COMPARATIVE TRANSCRIPTOMIC ANALYSIS OF BRAIN AND SPLEEN IN TRIM47 KNOCKOUT ZEBRAFISH (DANIO RERIO)
2021-06-02YAOJianWANGYeDaWANGFangLIULiYueLUYuanAnandLIUXueQin
YAO Jian , WANG Ye-Da , WANG Fang , LIU Li-Yue, LU Yuan-An and LIU Xue-Qin
(1. College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China; 2. Hubei Engineering Technology Research Center for Aquatic Animal Diseases Control and Prevention, Wuhan 430070, China; 3. Technical Staff China Zebrafish Resource Center, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China; 4. Department of Public Health Sciences, University of Hawaii at Manoa, Honolulu, HI, 96822, USA)
Abstract: Tripartite motif (TRIM) proteins is a structurally conserved protein group that plays an important role in many biological processes, such as apoptosis, cycle regulation, cellular responses to viruses and inflammatory response. Trim47 is a member of the TRIM family. In this study, the CRISPR/Cas9 technique was employed to knockout trim47 of zebrafish. Brain and spleen of TRIM47–/–(trim47 knockout) and WT(wildtype) zebrafish were collected for RNA-seq analysis to identify differentially expressed genes (DEGs).A total of 271 DEGs were identified. After a brief analysis, these DEGs were annotated into Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and three ontologies following gene ontology (GO) enrichment analysis. DEGs were more concentrated in cell adhesion and glutamatergic synapse signaling pathway in brain of TRIM47–/– group as compared with WT group. In spleen, DEGs of TRIM47–/– group changed in complement and coagulation cascades signaling pathway as compared with WT group. The complement pathway-associated genes in brain and spleen were verified using qRT-PCR, which were consistent with the transcriptome data. These results indicated that Trim47 played an important biological role in spleen and brain,particularly involved in innate immune function through complement pathway. In vivo infection experiments showed that trim47 knockout increased the infection rate of Spring Viremia of Carp Virus (SVCV) in zebrafish. In conclusion, these findings provided new insight into the function of the TRIM members in innate immunity.
Key words: TRIM47; Knockout; DEGs; Zebrafish
The members of the TRIM family were reported to play many important roles involved in many cellular processes, such as developmental courses, tumor suppression, cell cycle regulation and viral response[1].In mammals, most TRIM members are associated with cancer. TRIM50 is known to inhibit ovarian cancer progression by targeting Src and reducing its activity, which provides a novel therapeutic strategy for Src over-activated cancers by positive regulation of TRIM50[2]. TRIM31 was reported to promote glioma proliferation and invasion through activating NF-κB activity[3]. Some TRIM members were also reported to have antiviral effect in fish. Grouper TRIM8, TRIM32 and TRIM39 have been identified as antiviral effectors against DNA and RNA viruses[4]. In common carp, TRIM32 was described as antiviral effectors and inducers of type I-IFN response, interfering with SVCV replicationin vitro[5]. Tripartite motif 47 is a member of the TRIM family proteins and increased TRIM47 expression is a negative prognostic predictor in human prostate cancer[6]. Common carp TRIM47 was identified to play an important role in viral resistance processes[7]. Most experimental studies on TRIM molecules remainin vitrocell with few attention paid toin vivoexperimental test, especially in fish.
The complement system known as an important part of the body’s innate immune response plays an irreplaceable role in host defense against pathogenic microorganisms and serves as a bridge between the innate and acquired immune responses[8]. The complement system is composed of more than 30 soluble and membrane-bound proteins. These proteins interact to form a complement cascade reaction[9]. There are three different pathways of complement activation: the classical pathway, the MB-lectin pathway and the alternative pathway. The complement system can be activated through “hard-wired” pattern recognition receptors that have been developed to recognize pathogenassociated molecular patterns (PAMPs)[8,10,11].
In recent years, CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat)/Cas9 (CRISPRassociated) genome editing systems have become one of the most robust platforms in basic biomedical research and therapeutic applications[12]. Recent studies have shown that the RNA-mediated Cas9 system can mediate genome editing of embryonic cells of the trophozoites, zebrafish, mice, rats, barley, pigs and goats[13—17]. Because of simple manufacturing, low cost, direct cellular target, and high efficiency (it can transmit multiple sgRNAs at the same time)[14], CRISPRCAS technology has made gene editing more efficient and more conducive to examine the function of target genes.
In this study, the CRISPR/Cas9 technique was employed and homozygous selection was performed to construct TRIM47–/–zebrafish. The brain and spleen from the TRIM47–/–group and WT group zebrafish were selected for RNA-seq. Sequencing was performed by using Illumina Hiseq4000 and differentially expressed genes and functional enrichment were analyzed. Findings of this study may provide a theoretical basis for further comprehensive study to understand the functions of the TRIM genes.
1 Materials and methods
1.1 Ethics statement
All experimental animals were handled in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal infection tests did not involve any endangered or protected species. Experiments using zebrafish were performed under the approval of the Animal Ethics Committee of Huazhong Agriculture University (HZAU). The infection and dissection experiments were performed under 3-Aminobenzoic acid ethyl ester methane sulfonate (MS-222) (Sigma, USA) anesthesia to minimize fish suffering.
1.2 Gene knockout
According to Ensembl ID (ENSDART000000 79207), the genomic sequence of the zebrafishtrim47 gene was downloaded from the Ensembl website. Thetrim47 gene contains 8 exons and 7 introns and a gRNA targeting a portion of the first exon was designed. The sequence was GGAAAATGAGAGGCG ATGATG, which was unique to the zebrafish genome. The target sequence was amplified from the wildtype DNA and confirmed by sequencing. Then, gRNA and zCas9 mRNA were synthesizedin vitro. The gRNAzCas9 mRNA complex was micro-injected into onecell stage zebrafish embryos and PCR was used to identify the P0 embryonic population that generates targeted mutations. The zebrafish of heterozygous P0 generation were crossed with wild type to obtain F1 generation. The genotypes of F1 zebrafish were verified and multiple mutation types were screened according to different types. The selected F1 generation fish were reared separately according to the mutation type.After the F1 generation reached sexually mature, the F2 generation were obtained by inbreeding within type. Recovered homozygotes were identified by cutting the fish tail sequence.
1.3 Total RNA extraction, library construction and sequencing
The TRIM47–/–group and the WT group were set up in the experiment. The brains of the four fish were taken as one sample, and the spleens were the same.Three samples were set for each group, four groups(WT-brain, WT-spleen, TRIM47–/–-brain and TRIM47–/–-spleen) in total, 12 samples in total. Samples (brain and spleen) from zebrafish of TRIM47–/–and wild type were gotten. Tissues were ground to extract RNA,and the RNA integrity was assessed using the nanodrop, Agilent 2100 Bioanalyzer, and ABI StepOne Plus real-time PCR system. Finally, libraries were constructed and RNA-seq was performed using the Illumina HiSeqTM4000 platform.
1.4 Bioinformatics analysis
Samples were measured using the Illumina Hiseq4000 platform. Some low-quality of sequenced data with linker-contamination, and a total of 361588720 clean reads were obtained. HISAT[18]was used to map clean reads toDanio reriogenome reference. Reconstructed the transcripts with StringTie[19]and compared the reconstructed transcripts with the reference annotation with cuffcompare. Clean reads were mapped to reference using Bowtie2[20], and gene expression level was calculated with RSEM[21]. The DEG was screened according to the NoIseq[22]packaging method. The default criterion for screening DEG are multiples of≥2 and dispersion probability of≥0.8. With the GO annotation result, DEGs were classified according to official classification, and also performing GO functional enrichment using phyper, a function of R. With the KEGG annotation result, DEGs were grouped according to official classification, and pathway functional enrichment was conducted by using phyper, a function of R.
1.5 Quantitative real-time PCR (qRT-PCR) validation
Transcriptional levels of five complement-associated genes (C1q-cln8, C3a, C9, MASP2, HBL4)were detected using qRT-PCR. All the primers used in this study were presented in Tab. 1. The enzyme we used in all qRT-PCR assays was SYBR Green qPCR SuperMix (TaKaRa). The Roche-Light-CyclerR480IIreal-time detection system (Roche, Germany) was used to detect gene expression levels[23]. We used the reaction system and procedures recommended by the reagent manufacturer and make appropriate adjustments. The reaction volume was 20 μL at 95℃ for 5min, 41 cycles, 94℃ for 15s, 58℃ for 20s, and 72℃for 20s. Cycling conditions included three parallel experiments to confirm the results. The results were calculated using a 2−ΔΔCtanalysis[24], and TBP serves as a reference gene.

Tab. 1 Primer sequences used for qRT-PCR
1.6 Western blot
Western blot was used to detect protein changes.SDS-PAGE gel was prepared. After gel electrophoresis, the protein was transferred to polyvinylidene fluoride membrane (PVDF) (Bio-Rad, USA) using transfer buffer. Following blocking with TBST solution containing 3% bovine serum albumin (BSA) at room temperature for 3h, the membrane was immersed with the primary antibody [TRIM47 polyclonal antibody (1∶1000 dilution), anti-β-actin (ABclonal, 1∶10000 dilution)] for 2h at room temperature.After washing three times with TBST solution for 10min each, the membrane was incubated with secondary antibody (anti-rabbit IgG, 1∶3000 dilution;ABclonal, China) for 45min. Washing three times with TBST buffer for 10min each. Chemiluminescence detection results were used finally.
1.7 Infection experiment
Adult wild-type AB strain male zebrafish were purchased from the China Zebrafish Resource Centre(CZRC) to ensure a clear genetic background and no pathogenic infection. The purchased zebrafish were infected for 7 days after adaptation at 17℃. The TRIM47–/–group and the WT group were set up in the experiment. There were two groups of 30 zebrafish in each group. Intraperitoneal injection of SVCV (ATCC:VR-1390). Using anesthetic before injection, and MEM medium was injected into the control group.The survival rate was calculated.
1.8 Statistical analysis
The results of the analysis of the experimental data were expressed as mean±SEM, and survival rates were compared using two-way ANOVA. For qRTPCR results analysis,P<0.05 was considered significant and marked with *, andP<0.01 was marked with**, 0.001<P<0.01 with ***, andP<0.001 with ****.
2 Results
2.1 Successful knockout of trim47 in zebrafish
A gRNA target for exon 1 oftrim47 was designed and CRISPR/Cas9 was used to knockouttrim47. Followed observation revealed no significant difference in growth and reproduction between the two groups.The tails of TRIM47–/–and WT zebrafish were cut off to sequence. One mutant line oftrim47 was identified as 5 bp (–5) deletion. Compared with WT, this TRIM47–/–group contains 5 nucleotides (TGATG) deletion at positions 153—157 of the wild-typetrim47 coding sequence (Fig. 1A). A peak analysis of the sequencing results was performed and confirmed that both WT zebrafish and mutant TRIM47–/–groups had a single sequencing peak (Fig. 1B). Zebrafish from the TRIM47–/–and the WT groups were taken respectively, and RIPA Lysis Buffer was used to grind the lysed tissue. Trim47 polyclonal antibody was used to detect Trim47 protein. The results showed that the expression of Trim47 protein in TRIM47–/–group was not detectable (Fig. 1C), indicating a successful knockout of thetrim47.
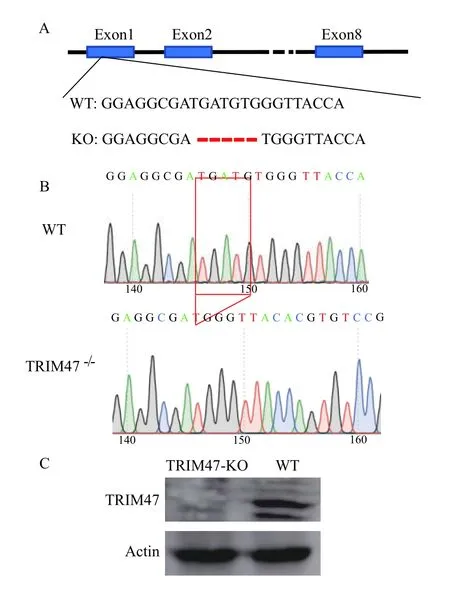
Fig. 1 Sequencing results of TRIM47–/– and WT groups
2.2 Transcriptome sequencing summary
All samples were sent to Huada Company for sequencing on the Hiseq4000 platform. The sequencing results were analyzed and summarized in Tab. 2. The sequencing error rate of 95% of bases was less than 1%, and the sequencing error rate of more than 90%of bases was less than 1‰. These data were used as preliminary sequencing results. The high-quality clean readings were then compared to the corresponding reference genome. The genomic localization rates,gene numbers, expressed transcripts and new transcripts for the aligned information and samples were shown in Tab. 3. These results indicated that our sequencing results were accurate and the sequencing quality is high.
2.3 Analysis of differentially expressed genes
The HISAT was used to compare the control clean reads to the reference genome to obtain a new transcript. Then differentially expressed genes were obtained by analyzing the expression of the transcript.Bioinformatics methods were used for DEGs analysis.As compared to WT group with 156 genes in brain and 115 genes in spleen differentially expressed, there are 121 genes up-regulated and 35 genes down-regulated in brain and 24 up-regulated and 91 down-regulated in spleen, respectively, in TRIM47–/–group (Fig. 2A).Volcanoes and MA maps were identified based on the expression levels of differential genes after knocking outtrim47 in brain (Fig. 2B and 2C) and spleen (Fig. 2D and 2E). These results showed that there were significant differences in expression levels of these differentially expressed genes both in the brain and spleen between the two groups after knocking outtrim47.

Tab. 2 Summary of the Illumina HiSeqTM 4000 sequencing output for all samples
2.4 GO analysis
To further understand the function of Trim47,GO functional analysis and hierarchical clustering analysis were performed on differentially expressed genes in brain and spleen between TRIM47–/–group and WT group. After GO analysis, DEGs in brain between TRIM47–/–group and WT group distributed biological process category: cellular process (most),single-organism process (second). In cellular component category: cell (most), cell part (second). And in molecular function category: binding (most), catalytic activity (second) (Fig. 3A). For spleen, biological process category: cellular process, metabolic process. In cellular component category: cell, cell part. In molecular function category binding and catalytic activity (Fig. 3B).
2.5 KEGG pathway enrichment based on DEGs
For understanding the genetic information, analysis of differential genes using the KEGG database.The cell adhesion and glutamatergic synapse signaling pathway play an important role in the brain of both TRIM47–/–and WT groups (Fig. 4A). However,complement and coagulation cascades and staphylococcus aureus infection signaling pathway response totrim47 knockout was different in the spleen between TRIM47–/–- and WT-group (Fig. 4B). These results indicated an important function of Trim47 on affecting various life processes. In addition to participating in the cellular process, knocking out test also indicated that Trim47 may exert immune function through complement-related pathways. In order to further study the changes of differential genes in the signaling pathway after knocking outtrim47, we performed heat map analysis on cell adhesion and glutamatergic synapse signaling pathway (brain, Fig. 4C) plement and coagulation cascades signaling pathway (spleen,Fig. 4D).
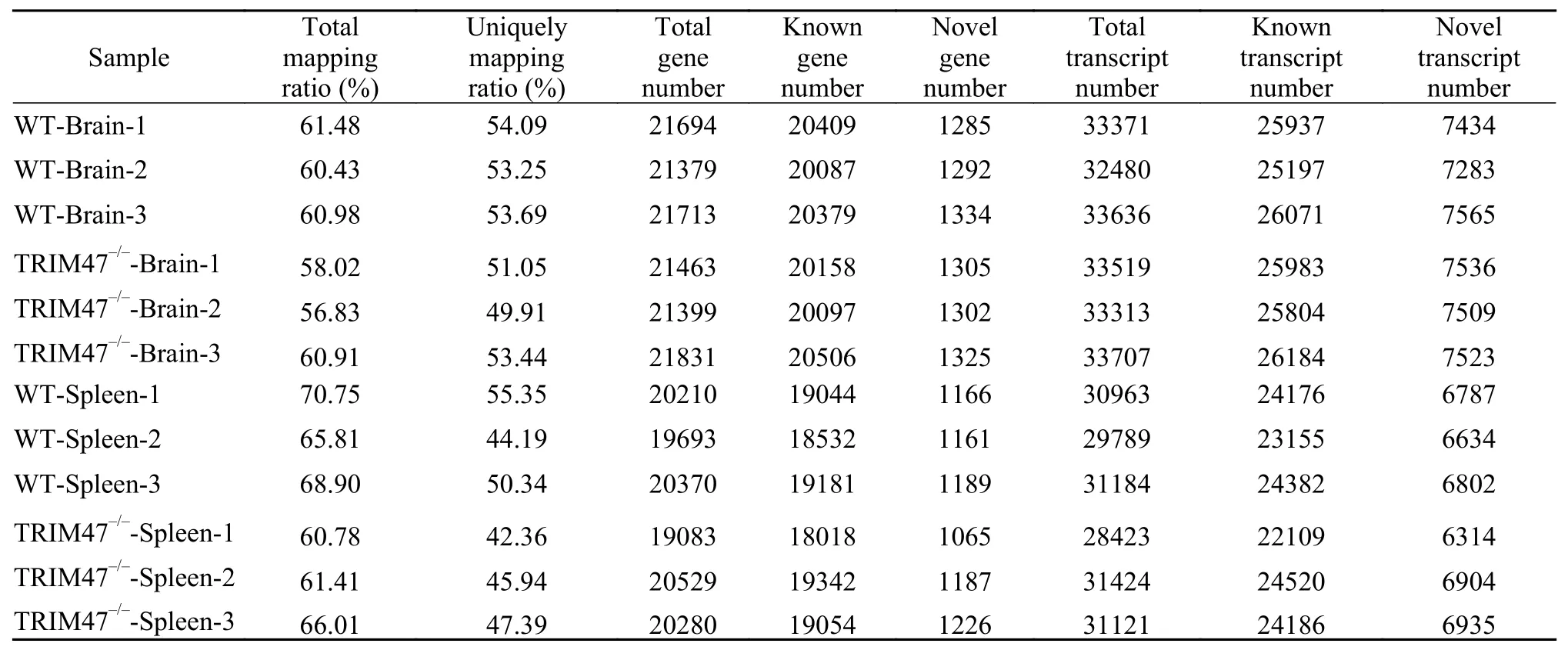
Tab. 3 Summary of the transcriptome data
2.6 Validation of DEGs by qRT-PCR
After pathway analysis of the differential genes,the differential genes were validated because of our interest in the enrichment of the complement hemagglutinin pathway after deletion oftrim47 in spleen. To further validate this conjecture, some genes related to complement were selected after knocking outtrim47 to detect their mRNA expression levels in the brain and spleen. The differential expression levels of the 5 genes associated with complement were comparatively quantified by qRT-PCR between the WT and the TRIM47–/–groups. The fold change of the target genes was compared through the RNA-Seq expression profile and presented in Fig. 5 (A for brain and B for spleen). There was a down-regulation of the gene between the WT and TRIM47–/–groups. The results indicated that the RNA-Seq data was reliable, which further verified the conjecture.
2.7 Survival curve
To further determine the role of Trim47 in immunization, survival experiments were performed(Fig. 6). The WT group and TRIM47–/–group zebrafish were intraperitoneally injected with 105pfu/mL of SVCV, and the control group was injected with 10 μL MEM medium. Based on the pre-experiment results of the previous period and combining the existing publications[25,26], we finally selected 105pfu/mL as the infection dose for subsequent experiments. In the virus infection experiment, we cansee the typical symptoms of SVCV infection in the infected group. For example, protruding eyeballs,bleeding, bleeding on the body surface, bleeding in the head, and ascites[27]. The uninfected group had no such symptoms. Zebrafish died from the third day, and the death rate accelerated from the third to the fifth day, which was consistent with the report[26].After 7 days of infection, the zebrafish survival rate in the experimental group was lower than that in the control group.
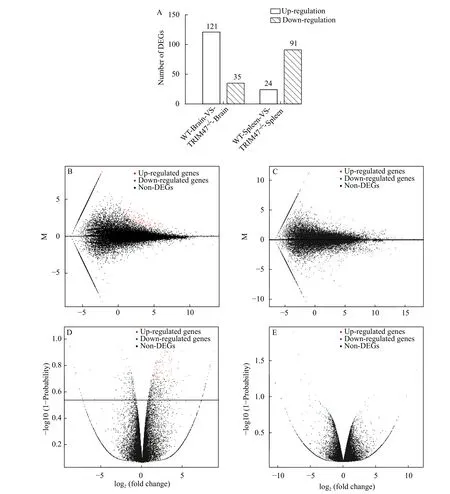
Fig. 2 Analyzing DEGs in brain and spleen between TRIM47–/– group and WT group
3 Discussion
In the transcriptome results, 156 up- and 115 down-regulated genes were differentially expressed and detected in the brain and spleen from knocking outtrim47, respectively. These differentially expressed genes were enriched into the signaling pathway. After knocking outtrim47, cell adhesion and glutamatergic synapse were enriched in the brain, indicating its important role in these cellular processes.In the spleen, these differentially expressed genes were enriched into the complement and coagulation cascades signaling pathway, which was novel and interesting. Complement system is a central component of innate immunity and can function as an important defender against pathogens, which is now known as an essential humoral system of innate immunity as well as a link between innate and adaptive immune responses[28—30]. Complement not only kills pathogenic microorganisms within an organism, but also participates in a variety of humoral and cellular immune responses[31]. Complement can transmit stimulation and inhibition signals to many cell types, regulating multiple life activities of cells, including cellular activation, differentiation, proliferation, cell movement,adhesion, secretion, and programmed death. Complement transmits signals through traditional cell membrane receptors, and signals can also be delivered to cells via membrane regulatory proteins or MAC complexes[32]. We selected complement-associated genes to validate transcriptome data. respectively, for two purposes: to verify the sequencing results and to determine the spleen differential gene enrichment pathway after knocking out TRIM47. it was hypothesized that TRIM47 may function through the complement pathway in the zebrafish spleen.

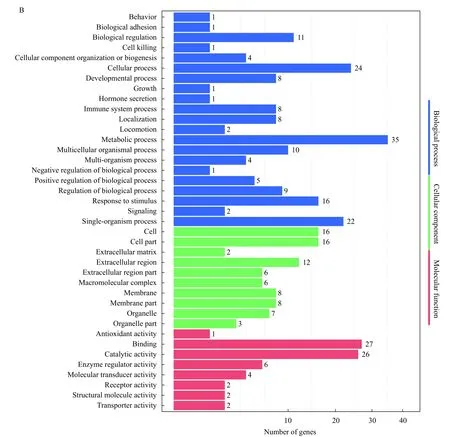
Fig. 3 GO enrichment for DEGs in brain and spleen between TRIM47–/– group and WT group
For infection experiments in zebrafish, we chose to use the SVCV, a virus caused severe disease in common carp[23]. The results showed that there was a difference in the mortality rate after knocking out thetrim47. Survival rate test was designed to simulate the influence of infection on survival rate under natural conditions. It is undeniable that after the Trim47 knockout, the susceptibility of zebrafish to SVCV is enhanced, and the mortality rate is higher. But this difference has not reached 50% or more. The function of single gene knockout is limited. Whether to apply the gene knockout technology in the prevention and control of fish diseases requires further research, and whether knocking out a single gene can promote other diseases also needs further investigation.
In summary, the model organism of zebrafish was used as the experimental object in this study, and knocked outtrim47 at the individual level, and performed transcriptome analysis. Differential gene analysis, signal pathway analysis, and functional cluster analysis were all performed to characterize the differentially expressed genes. The transcriptome data was validated and the function of Trim47 at the individual level was determined. The zebrafish infection experiments also confirmed that Trim47 played a significant role in the body of the fish. Findings from this study provide some essential basis for future in-depth study to verify the functions of the Trim47 and other TRIM proteins.
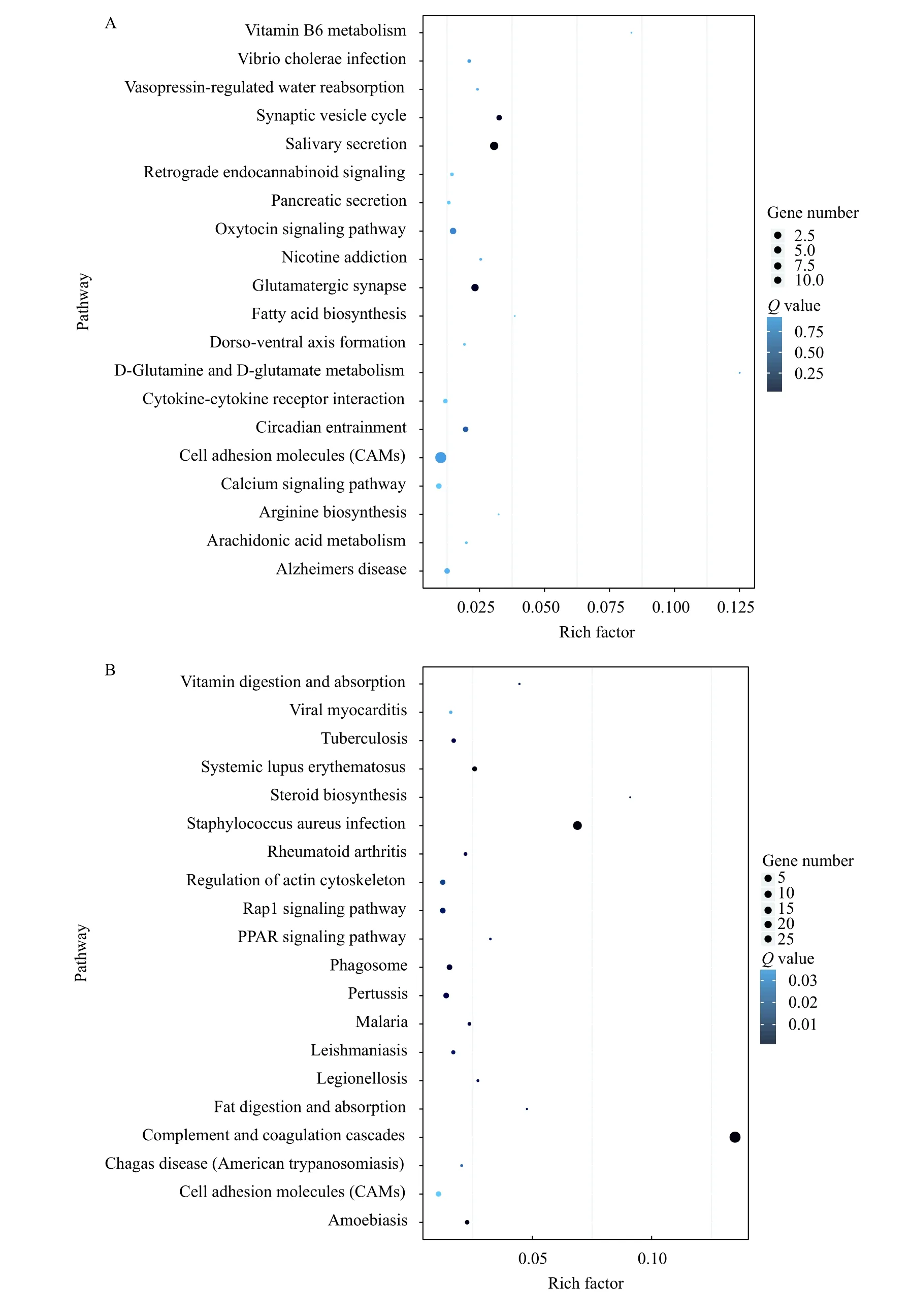

Fig. 4 Pathway functional enrichment of DEGs in brain and spleen between TRIM47–/– group and WT group

Fig. 5 Validation of differentially expressed genes by qRT-PCR in brain (A) and spleen(B) between TRIM47–/– group and WT group

Fig. 6 Survival rate after SVCV infection in TRIM47–/– group and WT group
