO2peakof HF patients, and the training frequency of HF patients > 3 days/week [67]. A meta-analysis showed that aerobic interval training led to greater increases in VO2peakthan aerobic continuous training in CHF patients [68]. Cardiac mechanical efficiency and ventricular-arterial coupling are also important dimensions of the pathophysiology of HF. In patients with CR with an ejection fraction 45%, ventricular-arterial coupling and LV showed an improved mechanical efficiency curve [69].
Effects of cardiac rehabilitation on peripheral muscle
Measurements of exercise capacity are independently associated with the risk of mortality and HF hospitalization. METs achieved at the end of CR had the strongest independent association with all-cause mortality (adjusted HR, 95% CI: 0.58, 0.48-0.70) and HF hospitalization (adjusted HR, 95% CI: 0.62, 0.52-0.74) [70]. HF patients lack a reserve of myocardial contractility during incremental exercise, so skeletal muscle pump function may play a key role in maintaining cardiac output. Exercise intolerance remains a major feature of all patients with HF. Damage to a single organ system cannot independently explain exercise intolerance and loss of function for daily activities in patients with HF. The progression of HF is often accompanied by weight loss and decreased muscle mass and physical capacity, powerful predictors of QoL and mortality [71]. Patients with HF who have a high risk of malnutrition (MNA-SF score < 7) have lower body mass index, muscle mass, and strength and are unable to walk long distances [72]. Protein supplementation can increase the effects of exercise on strength and muscle mass and promote improved endothelial function, body composition, and QoL [73]. Therefore, early detection of malnutrition and increased nutritional support may contribute to functional recovery after CR.
In addition to improved cardiac function, changes in skeletal muscle function and structure are associated with impaired exercise capacity for patients with CHF, and improvement in skeletal muscle strength is therefore also one of the benefits of CR for patients with HF [74]. CHF patients can use resistance bands for peripheral muscle training in order to initially improve and maintain walking distance and health-related QoL [75]. One study suggested the introduction of exercise rehabilitation for muscle hypertrophy, especially for the calf muscles, which promotes muscle elongation [76]. HIIT has a positive impact on skeletal muscle strength in patients with advanced symptomatic HF in the hospital, and HIIT in patients with advanced HF significantly reduces atrial natriuretic peptide levels and significantly increases knee extensor strength, which was significantly associated with hemoglobin A1c levels at the onset of exercise [77]. HIIT improves exercise performance and improves resting heart function in patients with CHF, likely through mechanisms involving hemodynamics and skeletal muscle deoxidation at peak exercise [78]. During the Cardiopulmonary Exercise Test, the lower leg ejection fraction of HF patients was associated with VO2peakand peak oxygen pulses. Calf skeletal muscle function may promote exercise capacity by indirectly acting on cardiac function in patients with HF [79].
Acute heart failure is associated with decreased peripheral perfusion, leading to dysfunction of multiple organs. Improving muscle tissue perfusion is an important goal during the rehabilitation of patients with AHF [80]. Gordon R Reeves studies have shown that Rehabilitation Therapy in Older Acute Heart Failure Patients is the first randomized trial of a physical function intervention in older patients with hospitalized acute heart failure designed to determine if addressing deficits in balance, mobility, strength and endurance improves physical function and reduces rehospitalizations [81]. (table 1)
Conclusion
The benefits of center-based CR include improved QoL for patients HF as well as improved cardiopulmonary function, decreased mortality, symptomatic relief, and increased exercise tolerance. These findings underscore the importance of CR for patients with HF and suggest that new CR programs, such as home-based CR and traditional exercises could be potentially beneficial for HF. Unfortunately, CR is still not fully applied, and there is much less literature on HFpEF, Despite the better rehabilitation prospects. The development of alternative therapies and the use of telecommunication tools and other monitoring methods will help the development of CR. In the future, we can further carry out the research on cardiac rehabilitation of HF with preserved ejection fraction in order to guide clinical and later rehabilitation.
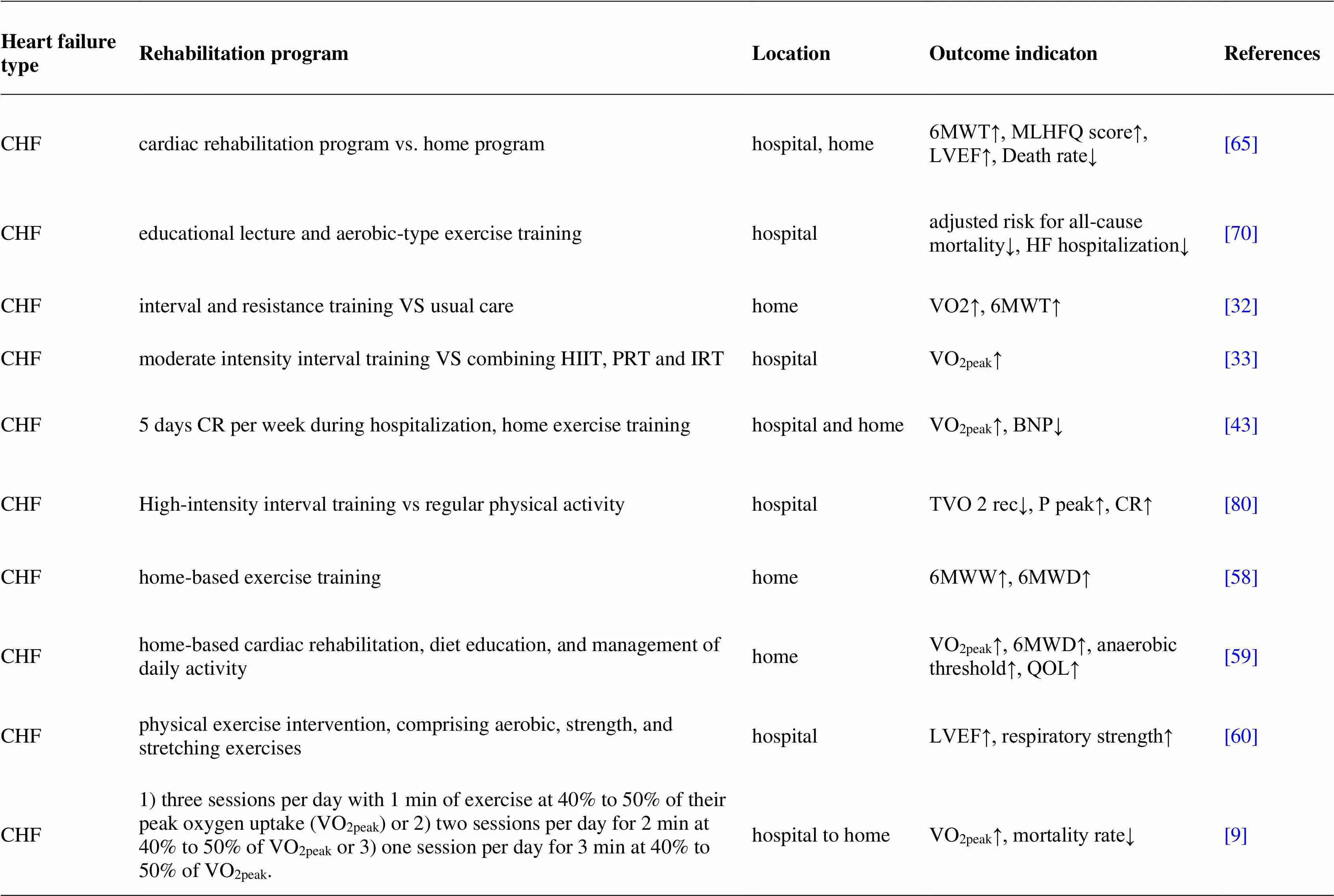
Table 1 Status of cardiac rehabilitation in patients with different types of heart failure
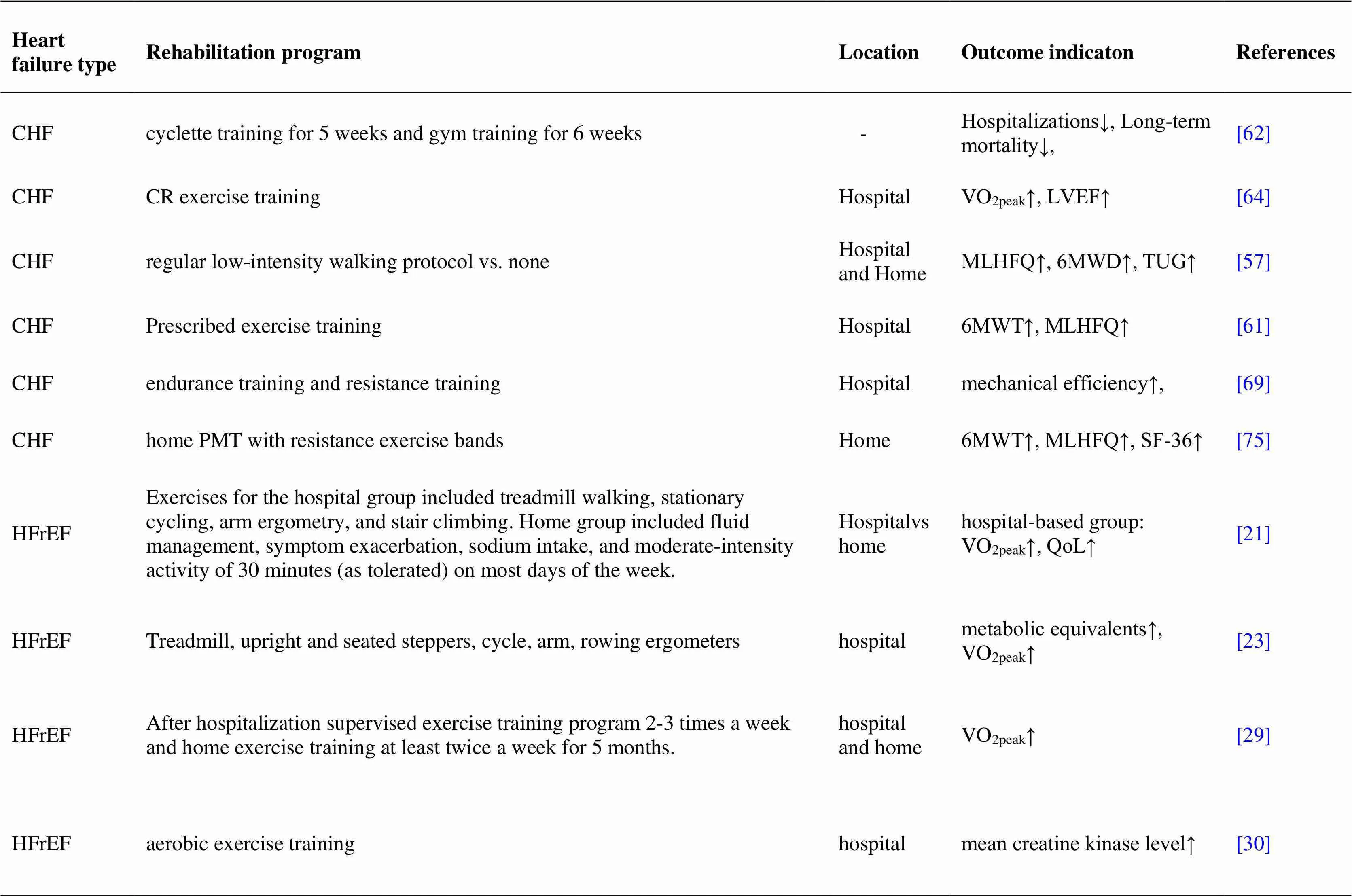
Table 1 Status of cardiac rehabilitation in patients with different types of heart failure (continued)
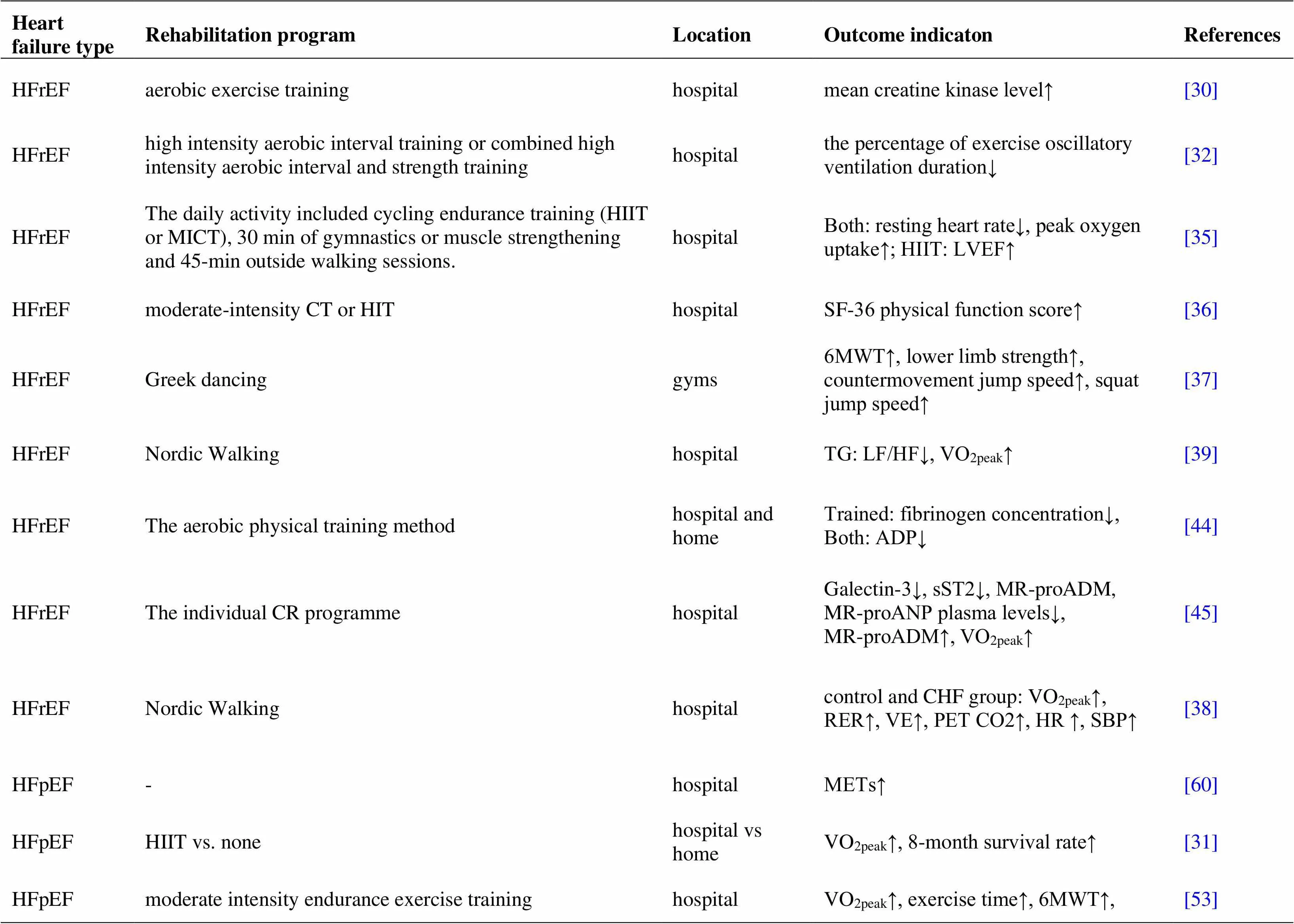
Table 1 Status of cardiac rehabilitation in patients with different types of heart failure (continued)
1. Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature.. 2001;80(2-3):213−219.
2. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR).. 2016;37(29):2315−2381.
3. Slimani M, Ramirez-Campillo R, Paravlic A, Hayes LD, Bragazzi NL, Sellami M. The Effects of Physical Training on Quality of Life, Aerobic Capacity, and Cardiac Function in Older Patients With Heart Failure: A Meta-Analysis.. 2018;9:1564.
4. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries.. 2014;63(12):1123−1133.
5. Mohammed H G, Shabana A M. Effect of cardiac rehabilitation on cardiovascular risk factors in chronic heart failure patients.. 2018;70(2):77−82.
6. Sabbag A, Mazin I, Rott D, et al. The prognostic significance of improvement in exercise capacity in heart failure patients who participate in cardiac rehabilitation programme.. 2018;25(4):354−361.
7. Cacciatore F, Amarelli C, Ferrara N, et al. Protective effect of physical activity on mortality in older adults with advanced chronic heart failure: A prospective observational study.. 2019;26(5):481−488.
8. Scalvini S, Grossetti F, Paganoni AM, La Rovere MT, Pedretti RF, Frigerio M. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: A population study in Lombardy, Italy, from 2005 to 2012.. 2019;26(8):808−817.
9. Rovere MT, Pedretti RF, Frigerio M. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: A population study in Lombardy, Italy, from 2005 to 2012.. 2019;26(8):808−817.
10. Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in Cardiac Rehabilitation Among Patients with Heart Failure.. 2017;23(5):427−431.
11. Buttery AK, Carr-White G, Martin FC, Glaser K, Lowton K. Limited availability of cardiac rehabilitation for heart failure patients in the United Kingdom: findings from a national survey.. 2014;21(8):928−940.
12. Piepoli MF, Binno S, Corrà U, et al. ExtraHF survey: the first European survey on implementation of exercise training in heart failure patients.. 2015;17(6):631−638.
13. Ma LY, Chen WW, Gao RL, et al. China cardiovascular diseases report 2018: an updated summary.2020;17(1):1−8.
14. Yuan D, Hai J. A review of cardiac rehabilitation concept, patients and models.. 2018;358-361.
15. Peng X, Su Y, Hu Z, et al. Home-based telehealth exercise training program in Chinese patients with heart failure: A randomized controlled trial.. 2018;97(35):e12069.
16. Tang XY, Gao JH. Clinical observation on 30 cases of chronic heart failure treated by xinkangcao combined with Jianpi Yangxin Decoction.2018;55−57.
17. Zhao J, Chau JPC, Lo SHS, Choi KC, Liang S. The effects of sitting Tai Chi on physical and psychosocial health outcomes among individuals with impaired physical mobility: A systematic review and meta-analysis [published online ahead of print, 2021 Mar 3].. 2021;118:103911.
18. Keteyian SJ, Kerrigan DJ, Lewis B, Ehrman JK, Brawner CA. Exercise training workloads in cardiac rehabilitation are associated with clinical outcomes in patients with heart failure.. 2018;204:76−82.
19. Sutherland N, Harrison A, Doherty P. Factors influencing change in walking ability in patients with heart failure undergoing exercise-based cardiac rehabilitation.. 2018;268:162−165..
20. Snoek JA, Eijsvogels TMH, VAN 't Hof AWJ, et al. Impact of a Graded Exercise Program on V˙O2peakand Survival in Heart Failure Patients.. 2018;50(11):2185-2191.
21. Kim M, Kim MS, Lim SJ, Ahn JM, Kim JJ, Park SJ. Comparison of Supervised Hospital-based versus Educated Home-based Exercise Training in Korean Heart Failure Patients.. 2017;47(5):742−751.
22. Acanfora D, Scicchitano P, Casucci G, et al. Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: A randomized, controlled trial.. 2016;225:313−323.
23. Rengo JL, Savage PD, Barrett T, Ades PA. Cardiac Rehabilitation Participation Rates and Outcomes for Patients With Heart Failure.. 2018;38(1):38−42.
24. Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial [published correction appears in Eur J Prev Cardiol. 2020 Dec;27(18):NP17].. 2019;26(3):262−272.
25. Peng X, Su Y, Hu Z, et al. Home-based telehealth exercise training program in Chinese patients with heart failure: A randomized controlled trial.. 2018;97(35):e12069.
26. Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial [published correction appears in Eur J Prev Cardiol. 2020 Dec;27(18):NP17].. 2019;26(3):262−272.
27. Wingham J, Frost J, Britten N, et al. Caregiver outcomes of the REACH-HF multicentre randomized controlled trial of home-based rehabilitation for heart failure with reduced ejection fraction.. 2019;18(7):611−620.
28. Ambrosetti M, Sarzi Braga S, Giada F, Pedretti RFE. Exercise-based cardiac rehabilitation in cardiac resynchronization therapy recipients: A primer for practicing clinicians.. 2017;87(3):791.
29. Kusunose K, Seno H, Yamada H, et al. Right Ventricular Function and Beneficial Effects of Cardiac Rehabilitation in Patients With Systolic Chronic Heart Failure.. 2018;34(10):1307−1315.
30. Iliou MC, Vergès-Patois B, Pavy B, et al. Effects of combined exercise training and electromyostimulation treatments in chronic heart failure: A prospective multicentre study.. 2017;24(12):1274−1282.
31. Hsu CC, Fu TC, Yuan SS, et al. High-Intensity Interval Training is Associated with Improved Long-Term Survival in Heart Failure Patients.. 2019;8(3):409.
32. Panagopoulou N, Karatzanos E, Dimopoulos S, et al. Exercise training improves characteristics of exercise oscillatory ventilation in chronic heart failure.. 2017;24(8):825−832.
33. Safiyari-Hafizi H, Taunton J, Ignaszewski A, Warburton DE. The Health Benefits of a 12-Week Home-Based Interval Training Cardiac Rehabilitation Program in Patients With Heart Failure.. 2016;32(4):561−567.
34. Hornikx M, Buys R, Cornelissen V, Deroma M, Goetschalckx K. Effectiveness of high intensity interval training supplemented with peripheral and inspiratory resistance training in chronic heart failure: a pilot study.. 2020;75(4):339−347.
35. Besnier F, Labrunée M, Richard L, et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial.. 2019;62(5):321−328.
36. Benda NM, Seeger JP, Stevens GG, et al. Effects of High-Intensity Interval Training versus Continuous Training on Physical Fitness, Cardiovascular Function and Quality of Life in Heart Failure Patients.. 2015;10(10):e0141256.
37. Vordos Z, Kouidi E, Mavrovouniotis F, et al. Impact of traditional Greek dancing on jumping ability, muscular strength and lower limb endurance in cardiac rehabilitation programmes.. 2017;16(2):150−156.
38. Lejczak A, Josiak K, Węgrzynowska-Teodorczyk K, et al. Nordic Walking May Safely Increase the Intensity of Exercise Training in Healthy Subjects and in Patients with Chronic Heart Failure.. 2016;25(1):145−149.
39. Piotrowicz E, Buchner T, Piotrowski W, Piotrowicz R. Influence of home-based telemonitored Nordic walking training on autonomic nervous system balance in heart failure patients.. 2015;11(6):1205−1212.
40. Mazzoni G, Sassone B, Pasanisi G, et al. A moderate 500-m treadmill walk for estimating peak oxygen uptake in men with NYHA class I-II heart failure and reduced left ventricular ejection fraction.. 2018;18(1):67.
41. Notarius CF, Millar PJ, Keir DA, et al. Training heart failure patients with reduced ejection fraction attenuates muscle sympathetic nerve activation during mild dynamic exercise.. 2019;317(4):R503−R512.
42. Haack KK, Zucker IH. Central mechanisms for exercise training-induced reduction in sympatho-excitation in chronic heart failure.. 2015;188:44−50.
43. Takagawa Y, Yagi S, Ise T, et al. Improved Exercise Capacity After Cardiac Rehabilitation Is Associated with Reduced Visceral Fat in Patients with Chronic Heart Failure.. 2017;58(5):746−751.
44. Mongirdienė A, Kubilius R. Effect of physical training on indices of platelet aggregation and fibrinogen concentration in patients with chronic heart failure.. 2015;51(6):343−350.
45. Billebeau G, Vodovar N, Sadoune M, Launay JM, Beauvais F, Cohen-Solal A. Effects of a cardiac rehabilitation programme on plasma cardiac biomarkers in patients with chronic heart failure.. 2017;24(11):1127−1135.
46. da Rocha AL, Teixeira GR, Pinto AP, et al. Excessive training induces molecular signs of pathologic cardiac hypertrophy.. 2018;233(11):8850−8861.
47. Andrzejczak-Karbowska M, Irzmański R. Wpływ dozowanego 12-tygodniowego treningu fizycznego na stężenia NT-proBNP i D-Dimer u chorych z niewydolnością serca i zaburzeniami sprawności funkcjonalnej w VII–X dekadzie życia [The impact of the dosing, a 12-week physical training on the concentration of NT-proBNP and D-Dimer in patients with heart failure and impaired functional capacity in VII-X decade of life].. 2016;41(241):11−15.
48. Pagan LU, Damatto RL, Cezar MD, et al. Long-term low intensity physical exercise attenuates heart failure development in aging spontaneously hypertensive rats.. 2015;36(1):61−74.
49. Sutherland N, Harrison A, Doherty P. Factors influencing change in walking ability in patients with heart failure undergoing exercise-based cardiac rehabilitation.. 2018;268:162−165.
50. do Prado DML, Rocco EA. The Benefits of Exercise Training on Aerobic Capacity in Patients with Heart Failure and Preserved Ejection Fraction.. 2017;1000:51−64.
51. Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial.. 2018;64:118−127.
52. Klempfner R, Tzur B, Sabbag A, et al. Participation in an Exercise-Based Cardiac Rehabilitation Program and Functional Improvement of Heart Failure Patients with Preserved Versus Reduced Left Ventricular Systolic Function.. 2018;20(6):358−362.
53. Pandey A, Kitzman DW, Brubaker P, et al. Response to Endurance Exercise Training in Older Adults with Heart Failure with Preserved or Reduced Ejection Fraction.. 2017;65(8):1698−1704.
54. Asiri FY, Marchetti GF, Ellis JL, et al. Effect of home-based rehabilitation on activities of daily living and gait in older adults with heart failure at risk for falling: A retrospective cohort study.. 2017;33(12):943−953.
55. Mediano MFF, Mendes FSNS, Pinto VLM, Silva PSD, Hasslocher-Moreno AM, Sousa AS. Reassessment of quality of life domains in patients with compensated Chagas heart failure after participating in a cardiac rehabilitation program.. 2017;50(3):404−407.
56. Motoki H, Nishimura M, Kanai M, et al. Impact of inpatient cardiac rehabilitation on Barthel Index score and prognosis in patients with acute decompensated heart failure.. 2019;293:125−130.
57. Li XY, Hao Y, Xu SL, Li RB, Gao Y. Effects of Low-Intensity Exercise in Older Adults With Chronic Heart Failure During the Transitional Period From Hospital to Home in China: A Randomized Controlled Trial.. 2017;10(3):121−128.
58. Babu AS, Desai CV, Maiya AG, Guddattu V, Padmakumar R. Changes in derived measures from six-minute walk distance following home-based exercise training in congestive heart failure: A preliminary report.. 2016;68(4):527−528.
59. Chen YW, Wang CY, Lai YH, et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure.. 2018;97(4):e9629.
60. Mediano MF, Mendes Fde S, Pinto VL, et al. Cardiac rehabilitation program in patients with Chagas heart failure: a single-arm pilot study.. 2016;49(3):319−328.
61. Mohammed HG, Shabana AM. Effect of cardiac rehabilitation on cardiovascular risk factors in chronic heart failure patients.. 2018;70(2):77−82.
62. Doimo S, Fabris E, Chiapolino S, et al. Prognostic Role of Left Ventricular Dysfunction in Patients With Coronary Artery Disease After an Ambulatory Cardiac Rehabilitation Program.. 2019;124(3):355−361.
63. Mediano MF, Mendes Fde S, Pinto VL, et al. Cardiac rehabilitation program in patients with Chagas heart failure: a single-arm pilot study.. 2016;49(3):319−328.
64. Choi HE, Kim C, Sohn Y. Cardiac Rehabilitation Exercise Training for High-Risk Cardiac Patients.. 2017;41(4):650−658.
65. Koukoui F, Desmoulin F, Lairy G, et al. Benefits of cardiac rehabilitation in heart failure patients according to etiology: INCARD French study.. 2015;94(7):e544.
66. Haykowsky MJ, Daniel KM, Bhella PS, Sarma S, Kitzman DW. Heart Failure: Exercise-Based Cardiac Rehabilitation: Who, When, and How Intense?. 2016;32(10 Suppl 2):S382−S387.
67. Ballesta García I, Rubio Arias JÁ, Ramos Campo DJ, Martínez González-Moro I, Carrasco Poyatos M. High-intensity Interval Training Dosage for Heart Failure and Coronary Artery Disease Cardiac Rehabilitation. A Systematic Review and Meta-analysis.. 2019;72(3):233−243.
68. Pattyn N, Beulque R, Cornelissen V. Aerobic Interval vs. Continuous Training in Patients with Coronary Artery Disease or Heart Failure: An Updated Systematic Review and Meta-Analysis with a Focus on Secondary Outcomes.. 2018;48(5):1189−1205.
69. Aslanger E, Assous B, Bihry N, Beauvais F, Logeart D, Cohen-Solal A. Effects of Cardiopulmonary Exercise Rehabilitation on Left Ventricular Mechanical Efficiency and Ventricular-Arterial Coupling in Patients With Systolic Heart Failure.. 2015;4(10):e002084.
70. Keteyian SJ, Kerrigan DJ, Lewis B, Ehrman JK, Brawner CA. Exercise training workloads in cardiac rehabilitation are associated with clinical outcomes in patients with heart failure.. 2018;204:76−82.
71. Dos Santos EM, de Moraes R, Tibiriça EV, Huguenin GVB, Moreira ASB, De Lorenzo AR. Whey protein supplementation for the preservation of mass and muscular strength of patients with heart failure: study protocol for a randomized controlled trial.. 2018;19(1):431.
72. Matsuo H, Yoshimura Y, Fujita S, Maeno Y. Risk of malnutrition is associated with poor physical function in patients undergoing cardiac rehabilitation following heart failure.. 2019;76(1):82−88.
73. Dos Santos EM, de Moraes R, Tibiriça EV, Huguenin GVB, Moreira ASB, De Lorenzo AR. Whey protein supplementation for the preservation of mass and muscular strength of patients with heart failure: study protocol for a randomized controlled trial.. 2018;19(1):431.
74. Van Iterson EH, Olson TP. Therapeutic Targets for the Multi-system Pathophysiology of Heart Failure: Exercise Training.. 2017;19(11):87.
75. Lans C, Cider Å, Nylander E, Brudin L. Peripheral muscle training with resistance exercise bands in patients with chronic heart failure. Long-term effects on walking distance and quality of life; a pilot study.. 2018;5(2):241−248.
76. Panizzolo FA, Maiorana AJ, Naylor LH, et al. Muscle size explains low passive skeletal muscle force in heart failure patients.. 2016;4:e2447.
77. Taya M, Amiya E, Hatano M, et al. High-intensity aerobic interval training can lead to improvement in skeletal muscle power among in-hospital patients with advanced heart failure.. 2018;33(7):752−759.
78. Spee RF, Niemeijer VM, Wijn PF, Doevendans PA, Kemps HM. Effects of high-intensity interval training on central haemodynamics and skeletal muscle oxygenation during exercise in patients with chronic heart failure.. 2016;23(18):1943−1952.
79. Kondo T, Yamada S, Asai C, Okumura T, Tanimura D, Murohara T. Skeletal Muscle Pump Function Is Associated With Exercise Capacity in Patients With Heart Failure.. 2018;82(4):1033−1040.
80. Węgrzynowska-Teodorczyk K, Siennicka A, Josiak K, et al. Evaluation of Skeletal Muscle Function and Effects of Early Rehabilitation during Acute Heart Failure: Rationale and Study Design.. 2018;2018:6982897.
81. Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale.. 2017;185:130−139.
10.12032/TMRCR20210515007
1Jing-Yuan Mao.Cardiovascular Department, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine. No. 88, Changling Road, Xiqing District, Tianjin 300381, China. Email: jymao@126.com.2Xian-Liang Wang. Cardiovascular Department, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine. No. 88, Changling Road, Xiqing District, Tianjin 300381, China. Email: xlwang1981@126.com.
:HF, heart failure; VO2peak, peak oxygen uptake; QoL, quality of life; CR, cardiac rehabilitation; HFrEF, heart failure with reduced ejection fractio; HFpEF, heart failure with preserved ejection fraction; ET, exercise training; CHF, chronic heart failure; HFrEF, heart failure with reduced ejection fraction; MLHFQ, per Minnesota Living with Heart Failure questionnaire; MSNA, muscle sympathetic nerve activity; ADL, activities of daily living; LV, left ventricular;
:This work was supported by the National Natural Science Foundation of China (NO. 81904153, NO. 81603568), the Tianjin science and technology project: clinical medicine research center of Internal medicine of TCM in Tianjin (15ZXLCSY00020), the “Innovation team development Plan” of Ministry of Education-Research on the prevention and treatment of cardiovascular diseases in traditional Chinese medicine (IRT 16R54), the State Administration of traditional Chinese Medicine (SATCM), the National Clinical Research Base of Chinese Medicine (No. JDZX2015005), and the Najor Science and Technology Project of Tianjin (No. 16zxmjsy00050).
: Yu Liu originally conceived the research. Jing-Yuan Mao and Xian-Liang Wang were responsible for overall design guidance. All authors provided comments and suggestions. Yu Liu was responsible for writing the manuscript. All authors have read, reviewed and approved the final manuscript.
:The authors declare that they have no conflict of interest.
: Liu Y, Wang XL, Bi YF, et al. Cardiac exercise rehabilitation for heart failure: a review of the literature.. 2021;4(2):7.
:Ying Chen.
:05 March 2021,
30 April 2021,
:15 May 2021
© 2021 By Authors. Published by TMR Publishing Group Limited. This is an open access article under the CC-BY license (http://creativecommons.org/licenses/BY/4.0/)
杂志排行
Clinical Research Communications的其它文章
- Role of herbal medicines to treat the symptoms of COVID-19 disease
- Screening of acupuncture treatment for simple obesity
- Clinical observation of Shengxuebao Mixture in treating anemia after concurrent chemoradiotherapy for cervical cancer
- Evaluation of autophagy-related genes and lncRNAs signature for prognositic prediction in thyroid carcinoma via bioinformatics analysis
