复合井修复地下水硝酸盐污染的效果
2021-06-01刘佩贵曾康辉尚熳廷刘湘伟
刘佩贵,曾康辉,尚熳廷,刘湘伟,阳 辉
复合井修复地下水硝酸盐污染的效果
刘佩贵1,曾康辉1,尚熳廷2※,刘湘伟3,阳 辉3
(1. 合肥工业大学土木与水利工程学院,合肥 230009;2. 合肥工业大学汽车与交通工程学院,合肥 230009;3. 西藏自治区水文水资源勘测局,拉萨 850000)
为探寻更适用于农田周边硝酸盐污染地下水的原位生物修复技术,该研究构建了A、B、C3套试验装置,分别刻画管井(A)、大口井与管井组成的复合井(B、C)。基于3套物理试验模型,定量对比分析了管井与复合井修复地下水硝酸盐污染的效果。结果表明:受水力停留时间的影响,相同流速条件下,A、B、C三套修复系统的硝酸盐负荷分别介于75~100、100~125、125~150 mg/L之间;在允许硝酸盐负荷范围内,去除率均可达到95%以上,且不会出现亚硝酸盐累积及氨氮超标现象,表明了复合井修复系统的可行性,可以实现地下水开采与修复同步进行,提高了地下水水源地供水安全保证率。
地下水;污染;硝酸盐去除;反硝化作用;复合井;水力停留时间
0 引 言
受农业长期施肥及土壤中微生物的影响,迁移能力较强的硝酸盐易随水分运动进入到饱和带,致使农田周边地下水面临被硝酸盐氮污染的威胁,影响到地下水水源地的供水安全[1-4]。为解决该问题,众多研究学者基于物理吸附[5-8]、化学反应[9-12]和生物转化[13-16]等原理提出了相应的原位或异位修复技术,其中,原位生物修复方法以去除率高、碳源经济安全、无二次污染或二次污染危害程度低、占地空间小等优点成为研究热点。现阶段常用的修复介质载体可概括为原位反应带(In-situ Reactive Zone,IRZ)[17-18]和可渗透反应墙(Permeable Reactive Barrier,PRB)[19-20]两大类,通过扩大IRZ的面积或者增加PRB的厚度均可以有效提高硝酸盐污染地下水的修复效果,但受含水层空间展布情况和修复工程经济成本等的影响,实际工程运行过程中,多存在去除效率与修复介质载体体积及工程运维成本之间的矛盾,制约了该技术的推广与应用。此外,已有的修复技术和方法多是围绕切断污染源展开,然而,因农作物生长和产量需求,农业化肥面源污染不易阻截,进一步增加了农田区域地下水硝酸盐污染修复的难度。刘明朝[21]设计了原位水平井修复系统,该系统实现了地下水开采与修复的同步进行,破解了需要切断污染源才可修复地下水污染的问题,但该系统的不足是水平井成井结构技术要求较高,操作复杂,亟需寻求施工工艺相对简单、修复效果佳的原位修复介质。
为此,本文基于管井和大口井的适用条件,借助生物修复技术,通过设计管井与大口井组成的复合井,构建室内物理试验模型,研究复合井原位生物修复地下水硝酸盐污染的效果,探讨该类井结构应用于实际工程的可行性和适用性,以期提出一种简便易操作的原位生物修复地下水污染系统,为解决农田周边地下水硝酸盐污染问题提供切实可行的技术与方法。
1 材料与方法
1.1 试验装置与运行
为构建复合井原位修复系统,自制了3套试验装置(图1和表1),每套装置均由井、储液池、蠕动泵、乙醇注入桶组成,其中装置A模拟的是一口直径7 cm的管井,装置B和C模拟的是由直径14 cm的大口井和直径7 cm的管井组成的复合井,井的材料均为亚克力板材。
试验所用含水介质为粒径<0.25 mm的均质细砂,为保证3个系统的渗透性能一致,试验中采用相同均质砂的干容重分层填砂,边压实边填入A、B、C试验装置的管井中,保证管井中均填入相同体积和高度的细砂(细砂高度均为12 cm),装置B和C的大口井中介质填充高度分别为4 cm和8 cm的介质,压实度与管井相同。管井和大口井底部密封,侧壁通过过滤器进水,每个管井上部安装蠕动泵模拟抽水,3组试验装置抽水期间不形成干扰井。本文的目标是分析井的类型对硝酸盐修复效果的影响,为了保证其他因素完全相同,3套试验装置放置于同一模拟硝酸盐污染地下水的储液池中,尽可能保证外界环境完全相同。

表1 试验运行参数
注:S1~S6表示反应阶段。
Note : S1-S6 represent the reaction stages.
1.2 试验方法
自然界中广泛存在反硝化微生物,为提高复合井原位系统中生物反硝化作用的效果及反应速率,需要人为添加碳源,对比固态、液态碳源的优缺点[22-24],本次选用无毒无害、经济实惠的乙醇作为反硝化碳源,根据公式(1)计算试验运行一定时间内需要注入的乙醇量
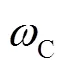
为避免地下水中其他离子成分对反硝化作用的干扰,采用去离子水与KNO3(分析纯)配置模拟不同浓度硝酸盐污染地下水。试验开始前将3套试验装置静置于模拟硝酸盐污染地下水的储液池中,使水流从下往上缓慢饱和介质,并保证排空介质中的空气,待试验柱充分饱水后开始试验。首先,进行第一个试验段(S1),启动乙醇注入泵,为保证碳源分析的均匀性,将传输乙醇直径为1cm的软管在大口井中部缠绕一圏,软管侧壁均匀打孔,以便使乙醇均匀扩散到介质中;然后启动补水泵和抽水泵。该阶段为生物自然挂膜阶段,通过自然驯化优势菌种实现反应系统中的反硝化作用[25]。试验期间每天8:00从抽水泵中取样,使用哈希DR6000型紫外可见光分光光度计检测NO3--N、NO2--N、NH4+-N(总称为三氮)的浓度,待三氮浓度稳定后结束该阶段。然后,保持所有泵正常工作,增大储液池内NO3--N浓度至50 mg/L(S2阶段),乙醇的注入量根据公式(1)作相应的调整,继续试验过程,重复取样和检测过程,待出口处三氮浓度稳定后再进入S3阶段,依次进行,直至出口处硝酸盐的浓度超过标准限值才停止试验。本文硝酸盐的限值采用世界卫生组织(WHO)规定的硝酸盐氮浓度(11.3 mg/L)。
2 结果与分析
2.1 修复效果分析
根据生物反应过程,将原位生物修复硝酸盐过程分为自然挂膜和正常运行两个阶段。为保障自然挂膜效果,初始管井进水流速为0.13 m/d,NO3--N浓度为25 mg/L,3套反应系统的NO3--N浓度均在第4 d降至1.0 mg/L以下,去除率大于97%,持续稳定4 d后,认为挂膜成功。由于进水流速将对反硝化作用效果产生一定的影响[21],硝酸盐修复过程中不宜使进水流速过大,根据本次试验的介质条件,正常运行阶段进水流速控制为0.26 m/d。根据反硝化作用程度及NO3--N浓度,本次试验自然挂膜和正常运行共包括6个阶段。
由图2的NO3--N浓度变化过程可以看出,每个反应阶段NO3--N浓度均随着反应时间的增加和进水NO3--N浓度的变化,出现先上升后逐渐下降并逐渐趋于稳定的状态。当反应阶段发生变化,即瞬时增大硝酸盐浓度时,浓度变化情况表明反应介质中的微生物基本在1d内即可完成筛选优势菌种,以便适应硝酸盐负荷条件的变化,提高反硝化能力。
S1和S2两个阶段A、B、C3套修复系统NO3--N的去除率均大于97%,当进水NO3--N浓度增大至75 mg/L时,仅反应系统A的去除率略有降低,约为89%,但此时硝酸盐氮浓度的检测值为7.94 mg/L,仍小于WHO规定的限值11.3 mg/L,满足水质要求。继续增大进水NO3--N浓度至100 mg/L时(S4),系统A、B、C持续反应11d后NO3--N浓度分别稳定在50 mg/L、3 mg/L、0.5 mg/L,表明A系统内的反硝化菌群的处理能力已不能满足标准限值的要求,超出了其最大硝酸盐处理负荷。其他两个复合井系统去除率仍大于97%,修复效果仍非常好,此后停止试验装置A的运行。当进水NO3--N浓度增大至125 mg/L(S5)时,装置B的NO3--N去除率由97%降至68%(图3),稳定浓度超过了11.3 mg/L,表明地下水硝酸盐浓度超过了装置B的处理能力即承载负荷,停止装置B的试验。此时,系统C的去除率仍接近98%,表明硝酸盐浓度还可以继续增大。当进水NO3--N浓度持续增大至150 mg/L,待试验稳定后去除率下降至70%,出水浓度约45 mg/L,超过了WHO的标准限值。由此得出,A、B、C3套试验装置,以0.26 m/d的流速稳定运行时的硝酸盐负荷分别介于75~100、100~125、125~150 mg/L之间。
NO2--N是微生物反硝化过程的中间产物,装置A、B、C分别在S3、S4、S6阶段出现NO2--N的累积现象(图3),特别在S4阶段A和B两个系统中NO2--N浓度均大于10 mg/L,出现了NO2--N严重累积问题。但S1-S5反应阶段3个修复系统的NH4+-N浓度基本在0~1 mg/L之间,仅在装置处理能力小于硝酸盐负荷时NH4+-N浓度才增大至地下水III类水限值(0.5 mg/L)以上。
2.2 修复效果差异性分析
综合图2~图3的结果可以看出,3套试验装置的硝酸盐负荷和去除率总体效果C>B>A。造成系统间差异的主要原因是由于B、C两个装置中的取水建筑物为管井与大口井组成的复合井。从管井中抽水,被硝酸盐污染的地下水通过过滤器首先进入大口井,经过反硝化作用后才进入管井,管井中的地下水在微生物的作用下进一步降低了硝酸盐的浓度,复合井增大了反硝化作用的面积、延长了微生物反硝化时间。三个试验装置反应介质的体积比为1﹕2﹕3,管井和大口井的直径相同,试验装置表现在介质填充厚度不同,装置C大口井的厚度是装置B的2倍,流速相同条件下,水力停留时间()之比为1 ﹕2﹕4,C>B>A,而水力停留时间是决定修复效果的重要因素[26]。从3套试验装置可处理的硝酸盐负荷(NL)也可以看出,NLC>NLB>NLA。由此得出,装置C的修复效果最优,B次之,A最差,复合井的修复能力和效果明显优于管井。
NO2--N的浓度主要受反应速率、生物菌群、还原酶的影响,试验后期出现了NO2--N积累现象,可能是由于NO2--N的降解速率小于NO3--N的降解速率[27],因为进行异化硝酸盐还原的异样细菌可分为两类,a类菌群只含有硝酸盐还原酶,b类菌群含有反硝化中的全部酶系。当某些因素抑制b类菌群的生长而对a类菌群影响较小时,就会造成NO2--N积累[28],且硝酸盐还原酶的活性比亚硝酸盐还原酶的活性更高[29],随着微生物的不断生长,修复系统内能够反应NO3--N的生物量远远多于能够反应NO2--N的生物量,反应优先进行NO3--N→NO2--N,此后才发生NO2--N→NO的过程。
造成部分阶段NH4+-N浓度增大的主要原因是由于异化还原成铵作用(Dissimilatory Nitrate Reduction to Ammonium, DNRA)[30-31]。由于反硝化作用与DNRA作用均需要有机质提供电子供体,故二者呈竞争关系。根据细菌将选择获取能量较大的反应这一理论[32],细菌从反硝化作用中获得的能量(2 333.84 kJ/mol)远高于从DNRA作用中获得的(679.605 kJ/mol),因此优先发生反硝化作用。故在修复系统的硝酸盐负荷范围内,NH4+-N积累程度较低,DNRA作用较弱,反硝化作用呈主体作用,而超过硝酸盐负荷范围后,反硝化作用减弱,DNRA作用增强,NH4+-N积累程度有所增加。
2.3 复合井的应用性探讨
常见的取水建筑物有管井、大口井、渗渠、辐射井,其中管井和大口井适用范围广、适用性强[33],因此,本次研究过程中选用了管井和大口井。大口井一般适用于地下水埋藏较浅、含水层厚度不大和富水性好的地区,实际应用中井径多在5~10m,具有较大的反硝化面积,较管井存在施工条件要求高、基建费用高的缺点。管井适用范围较广、成井工艺相对简单,但反硝化面积有限,影响修复效果,因此,本文结合两种类型井的优势,构建了大口井与管井组成的复合井,用其开展原位修复地下水硝酸盐污染,试验结果表明了复合井的去除率和处理负荷明显优于管井。
决定装置修复效果的关键因素是反硝化面积和时间,类比分析,理论上可以仅建立一个大口井开展地下水的修复和开采,但从成井成本和便于管理角度,一般不采用大口井开采地下水,但为了满足地下水供水中硝酸盐标准限值的要求,建议借助本次研究中提出的复合井结构提升修复效果。实际成井结构示意图如图4所示,施工过程中底部的大口井可以通过扩孔解决,实际应用中大口井的口径由含水层中地下水硝酸盐污染的浓度和去除率共同决定。
3 结 论
本文通过构建管井和大口井组成的复合井原位生物修复系统,研究了复合井原位生物修复地下水硝酸盐污染的效果及硝酸盐负荷。在试验装置尺寸条件下,当以0.26 m/d的水流流速抽水时,三个试验装置的硝酸盐负荷分别介于75~100、100~125、125~150 mg/L之间。因复合井的水力停留时间和体积是管井的2倍,增加了反硝化时间和面积,修复能力和效果明显优于管井。当试验装置的硝酸盐负荷小于其处理能力时,去除率均可达到95%以上,且未出现亚硝酸盐累积及氨氮超标现象,从而表明了复合井修复系统的可行性,并对其应用性进行了探讨,设计了实际工程应用中建议的成井结构示意图,在不需要切断农业面源污染条件下,可以实现地下水开采与修复同步进行,提高了地下水水源地供水安全保证率。此外,复合井为管井与大口井的组合,不仅成井工艺相对简单,适用范围也广(包括潜水、承压水),可以根据含水层厚度、需水量大小、硝酸盐污染地下水的程度等调整大口井的井径及反应介质载体高度,在不影响开采量的同时保障硝酸盐的去除效果。
受试验时间的限制,物理试验不可能穷尽所有的试验方案,因此,基于物理试验模型,本文仅确定了三个修复系统的最大硝酸盐负荷区间,未明确具体的硝酸盐负荷。后续工作中将通过建立水文地球化学模拟模型,精确确定不同复合井修复系统的最大硝酸盐负荷,并探讨介质的非均质性对修复效果的影响程度。
[1]严冬冬,徐从海,黄永军,等. 郯城县农村饮用水源地硝酸盐污染及防治对策[J]. 环境研究与监测,2020,33(3):59-63. Yan Dongdong, Xu Conghai, Huang Yongjun, et al. Nitrate pollution in rural drinking water sources in Tancheng county and countermeasures[J]. Environmental Research and Monitoring, 2020, 33(3): 59-63. (in Chinese with English abstract)
[2]孟春霞,郑西来,马振宇,等. 青岛市农村供水中硝酸盐氮污染状况及健康风险评价[J]. 水利水电技术,2014,45(9):24-26. Meng Chunxia, Zheng Xilai, Ma Zhenyu, et al. Assessment on status of nitrate pollution in rural water supply of Qingdao and its healthy risk[J]. Water Resoures and Hydropower Engineering, 2014, 45(9): 24-26. (in Chinese with English abstract)
[3]Liu Jiutan,Peng Yuming,Li Changsuo,et al. Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health-ScienceDirect[J]. Environmental Pollution, 2021, 268: 115947.
[4]Cameira M D R, Rolim J, Valente F, et al. Translating the agricultural N surplus hazard into groundwater pollution risk: Implications for effectiveness of mitigation measures in nitrate vulnerable zones-ScienceDirect[J]. Agriculture, Ecosystems & Environment, 2021, 30: 107204.
[5]Bergquist A M, Choe J K, Strathmann T J, et al. Evaluation of a hybrid ion exchange-catalyst treatment technology for nitrate removal from drinking water[J]. Water Research, 2016, 96(1): 177-187.
[6]Shafiekhani H, Barjoizadeh R. Modification of activated carbon by ZnCl2, CaCl2, MgCl2and their applications in removal of nitrate ion from drinking water[J]. Asian Journal of Green Chemistry, 2019, 3: 1-12.
[7]Lékéné R B N, Nsami J N, R A, et al. Optimization conditions of the preparation of activated carbon based Egusi (Naudin) seed shells for nitrate ions removal from wastewater[J]. American Journal of Analytical Chemistry, 2018, 9(10): 439-463.
[8]赵爽. 给水厂污泥改性活性炭去除硝酸盐氮的性能研究[D]. 北京:北京建筑大学,2020. Zhao Shuang. Study on the Performance of Water Treatment Residuals Modified Granular Activated Carbon to Remove Nitrate[D]. Beijing: Beijing University of Civil Engineering and Architecture, 2020. (in Chinese with English abstract)
[9]沈静,项凯民,缪泽群,等. 钴卟啉催化硝酸盐电化学还原反应[J]. 湖南科技大学学报:自然科学版,2020,35(4):89-95. Shen Jing, Xiang Kaimin, Mou Zequn, et al. Nitrate electrochemical reduction catalyzed by cobalt protoporphyrin[J]. Journal of Hunan University of Science & Technology (Natural Science Edition), 2020, 35(4): 89-95. (in Chinese with English abstract)
[10]Sheydaei M, Ayoubi-Feiz B. Nitrate reduction through the visible-light photoelectrocatalysis and photoelectrocatalysis/reverse osmosis processes: Assessment of graphene/Ag/N-TiO2nanocomposite[J]. Journal of Water Process Engineering, 2021, 39: 101856.
[11]Dortsiou M, Katsounaros I, Polatides C, et al. Influence of the electrode and the pH on the rate and the product distribution of the electrochemical removal of nitrate[J]. Environmental Technology, 2013, 34: 373-381.
[12]王乐乐. 纳米电极制备及电化学去除地下水中硝酸盐行为与机理[D]. 北京:中国地质大学(北京),2020. Wang Lele. Preparation of Nano Electrode and Behavior and Mechanism for Electrochemical Reduction of Nitrate in Groundwater[D]. Beijing: China University of Geosciences(Beijing), 2020. (in Chinese with English abstract)
[13]张兰河,庄艳萍,王旭明,等. 温度对改良A2/O工艺反硝化除磷性能的影响[J]. 农业工程学报,2016,32(10):213-219. Zhang Lanhe, Zhuang Yanping, Wang Xuming, et al. Effect of temperature on denitrifying phosphorus removal efficiency using modified A2/O process[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(10): 213-219. (in Chinese with English abstract)
[14]Yan S, Cheng K Y, Ginige M P, et al. Optimization of nitrate and selenate reduction in an ethanol-fed fluidized bed reactor via redox potential feedback control[J]. Journal of Hazardous Materials, 2020, 402: 123770.
[15]Zhang Yichong, Xu Qiang, Huang Guangtuan, et al. Effect of dissolved oxygen concentration on nitrogen removal and electricity generation in self pH-buffer microbial fuel cell[J]. International Journal of Hydrogen Energy, 2020, 45(58): 34099-34109.
[16]易能,邸攀攀,王岩,等. 富氧灌溉池塘中反硝化细菌丰度昼夜垂直变化特征分析[J]. 农业工程学报,2015,31(15):80-86. Yi Neng, Di Panpan, Wang Yan, et al. Diel vertical variability analysis of denitrifying bacteria abundance in oxygen-enriched irrigation pond[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2015, 31(15): 80-86. (in Chinese with English abstract)
[17]Khan I A, Spalding R F. Development of a procedure for sustainable in situ aquifer denitrification[J]. Remediation Journal, 2003, 13(2): 53-69.
[18]Kim Y, Kim J H, Son B H, et al. A single well push-pull test method for in situ determination of denitrification rates in a nitrate-contaminated groundwater aquifer[J]. Water Science & Technology, 2005, 52(8): 77-86.
[19]何培芬. 丝瓜络作为模拟可渗透反应墙介质处理地下水中硝酸盐研究[D]. 广州:广州大学,2019. He Peifen, Study on Removal of Nitrogen from Groundwater by Loofah Sponge Solid Carbon as Simulate Permeable Reactive Barrier Media[D]. Guangzhou: Guangzhou University, 2019. (in Chinese with English abstract)
[20]Liu Yong, Liu Yajing, Ma Lei, et al. Corncob PRB for on-site nitrate removal in groundwater[J]. Arabian Journal of Geosciences, 2020, 13(20): 1-11.
[21]刘明朝. 地下水水源地硝酸盐污染的原位修复试验研究[D]. 合肥:合肥工业大学,2019. Liu Mingchao. Experimental Study on In-situ Remediation of Nitrate Pollution in Groundwater Well Field[D]. Hefei: Hefei University of Technology, 2019. (in Chinese with English abstract)
[22]姜婷婷. 乙醇作为生活污水同步硝化反硝化脱氮外碳源可行性研究[J]. 环境科学与管理,2019,44(1):128-131. Jiang Tingting. Feasibility study of using ethanol as external carbon source for simultaneous nitrification and denitrification of domestic sewage[J]. Environmental Science and Management, 2019, 44(1): 128-131. (in Chinese with English abstract)
[23]余猛,杨凤根. PRB修复受硝酸盐污染地下水的碳源材料研究进展[J]. 净水技术,2020,39(4):96-101,107. Yu Meng, Yang Fenggen. Research progress of PRB carbon source materials for groundwater rehabilitation polluted by nitrate[J]. Water Purification Technology, 2020, 39(4): 96-101, 107. (in Chinese with English abstract)
[24]黄骏宇. 利用米酒为碳源去除饮用地下水中硝酸盐的现场试验研究[D]. 桂林:桂林理工大学,2018. Huang Junyu. An In-situ Test Study on Nitrate Removal from the Drinking Groundwater by Using Rice Wine as Carbon Source[D]. Guilin: Guilin University of Technology, 2018. (in Chinese with English abstract)
[25]徐佩. 外加碳源生物滤池处理城市污水厂尾水脱氮试验研究[D]. 武汉:武汉科技大学,2011. Xu Pei. The Study on Nitrogen Removal in Biological Filter of Extra Carbon Source for The Secondary Effluent of Municipal Wastewater Treatment Plant[D]. Wuhan: Wuhan University of Science and Technology, 2011. (in Chinese with English abstract)
[26]Westerhoff P, James J. Nitrate removal in zero-valent iron packed columns[J]. Water Research, 2003, 37(8): 1818-1830.
[27]付昆明,曹相生,孟雪征,等. 污水反硝化过程中亚硝酸盐的积累规律[J]. 环境科学,2011,32(6):1660-1664. Fu Kunming, Cao Xiangsheng, Meng Xuezheng, et al. Characteristics of nitrite accumulation during wastewater denitrification[J]. Environmental Science, 2011, 32(6): 1660-1664. (in Chinese with English abstract)
[28]Payne W J. Reduction of nitrogenous oxides by microorganisms[J]. Bacteriol Rev, 1974, 37(4): 409.
[29]Bock E, Sundermeyer-Klinger H, Stackebrandt E. New facultative lithotrophic nitrite oxydizing bacteria[J]. Archives of Microbiology, 1983, 136(4): 281-284.
[30]Woods D D. The reduction of nitrate to ammonia by Clostridium welchii[J]. Biochemical Journal, 1938, 32(11): 2000-2012.
[31]Li Xiaowen, Song Chunlei, Zhou Zijun, et al. Comparison of community and function of dissimilatory nitrate reduction to ammonium (DNRA) bacteria in Chinese Shallow Lakes with Different Eutrophication Degrees[J]. Water, 2020, 12(1): 174.
[32]斯塔姆. 水化学:天然水体化学平衡导论[M]. 汤鸿宵,译. 北京:科学出版社,1987.
[33]王威,杨在文,杨广焱,等. 地下水取水工程验收技术要点分析[J]. 地下水,2017,39(6):98-99,157. Wang Wei, Yang Zaiwen, Yang Guangyan, et al. Analysis of technical points for acceptance of groundwater intake engineering[J]. Ground water, 2017, 39(6): 98-99, 157. (in Chinese with English abstract)
Remediation effects of compound well on nitrate pollution in groundwater
Liu Peigui1, Zeng Kanghui1, Shang Manting2※, Liu Xiangwei3, Yang Hui3
(1.,,230009,;2.,,230009,; 3.,,850000,)
Nitrate pollution has posed a great threat to groundwater near farmlands, due mainly to the long-term agricultural fertilization and soil microorganisms. Nitrates with strong migration ability have entered the zone of saturation along with water movement. High concentrations of nitrates aredirectly detrimental to the safety of groundwater source areas. In this study, three systems A, B, and C were constructed to explore an in-situ bioremediation technology for the detection of nitrate-contaminated groundwater around farmland. Every system consisted of wells, storage tanks, and peristaltic pumps. System A was used to simulate a tube well with a diameter of 7 cm. System B and C were used to simulate compound wells, where there were a large well with a diameter of 14 and a tube well with a diameter of 7 cm. Every tube well was filled with fine sand in the same volume and height, where the height of fine sand was 12cm. The large diameter wells in system B and C were filled with fine sand with the heights of 4 cm and 8 cm, respectively. Both tube wells and large wells were used to simulate the complete penetration wells. The bottom of the wells was sealed, where water flowed in from the side walls. The peristaltic pumps were installed on the top of wells to simulate water pumping. The volume ratio of the reaction medium was 1:2:3 in three systems. The ratio of hydraulic retention time was also 1:2:4 under the same flow rate. The biofilm was naturally domesticated. Ethanol was used as the carbon source. A total of 6 groups were set in the reaction stage, including 25, 50, 75, 100, 125 and 150 mg/L, according to the concentration gradients of nitrate nitrogen. The test results showed that the microorganisms in the reaction medium could basically select the dominant strains within one day when the nitrate concentration increased instantaneously, leading to match the changes in nitrate loading conditions for the better denitrification capacity. In-situ bioremediation systems were also constructed with compound wells including tube wells and large diameter wells, in order to repair nitrate-contaminated groundwater and the nitrate loading of every system. The nitrate loadings of three remediation systems A-C were 75-100 mg/L, 100-125 mg/L, and 125-150 mg/L at the flow rate of 0.26 m/d. The removal rate of remediation systems reached more than 95% within nitrate loading. There was no accumulation of nitrite and excessive ammonia nitrogen, indicating the feasibility of repair systems with compound wells. Groundwater mining and remediation were carried out simultaneously without the need to cut off agricultural non-point source pollution, indicating high security for groundwater source area. In addition, a combination of tube wells and large diameter wells can be installed to compound wells with a relatively simple well drilling (including phreatic water and confined water). The diameter of wells and height of the reaction medium can be adjusted for better removal of nitrates, according to the thickness of the aquifer, the amount of water demand, and the level of nitrate-contaminated groundwater. Physical test models were used to determine the nitrate loading intervals of three remediation systems. In the future work, the hydrogeochemical model will be established to accurately determine the maximum nitrate loadings of repair systems with compound wells, together with the influence of medium heterogeneity on the remediation performance.
groundwater; pollution; nitrate removal; denitrification; compound wells; hydraulic retention time
刘佩贵,曾康辉,尚熳廷,等. 复合井修复地下水硝酸盐污染的效果[J]. 农业工程学报,2021,37(6):214-219.doi:10.11975/j.issn.1002-6819.2021.06.026 http://www.tcsae.org
Liu Peigui, Zeng Kanghui, Shang Manting, et al. Remediation effects of compound well on nitrate pollution in groundwater[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(6): 214-219. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.06.026 http://www.tcsae.org
2020-01-21
2021-03-01
水文水资源与水利工程科学国家重点实验室“一带一路”水与可持续发展科技基金(2018nkms06)
刘佩贵,博士,副教授,主要研究方向为水资源评价。Email:liupg2512@163.com
尚熳廷,博士,副教授,主要研究方向为土壤水分运动物理规律模拟。Email:hfut_smt@163.com
10.11975/j.issn.1002-6819.2021.06.026
S152.7
A
1002-6819(2021)-06-0214-06
