Light-emitting MXene quantum dots
2021-05-21AnirSharbirinSophiaAkhtarandJeongyongKim
Anir S. Sharbirin, Sophia Akhtar and Jeongyong Kim
Keywords: MXene; quantum dots; light emission; MAX phase; 2D materials
Introduction
Since the discovery of graphene by Novoselov et al.1,2, extensive research has been conducted on two-dimensional (2D) materials owing to their excellent properties.Over the years, several other 2D materials have been investigated, namely, hexagonal boron nitride (hBN)3,transition metal dichalcogenides (TMDs)4, metal oxides,hydroxides5and their heterostructures6−8. In 2011,MXene, a new family of 2D materials that were produced by selective etching of the MAX phase (general formula of Mn+1AXn, where M is an early transition metal, A is main group elements 13 and 14, and X is carbon and/or nitrogen) in liquid at room temperature, was discovered by Naquib et al.9. Until now, approximately 100 MAX phases have been fabricated and up to 30 MXenes have been reported, making them the largest group among the 2D family10.
MAX phases are layered ternary carbides, nitrides, or carbonitrides with a hexagonal crystal structure with space group P63/mmc. Unlike other bulk 3D layered materials such as graphite and TMDs, which could be mechanically exfoliated with ease because of the weak van der Waals interactions that hold the structure together, the bonds between the layers in the MAX phase are too strong to be broken with similar means.However, by employing the relatively weaker M-A bonds compared to the M-X bonds, it is possible to selectively etch out the A layer by chemical means, leaving us with M-X layers of MXenes with a general formula Mn+1XnTx,where Txis the notation for a surface-terminating functional group (O, OH, F, H, etc.)10. Generally, aqueous solutions containing fluoride ions, such as aqueous hydrofluoric acid (HF), a mixture of lithium fluoride (LiF)or potassium fluoride (KF) with hydrochloric acid (HCl),or molten salts of fluoride, are used to selectively etch the A layers. After etching, the multilayer (ML)-MXenes are immersed in dimethyl sulfoxide (DMSO), tetrabutyl ammonium hydroxide, alcohols, choline hydroxide, or nbutylamine for further delamination through sonication or centrifugation9−15. Compared to that obtained using single-element monolayer graphene, layered structures that contain more than one element provide large opportunities for functionalization to achieve varying properties. This is why MXene has been a promising candidate for a myriad of applications including energy storage devices, sensors, catalysis, water purification, transparent conductors, electromagnetic interference shielding,thermoelectrics, and plasmonics. One of the most widely studied applications of MXene is their use in energy storage devices mainly because of their high electronic conductivity9,10,14,16−22. Despite that there are many applications taking advantages of its various excellent properties, the application of MXenes in optoelectronic devices is still lacking. Although there are various theoretical reports on semiconducting MXenes, to the best of our knowledge, experimental work on these materials has not yet seen any success yet10,23. A breakthrough in the synthesis of light-emitting MXene-based materials is in demand.
Quantum dots derived from 2D materials (2D-QDs)have shown promising prospects for applications in nanomaterial-based devices. They not only inherit the merits of their 2D counterparts but also exhibit improved properties such as better dispersibility, higher chemical stability, easier functionalization, larger surface-tovolume ratio, and stronger photoluminescence (PL) after a size reduction (typically <10 nm) resulting from the strong quantum confinement and edge effect24. One of the widely known 2D-QDs that has benefitted from the quantum confinement and edge effect is graphene quantum dots (GQDs). When a few layers graphene is reduced to a size ranging from a few nm to 100 nm, they show strong PL25. Other 2D-QDs derived from TMDs and hBN have shown enhancement in PL, bandgap tunability, and functionality relative to those of their 2D counterparts26−28. By utilizing the same idea of reducing the dimensionality, it is possible to fabricate MXenebased 2D-QDs. The first reported work on MXene-derived QDs was in 2017 by Xue et al.29by using Ti3C2MXene as the precursor; blue PL emission with an absolute quantum yield (QY) of 10% was observed after the Ti3C2MXene was broken down into a smaller size. As it retained the structure of its 2D counterpart in a smaller size, it is reasonable to use MQDs as the abbreviation for MXene-derived quantum dots (MQDs). Like other 2DQDs, the emergence of MQDs has garnered the attention of researchers owing to their excellent properties,such as hydrophilicity, biocompatibility, functionalization, and more importantly, the PL property29−31.
More and more studies on MQDs are published in recent years; however, comprehensive reviews on MQDs are rarely found, and only one review has been published recently32. However, light-emitting properties of MQDs require special attention because MQDs can replace conventional luminescent QDs in photonic or bioapplications that require non-toxic and biocompatible materials. In this review, we provide a general overview of the synthesis methods, physical, chemical, and structural properties, and the applications of MQDs with a focus on their light-emitting aspects and also provide a perspective for future opportunities and challenges of light-emitting MQDs for photonic and bio-applications.
Synthesis of MAX phase and MXene
Even though there are numerous techniques and methods available to synthesize MQDs, the basis of synthesizing light-emitting MQDs is the same which is reducing the size of MXene (typically <10 nm) to induce bandgap expansion by quantum confinement effect29,30,33−38. Thus,any method could be used to synthesize light emitting MQDs as long there is a size reduction while maintaining the structure of the precursor (MXene). However, the properties of MQDs are heavily dependent on the parent precursors used for MQD synthesis, which are the MAX phase and MXene. Only several methods of specific synthesis conditions and parent MXene precursors lead to the synthesis of light-emitting MQDs with desired properties. Various Mn+1AXnphases have been obtained by selecting differentnvalues and/or different M, A, or X elements. The strong M–X covalent bond and M–A metallic bond of the MAX phase cannot be broken by mechanical exfoliation. To prepare MXenes from the MAX phase, strong etchants such as lithium fluoride-hydrochloric acid mixtures (LiF-HCl), ammonium hydrogen bifluoride (NH4HF2), and HF are employed9−11,13,39,40.In this review, we limit our review to MQDs derived from Ti3C2, V2C, and Nb2C MXenes. These MQDs exhibit great light-emitting properties and high photoluminescence QY (PLQY).
Synthesis of MAX phase
The Ti3AlC2MAX phase was first discovered by Pietzka et al.41in 1994 while investigating the Ti–Al–C ternary phase diagram. The compound Ti3AlC2gained increasing attention owing to its lightweight and superior properties compared to other layered ternaries. A few methods used to synthesize the Ti3AlC2MAX phase, as well as other types, have been discussed below. The hot isostatic pressing (HIP) method42is used to densify the sintered components and enhance the interfacial bonding. HIP involves the simultaneous application of elevated temperature and high pressure in a sealed or an enclosed vessel. Usually, an inert gas is used to apply the pressure,as suggested by the name of isostatic. HIP is mainly concerned with the removal of pores that are induced during calcination from gas evolution, packing of the particles of powder, agglomeration of vacancies, and interdiffusion during bonding of dissimilar materials. Under high temperature and pressure, the internal defects or pore collapse and precursors are densified42. The bulk polycrystalline Ti3AlC2MAX phase was first synthesized by Tzenov and Barsoum43. A mixture of Ti, graphite, and Al4C3precursors was ball-milled, followed by HIP. The samples synthesized by HIP were observed to be single phase and fully dense with a grain size of nearly 25 μm.Ti3AlC2is isostructural and possesses the merits of both ceramics and metals: it exhibits high modulus, high strength at high temperatures, and low thermal expansion coefficient43. Wang and Zhou44also fabricated Ti3AlC2by solid–liquid reaction synthesis and simultaneous in situ hot pressing process.
The synthesis of the MAX phase is usually performed at very high temperatures. Gogotsi et al.45synthesized Ti3AlC2by using powder precursors of Ti, Al, and graphite at 1650 °C following a high-temperature synthesis method. Barosum et al.46synthesized V2AlC by ball milling of powders of V, Al, and C, followed by calcination at a high temperature of 1500 °C. Afterwards,the synthesized MAX phase was sieved, and a 400 mesh powder was used for MXene synthesis.
The Nb2AlC phase was synthesized for the first time in 1980 by Schuster and Nowotny47,48while investigating phase equilibrium in the Nb–Al–C system. Nb2AlC was prepared by arc melting and annealing. Barsoum et al.49used reactive HIP to fabricate bulk Nb2AlC from the precursors including Nb, C, and Al4C3. Zhang et al.50employed reactive hot pressing to synthesize Nb2AlC from NbC, Nb, and Al precursor powders. Kovalev et al.48synthesized Nb2AlC by using the self-propagating high-temperature synthesis method. They suggested that this method is highly productive and requires no complex equipment. The initial precursors were Nb(IV) oxide, Al,and C.
Zhou et al.51used the spark plasma sintering technique to synthesize Nb2AlC. The powdered precursors with a molar ratio of Nb∶Al∶NbC = 2∶1.2∶1 were employed and ethanol was used as a mixing medium. Afterwards, the obtained powder was compacted under 20 MPa pressure. Finally, sintering was done in a spark plasma sintering system at 1450 °C at a heating rate of 100 °C/min under 30 MPa. They concluded that the obtained Nb2AlC exhibited good electrical, thermal, and mechanical properties. Barosum et al.46synthesized Nb2AlC by mixing Nb, Al, and C.
Synthesis of MXene
For the synthesis of MXene, the MAX phase is etched using 10–50 wt.% HF as reported in most synthesis protocols9,40,52. The HF etching method includes the gradual addition of the MAX phase in the etchant while stirring at room temperature. The gradual addition of the MAX phase tends to minimize the bubbling, which is caused by the exothermic nature of this reaction. Ti3C2MXene was prepared by Xue et al.29by selectively etching the “A”layer in the Ti3AlC2MAX phase with 48% HF. Alhabeb et al.52prepared Ti3C2Txby etching Al from the Ti3AlC2MAX phase in the presence of HF. By using this etching process, ML-MXene powders are obtained with 2D layers held by van der Waals forces and hydrogen bonds52.After etching, the residual acid is removed by repeatedly washing the powders. Once the pH is neutral, MXene is obtained and filtered out by vacuum filtration. The two layers of exposed Ti atoms in the unit cell require passivation of dangling bonds53. As HF contains abundant hydroxyl and fluorine anions, the surface of the MXene nanosheets is terminated with -F, -O, and/or OH functional groups after the etching of the “A” layer from the MAX phase54. To etch out Al to make MXene, Naguib et al.46immersed V2AlC and Nb2AlC powders in 50% and 55% concentrated HF solutions, respectively, for different times along with continuous stirring at room temperature. After the HF treatment, the resulting suspensions were washed several times using deionized water and centrifuged to separate the settled powders from the supernatants. The settled powders were removed from the vials using ethanol and dried.
Owing to the hazardous nature of HF, a fluoride salt was employed to make a mild etchant. A mild etchant solution was prepared by mixing HCl and LiF under stirring at room temperature. This resulted in a clear HF solution with a low concentration, normally 3%–5%13.The Ti3AlC2powder was topo-chemically converted into MXene by Shuck et al.45. They reported the preparation of an etchant by mixing 1.6 g of LiF and 15 mL of HCl (37 wt.%) in 5 mL of deionized water. Afterwards,the as-synthesized Ti3AlC2powder was added gradually to a premixed etchant solution and stirred. After washing and centrifuging, the colloidal solution of delaminated Ti3C2Txwas obtained. The ratio of LiF to HCl in the etchant defines the quality and lateral size of MXene nanosheets. Large flakes with clean surfaces displayed higher electrical conductivity owing to the fewer defects on nanosheets compared with those on ones prepared by using HF as the etchant55.
Synthesis of MXene quantum dots
Multiple methods and approaches have been used to prepare MQDs. These methods can be categorized into topdown and bottom-up approaches56. Either of the methods can be used depending on the desired size, morphology, structure, and functionalization56−57.
Top-down approaches usually involve the cleavage of bulk MXene precursors by employing physical58, chemical26or electrochemical59methods. The top-down approach can be used for large-scale production, and the raw materials required are found abundantly in nature. It also has some limitations, such as low production yield56and requirement of post-synthesis treatments60. As shown in Fig. 1, the top-down synthesis mainly includes hydrothermal61, solvothermal62, ultrasonic63,64, ball milling34,64and intercalation methods65.
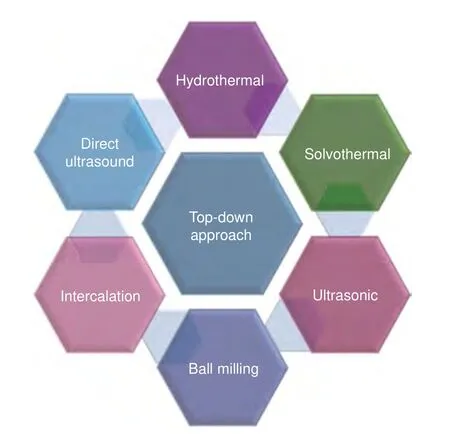
Fig. 1 | Top-down approaches for MQD synthesis.
Hydrothermal/solvothermal method
Hydrothermal/solvothermal is the most common approach that uses MXene as a precursor to synthesize MQDs29,30,66. The synthesis of MQDs via hydrothermal methods is a two-step crystallization process: crystal nucleation and subsequent growth. By controlling processing variables such as temperature, pH, reactant concentrations, and additives, desired particle sizes and morphologies of the final products can be obtained67.Xue et al.29synthesized Ti3C2MXene by selectively etching the A layer in the Ti3AlC2MAX phase with 48% HF(Fig. 2(a)). The MQDs were prepared through a facile hydrothermal method by cutting bulk layered Ti3C2MXene. Colloidal MQDs with different morphologies were obtained by changing the reaction temperature.The chemical structure and PL mechanism of the MQDs prepared at different temperatures were investigated.Blue fluorescence was obtained with a QY of 10%. Cao et al.68employed a hydrothermal method to synthesize V2C QDs by using a V2C nanosheet precursor (Fig. 2(b)). The average thickness of V2C QDs was 0.8 nm, indicating that the V2C QDs were single-layer atomic crystals.
The solvothermal synthesis method employs organic solvents as the reaction medium instead of water; ethanol, DMSO, and dimethylformamide (DMF) are commonly used solvents. The solvothermal method is advantageous over the hydrothermal synthesis in that the morphology69, size70and dispersion71of MQDs could be easily controlled. Feng et al.62prepared nitrogen-doped Ti3C2MQDs with an amine-assisted facile solvothermal method. They exfoliated the purchased MXene by using a solvent-aided sonication method to achieve few-layer MXene. N,N-DMF and diethylenetriamine were used as solvents for the exfoliated few-layered MXene, which resulted in nitrogen-doped Ti3C2MQDs with a size and thickness of 6.2 nm and 1 nm, respectively. Nitrogen doping in N-MQDs reduced the size and size distribution of the MQDs. The absolute fluorescence QY of nitrogen-doped MQDs was 16.9%62. The solvothermal route was employed by Xu et al.70to prepare three types of Ti3C2MQDs using three solvents: ethanol, DMF, and DMSO (Fig. 2(c)). All supernatants of the MQDs demonstrated good stability, dispersion, and different colors. The atomic thickness of the Ti3C2MQDs was 1.0–2.5 nm, indicating the presence of few layers. The size of MQDs was dependent on the solvent they used,and the sizes of Ti3C2MQDs in ethanol, DMF, and DMSO were 1.8 ± 0.1, 2.5 ± 0.2, and 3.3 ± 0.2 nm, respectively70. The variation in size could be attributed to the co-action of oxidation degree72, boiling point, and polarity of solvents73.
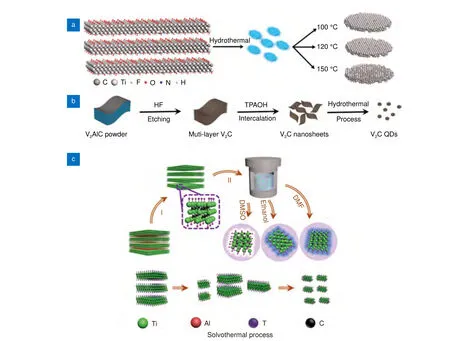
Fig. 2 | Hydrothermal and solvothermal synthesis processes. The figures were reproduced with permission from: (a) ref.29, Copyright 2017,John Wiley and Sons; (b) ref.68, Copyright 2019, Elsevier; (c) ref.70, Copyright 2018, John Wiley and Sons.
Hydrothermal/solvothermal-ultrasound method
Combining ultrasonication with the solvothermal/hydrothermal method is an efficient strategy to synthesize MQDs rather than using solvothermal or hydrothermal methods alone36,37,74,75. Ultrasonication can cause shockwaves and acoustic cavitation in certain solvents. It is also useful to suppress the oxidation of MXene and accelerate the fragmentation of MXene sheets to MQDs76,77.Usually, probe and bath ultrasonic methods are used for the synthesis of MQDs. MQDs prepared by probe ultrasonication are more dispersed than those prepared using bath ultrasonication78. The ultrasonic treatment of a liquid affects the intensity of mixing and heating of bulk liquid, as well as the concentration of energy in microscopic hot spots which are required to produce high-energy chemical reactions. Both the physical and chemical effects of ultrasound have been demonstrated in the production of nanostructured materials79. Li et al.66prepared Ti3C2MQDs by using the ultrasound-hydrothermal method, as shown in Fig. 3. The Ti3C2sheets were dispersed by tip sonication and bath sonication,and then, the dispersion was treated hydrothermally overnight. Zhou et al.75used the solvothermal-ultrasound method to synthesize Ti3C2-derived graphene QDs (GQDs). A product yield of 32.6% was obtained for GQDs, which is very high. Huang et al.37synthesized V2C MQDs by employing a hydrothermal method, followed by ultrasound treatment in ammonium hydroxide.Blue fluorescence was obtained with a QY of 15.88%.
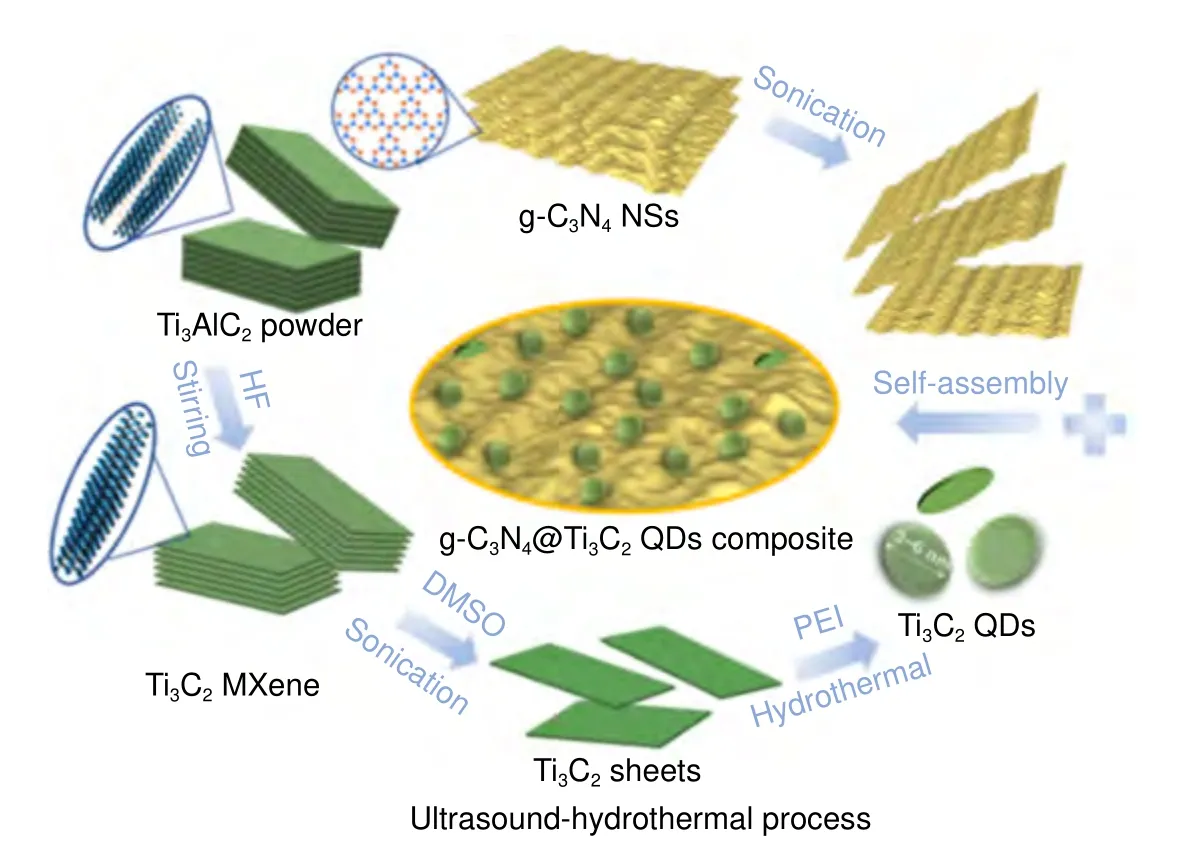
Fig. 3 | Solvothermal/hydrothermal-ultrasound synthesis process. The figure was reproduced from: ref.66, Copyright 2019, American Chemical Society.
Ultrasonic, ball milling, and intercalation methods
Several other top-down approaches to prepare MQDs include intercalation65, ball milling34,64and direct ultrasound. A two-step ultrasound approach was used by Yu et al.63to synthesize ultra-small Ti3C2MQDs. The probe sonication breaks down the bulk MXene, which increases the surface area, thereby providing more surfaces and edges available for reaction. Nb2C QDs were synthesized by Yang et al.38by employing a pulsed ultrasound method, followed by physicochemical exfoliation in tetrapropylammonium hydroxide (TPAOH). Wang et al.33synthesized Ti3C2MQDs by employing an intercalation-ultrasound method, followed by delamination of MXene layers, as shown in Fig. 4(a). MXene nanosheets were reacted with 25% tetramethylammonium hydroxide (TMAOH), followed by treatment in an ultrasonic bath to obtain MQDs. Blue fluorescence was observed for ultra-small Ti3C2sheets with a high QY of 8.9%. Additionally, Mo2C MQDs were synthesized by Dai et al.80using Mo2C powder as the starting material by following a one-step bath ultrasonic treatment, as shown in Fig.4(b). The synthesized MQDs had a diameter of ~6 nm,were highly stable and biocompatible, and possessed low cytotoxicity. The ball milling technique (Fig. 4(c)) was employed by Zhang et al.81to synthesize Ti3C2MQDs with different sizes. They ball-milled Ti3C2MXene with multiple elements in an inert environment. However,they concluded that this method is more suitable for the synthesis of composite materials.
Top-down synthesis methods are efficient for obtaining MQDs, but they possess drawbacks such as long synthesis time and low yield, as discussed above. Thus, advanced methods such as microwave synthesis or electrochemical synthesis have been used. These methods exhibit good reproducibility, involve simple operations,and are cost-effective and thus yield good results.
MQDs were also synthesized using the following bottom-up synthesis approach. The bottom-up method uses organic or inorganic molecular materials as precursors,which enables the precise manipulation of size distribution, morphology, or surface functionalization56−57. Until now, there have been no reports of light-emitting MQDs synthesized using bottom-up methods. However, it is worthwhile to discuss the utilization of the bottom-up method for the fabrication of MQDs so that we could increase the possibility of finding new synthesis methods.
Molten salt synthesis
In the molten salt synthesis method, a salt with a low melting point is added to the reactants. After the salt addition, the precursors are heated above the melting point of the salt. This causes the salt to melt and act as a solvent. Cheng et al.82synthesized a Mo2C QDs/carbon nanosheet (Mo2C/C) composite by using a molten salt synthesis approach (Fig. 5(a)). Molybdenum acetylacetonate, sucrose, and NaCl were used as parent precursors for the synthesis of MQDs. The characterization of Mo2C MQDs revealed a size of 2–3 nm and interplanar spacing of 2.37 Å (1 Å=10-10m), which was well corresponding with Mo2C (002) plane.
Pyrolysis method
Wang et al.83synthesized Mo2C QDs/carbon polyhedron composites by employing a pyrolysis method (Fig. 5(b)).Molybdic acid, zinc acetate, and 2-methylimidazole were used as precursors. Mo2C QDs exhibited an average diameter of 4.5 nm. High-resolution transmission electron microscopy (HRTEM) imaging revealed that lattice fringes of 0.24 nm were well associated with the (111)plane of cubic Mo2C. The bottom-up approach to synthesize MQDs is relatively simple compared with the top-down approach.
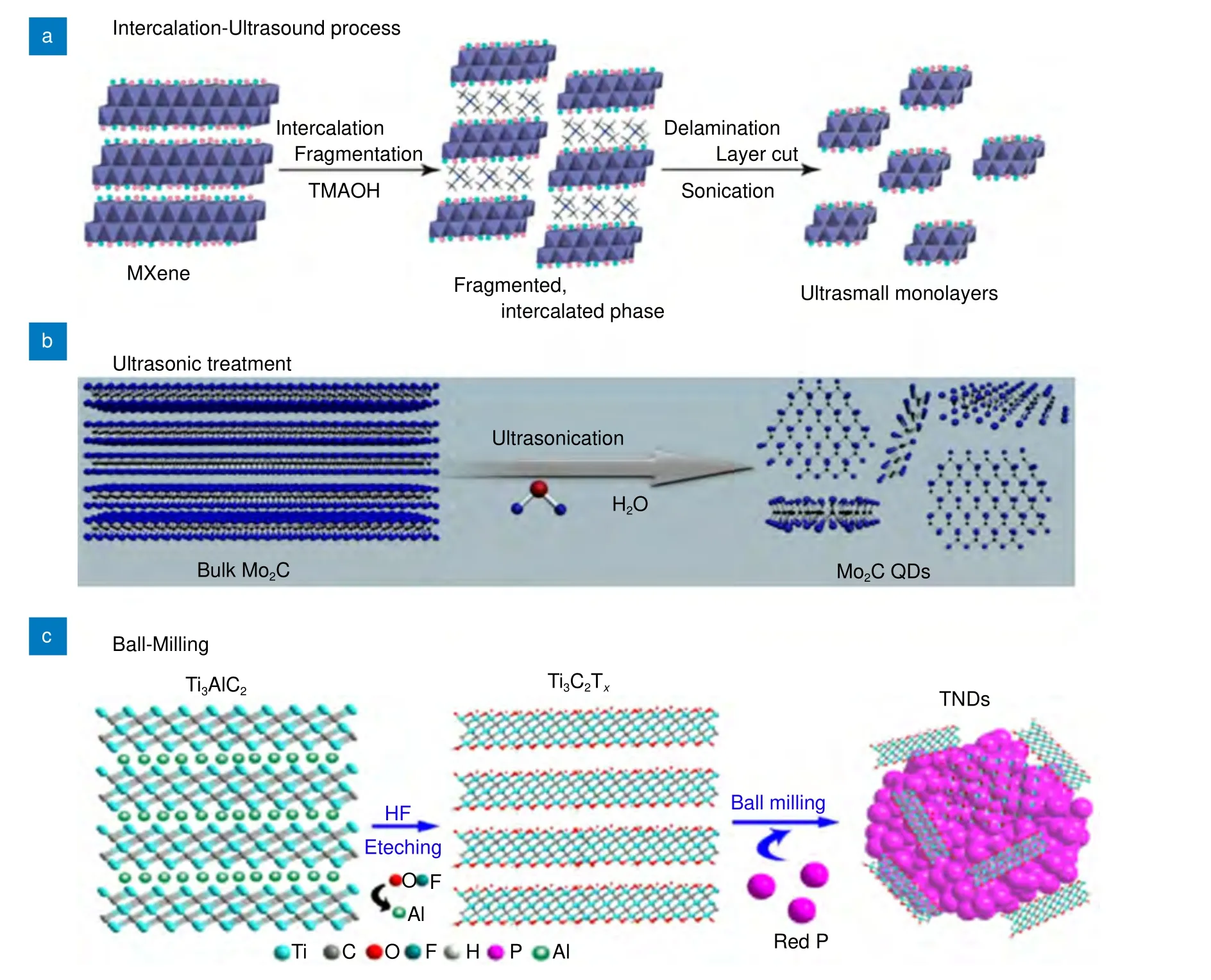
Fig. 4 | Ultrasonic, ball milling, and intercalation synthesis methods. The figures were reproduced from: (a) ref.33, Copyright 2017, American Chemical Society; (b) ref.80, Copyright 2018, under a Creative Commons Attribution lisence; (c) ref.81, Copyright 2018, Wiley-VCH.
Currently, only top-down method has successfully yielded light-emitting MQDs. This may be due to the precursor selection for MQDs synthesis. Mo2C is known to have metallic electronic properties84compared with other MXene such as Ti3C2that is semi-metallic9. Due to the limitation of precursor selection, there is no observation of light-emitting MQDs yielding from bottom-up method so far32,82,83. The following Table 1 lists few lightemitting MQDs along with their synthesis method and the PLQY achieved.
Light-emitting properties of MXene quantum dots
PL is the emission of light from matter after the absorption of incident light or photons. The reaction mechanism behind PL from MQDs is not yet completely clear.Factors such as functional groups, surface defects, degree of passivation, and quantum confinement have previously been proposed to be the origin of PL in MQDs85similar to the case of graphene and carbon QDs86−88.However, the PL reaction mechanism is still controversial and further studies are required for better understanding86−88. Following are the discussions about the PL behavior in MQDs.
Origin of photoluminescence, absorption, and quantum yield
It was theoretically (density functional theory, DFT) predicted that Ti3C2MXene has a small bandgap of ~0.1 eV,which could be further expanded by quantum effects,and light emission can be induced11,89. Xue et al.29explained the chemical structure and PL of Ti3C2MQDs prepared by the hydrothermal method (Fig. 6(a)) at various temperatures. They adjusted the temperature to obtain Ti3C2MQDs with different concentrations of Ti.The Ti3C2MQDs prepared at 100 °C, 120 °C, and 150 °C were named as MQD-100, MQD-120, and MQD-150, respectively. MQD-100 displayed a pristine structure of MXene, and the PL was proposed to arise from the MXene material itself (Fig. 6(e, h)). MQD-120 displayed a hybrid structure with TiO2at the surface and C-Ti in the core (Fig. 6(f, i)). The authors believe that the PL arises from the presence of a core-shell structure. In the case of MQD-150, amorphous carbon dots were obtained, and PL was ascribed to their presence (Fig. 6(g,j)). This study is the first to report the PL of Ti3C2MQDs with a QY of nearly 10%29. Xu et al.34prepared pristine Ti3C2MQDs and nitrogen-doped Ti3C2MQDs (NMQDs) using layered Ti3C2nanosheets as the parent precursors. It was proposed that the doping of heteroatoms such as nitrogen optimized the PL properties of Ti3C2MQDs. In addition, they attributed the excitationdependent PL of N-MQDs to quantum confinement,which resulted in a higher QY value of up to 18.7%. To understand the effect of nitrogen doping on the QY of N-MQDs, DFT calculations were employed and Ti3C2MQDs terminated with “O” group were used. As shown in Fig. 7(a), the density of states of pristine MQDs showed a bandgap and a sub-gap of 0.15 eV. With N doping, the bandgap of N-MQDs increased because of the combination of the existing bandgap and the subgap. Fig. 7(b) shows that a chemical bond existed between Ti and N in the N-MQDs. The presence of prominent gap states increased the lifetime of the carriers, thus improving the QY. In conclusion, nitrogen doping caused a widening of the energy gap, which resulted in an enhanced carrier lifetime and improved QY.The work function of the N-MQDs was smaller than that of Ti3C2MQDs (Fig. 7(c)), suggesting easier electron migration in N-MQDs34.
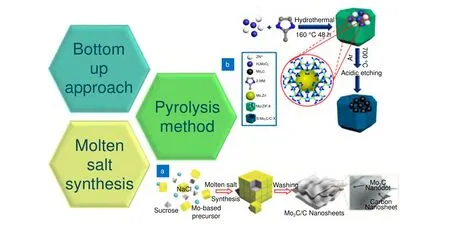
Fig. 5 | Types of bottom-up synthesis processes. The figures were reproduced from: (a) ref.82. Copyright 2018, John Wiley and Sons; (b)ref.83, Copyright 2018, American Chemical Society.
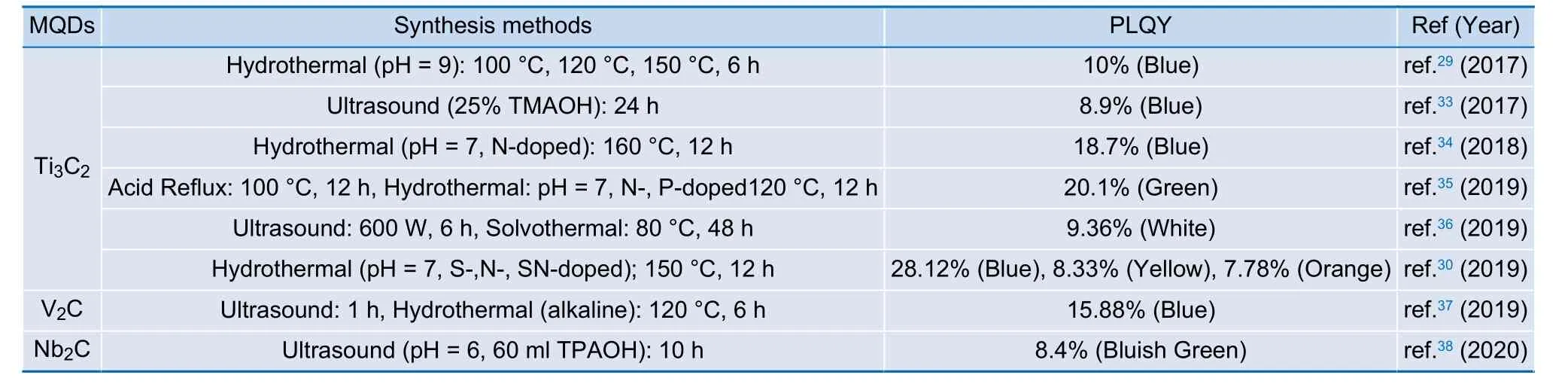
Table 1 | Current progress on MQDs exhibiting photoluminescence
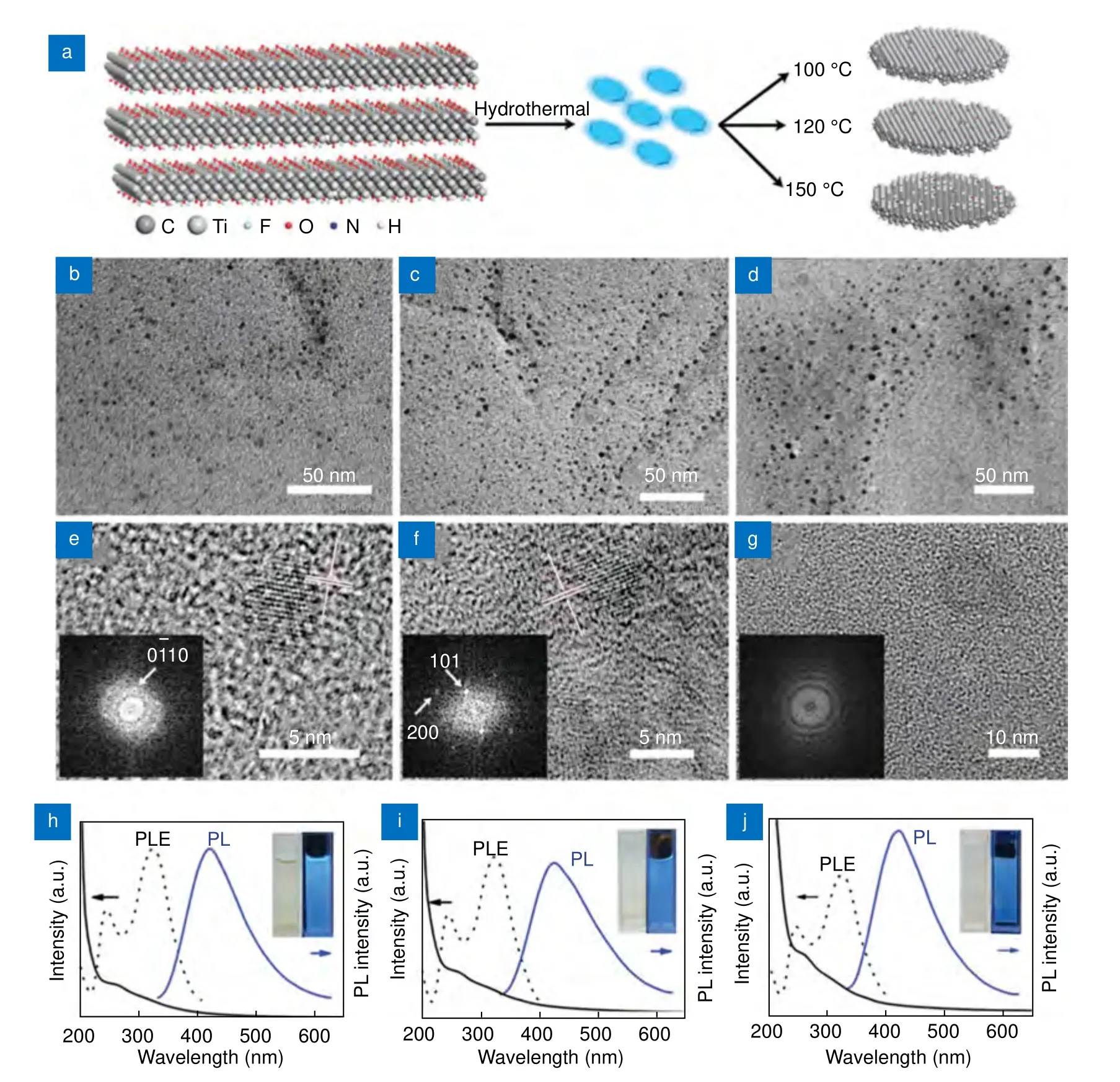
Fig. 6 | (a) MQD preparation by the hydrothermal method. TEM and HRTEM images of (b,e) MQD-100, (c,f) MQD-120, and (d,g) MQD-150.UV–vis spectra, PLE, and PL of (h) MQD-100, (i) MQD-120, and (j) MQD-150 in aqueous solutions. The figures were reproduced with permission from ref.29, Copyright 2017, John Wiley and Sons.
The presence of large heterogeneity during the synthesis of MQDs results in the PL properties of MQDs being affected by the size, defects, shape, functional groups,edge configuration, and heterogeneous hybridization of the carbon network90. Xu et al.30studied the PL mechanism of sulfur- and nitrogen-doped Ti3C2MQDs. They prepared sulfur- and nitrogen-doped Ti3C2MQDs (SMQDs, N-MQDs, SN-MQDs) by a hydrothermal synthesis approach. The MQDs exhibited fluorescence enhancement due to the formation of a stalwart bridge such as a hydrogen-bonded network among MQDs by bonding water on doped MQD surface. The possible molecular interaction and light emission mechanism in water were studied, as shown in Fig. 8(a). The doped Ti3C2MQDs (S-MQDs, N-MQDs, SN-MQDs) were connected with each other via hydrogen bonds with water molecules, which resulted in increased lateral size of MQDs(Fig. 8(b))30. R. Bailey and S. Nie explained that an increase in the lateral size and the decline in the band gap due to π-electron delocalization caused a red-shift in the emission spectra91. Figure 8(c) shows the dynamic light scattering (DLS) size distribution of the doped MQDs.Owing to the hydrogen-bonded network between the MQDs and water, the mean diameters of the SN-MQDs and N-MQDs hydrogen-bonded network were 129.3 and 101.7 nm, with the QY of 7.78% and 8.33%, respectively.S-MQDs did not form a hydrogen-bonded network because they had particle size distribution of less than 20 nm, except for the presence of TiO2in the pattern. SMQDs with the smallest among the three MQDs achieved a high QY of 28.12%. This suggests that with the help of surface functionalization it is possible to tune the bandgap of MQDs by controlling the lateral size during fabrication process. As the size of the MQDs became larger, the QY was reduced and the emission spectra was red-shifted. The formation of hydrogen-bonded networks led to the immobilization of the C=O and C-O bonds of the MQDs and strengthened the rigidity of the entire system. This resulted in an increased lateral size and enhanced fluorescence30.

Fig. 7 | DFT calculation of total and projected density of states of (a) Ti3C2O2 QDs and (b) Ti3C2-xNxO2 QDs. (c) Work function of pristine Ti3C2 QDs (MQDs) and N-MQDs. Figures were reproduced with permission from ref.34. Copyright 2018, Royal Society of Chemistry.
Modulation of photoluminescence by surface defects, functionalization, and passivation
Surface modification and engineering are employed to overcome the drawbacks of MQDs such as oxidation and aggregation. Surface engineering methods include functionalization of MQDs such as composite construction and hetero-atomic doping34,74,92,93. The surface-functionalized sites can affect the PL properties of MQDs. Chen et al.94functionalized the surface of Ti3C2QDs with polyethyleneimine by using a hydrothermal approach. The functionalized MQDs exhibited bright excitation-dependent blue PL, which was attributed to the bandgap transitions and surface defect emissions. The luminescence behavior of the Ti3C2QDs was pH-dependent,which was explained by the decrease in absorption and the increase in the non-radiation rate induced by the deprotonation of the surface defects of Ti3C2QDs. The surface defect sites originated from the functionalization of MQDs with the surface passivation agent. The functionalized Ti3C2QDs exhibited excitation wavelength- and pH-dependent blue PL, with a QY of 7.13%94. Liu et al.also functionalized Ti3C2MQDs with ɛ-poly-L-lysine(PLL) by sonication cutting of a Ti3C2film, followed by a hydrothermal approach. They explained that the surface defects originated from the PLL favored the excitation from wavelength-dependent emissions of PLL-protected Ti3C2MQDs. The PLL-protected Ti3C2MQDs exhibited a blue PL with a QY of 22% owing to the strong quantum confinement effect95.
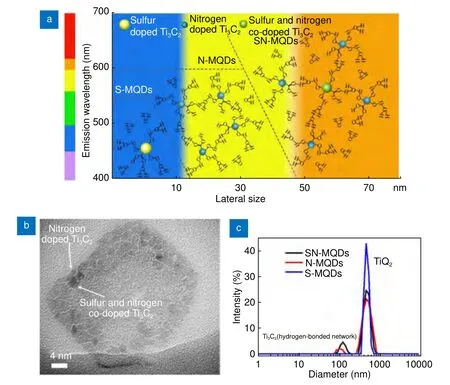
Fig. 8 | (a) Schematic of the molecular interactions and light-emitting mechanism of MQDs in water. (b) The corresponding HRTEM image of SNMQDs in relation to the mechanism. (c) DLS size distributions of the synthesized MQDs in deionized water. Figures were reproduced with permission from ref.30. Copyright 2019, Elsevier.
Yang et al. synthesized Nb2C QDs by employing a pulsed ultrasound method, followed by physicochemical exfoliation in TPAOH when the pH reached above 638.Nb2C QDs possess a hexagonal crystalline structure and good dispersibility. The synthesized Nb2C QDs are uniform ultra-small particles with a lateral size ranging from 1 to 5 nm. UV–Visible spectroscopy analysis revealed a significant increase in absorption below 400 nm. This spectral property was attributed to the size effect of Nb2C QDs, where the absorption edge of the Nb2C QDs blueshifted with a decrease in its dimensions. Strong PL excitation and emission peaks were observed at 370 nm and 485 nm, respectively. Nb2C QDs emitted a bluishgreen fluorescence at 365 nm with a QY of 8.4%. An increase in the excitation wavelength from 330 to 450 nm caused a red shift of the peak in the PL emission spectra of Nb2C QDs from 480 to 540 nm. This excitation-dependent PL emission property of QDs could be attributed to surface defects and size effects, whereas the bright PL emission is ascribed to the strong quantum confinement resulting from the surface defects and ultrasmall lateral dimension of Nb2C QDs. The effect of pH on the fluorescence stability of Nb2C QDs was studied,and the fluorescence intensity did not change at pH 5–11. However, a sharp decrease was observed at a pH below 5. The de/protonation effects of the Nb2C QDs might be a reason for this. The oxygen-containing groups are suitably deprotonated on the surface of Nb2C QDs at pH > 5. This deprotonation causes a high net surface negative charge, thus revoking the deterioration in fluorescence intensity. At pH < 5, however, the surface groups begin to be protonated, which leads to a reduction in the net surface charge and the formation of hydrogen bonds between the particles. As a result, the Nb2C QDs precipitate and their fluorescence intensity decreases.
Surface passivation is performed by coating the surface of QDs with another material to protect the QD core. It plays a vital role in improving the fluorescence of QDs by reducing surface defects96,97. Huang et al.37fabricated V2C MQDs via a hydrothermal method, followed by functionalization with -NH groups in an aqueous ammonia solution. The PL intensity of the passivated MQDs at 500 nm or longer wavelength was found to be enhanced five times as compared to that obtained for non-passivated MQDs. The full width at half maximum (FWHM) of the PL emission was observed to be broadened at longer wavelengths with a cutoff edge up to 700 nm. Also, the lifetime of passivated MQDs was 6.57 ns but that of non-passivated MQDs was 4.99 ns.According to the theory of finite atomic systems, V2C has few atomic layers, which makes it more susceptible to external passivation. An increase in the excitation wavelength caused luminescence peaks to red-shift,thereby decreasing the intensity. This could be attributed to the presence of different local electron states after passivation.
Applications
Optoelectronic applications
Currently, approximately 20% of the world’s electricity has been reported to be consumed for lighting purposes.As the world population has increased over time, lowcost and efficient artificial lights have been in high demand98. Inorganic semiconductor colloidal QDs have been of interest for next-generation light-emitting devices because of their high quantum efficiency and color purity, low-cost fabrication process, and bandgap tunability99.
However, most inorganic QDs are fabricated using heavy metals that are harmful to humans and the environment100. MQDs have been reported to be non-toxic or less toxic and thus have been used for several applications in the biomedical field29,68,101. MQDs can be used in light-emitting devices owing to their strong PL emission and non-toxicity.
MQDs have proven to be advantageous for LEDs because their convenient functionalization enables us to tune the emission wavelength and strong PL emission.Xu et al.30synthesized multi-color-emitting Ti3C2MQDs with a single excitation at 360 nm. The emission colors of S-, N-, and SN-doped MQD surface were blue, yellow,and orange with QYs of up to 28.12%, 8.33%, and 7.78%,respectively, making it a suitable material for multi-color LEDs. Furthermore, the differently colored MQDs can be homogenously mixed in polyvinylpyrrolidone to obtain a white LED (Fig. 9(b, c)). Huang et al.37significantly enhanced the PL of V2C MQDs through a passivation process. The MQDs have emissions that cover almost the entire visible wavelength region. The blue,green, yellow, and red lights could be amplified and lased simultaneously under excitation below 400 nm. This method was used to achieve a white laser using V2C MQDs. On the other hand, Lu et al.36fabricated whiteemitting Ti3C2MQDs with a QY of 9.36%, and twophoton fluorescence was also reported for these MQDs(Fig. 9(a)). The spectral full width at half maximum exceeded 220 nm, covering a large range of the visible wavelength region. The white LEDs fabricated using the MQDs with this method emitted stable white light close to the center of the chromaticity diagram (Fig. 9(d, e)).
Photoluminescence-based sensors
The detection of metal ions and biological components by monitoring the biological system at the cellular level is beneficial for a healthy life. Furthermore, the release of pollutants from industrial waste has caused serious environmental problems. Therefore, designing a sensitive and selective sensor for specific targets is important for maintaining the biological and environmental systems102.Among the methods developed for bio detection, PLbased sensors are preferred owing to their high sensitivity, good reproducibility, and short analysis time, along with a small sample size103−106. The fabrication of MQDs has further promoted the application of MXene-derived materials as they exhibit strong PL both in solid state and aqueous solution with small sizes (<10 nm)30. Furthermore, MQDs are known to be non-toxic due to the absence of heavy metal ions which made them less harmful to the environment than most inorganic QDs100,107,108,and thus promising materials for PL-based sensors.
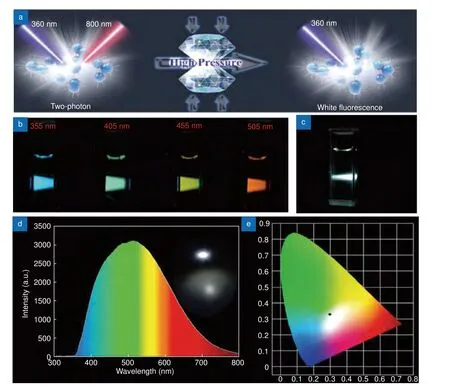
Fig. 9 | (a) White emission of Ti3C2. (b) PL color of the V2C QDs under different excitation wavelengths. (c) V2C MQDs colloid under 355 nm pulsed laser pumping. (d) Emission spectrum of white LED (e) Chromaticity diagram (CIE 1931) coordinates of the white LED (0.30, 0.34). Figures were reproduced with from: (a), (d), (e) ref.36. Copyright 2019, WILEY-VCH; (b), (c) ref.37. Copyright 2019, John Wiley and Sons.
MQDs were tested for their high sensitivity and selectivity by using different metal ions (Fe3+, Fe2+, Ca2+,Cd2+, Mg2+, Na2+, Sn2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Al3+,Cr3+)109,34. The MQDs showed efficient detection for Fe3+109,34, Zn2+29, Cu2+110, Ag+110, and Mn2+110. The readily functionalized MQDs have oxygenous groups, for example, hydroxyl, carboxyl, and carbonyl groups, at the surface and edge. These functional groups have a highaffinity interaction with metal ions, which induces aggregation on well-dispersed MQDs (Fig. 10(b)). The metal ions act as bridges connecting individual MQDs together. This interaction results in the PL quenching of the MQDs111. MQDs were also used for biological detection. Ti3C2MQDs were used to detect an alkaline phosphatase (ALP) assay112. The emission spectrum of Ti3C2overlapped with the absorption spectrum of ALP, which comes from p-nitrophenol (Fig. 10(d)), a chemical produced from the ALP-catalyzed dephosphorylation of the substrate. PL quenching of Ti3C2QDs was due to the inner filter effect. PL intensity of Ti3C2MQDs decreased with an increase in the ALP concentration (from 0 to 50 U L-1), and a good linearity between the quenching efficiency and ALP concentration was obtained in the range of 0.1–2.0 U L-1. A pH-dependent PL behavior was also observed based on ethylenimine-functionalized Ti3C2QDs (Fig. 10(c)), which could be used for pH sensing and quantitative detection of the intracellular pH values94.
It was observed that cysteine, serine, arginine, ascorbic acid, dopamine, H2O2, and various other metal ions have little or no effect on PL quenching of MQDs (Fig.10(a)). The presence of both H2O2and Fe2+was found to reduce the PL intensity of nitrogen-doped Ti3C2MQDs.However, there was no observable behavior in the presence of either H2O2or Fe2+34. Glutathione-functionalized Ti3C2(GSH-Ti3C2) MQDs were used to detect uric acid based on the oxidation of uric acid by uricase to allantoin and H2O2. The overlap of the PL emission of GSH-Ti3C2MQDs centered at 430 nm and large absorption of 2,3-diaminophenazine (oxOPD) around 425 nm induced a fluorescence resonance energy transfer between the GSH-Ti3C2MQDs. The emission of GSHTi3C2MQDs gradually decreased while the emission of oxOPD at 568 nm increased with an increase in uric acid concentration113.

Fig. 10 | (a) The normalized fluorescence intensity of MQDs at 380 nm in the presence of various analytes. (b) The MQD synthesis process and working principle for Fe3+ sensing. (c) Normalized PL spectra of [Ru(dpp)3]Cl2 and Ti3C2 QDs in a buffer solution with different pH values (λex =350 nm). (d) The principle of Ti3C2 QD-based fluorescence assay for ALP activity. The figures were reproduced with permission from: (a), (b)ref.109, (c) ref.94 and (d) ref.112, Royal Society of Chemistry.
Bioimaging
Bioimaging is a powerful technique that can effectively provide clear biological information114. Since the application of QDs in biological fields, extensive research has led to the discovery of more versatile applications such as bioimaging and biodetection115,116. However, issues related to toxicity and non-biodegradability remain a challenge in designing a biocompatible QD-based bioimaging tool117,108. MQDs are promising candidates for improving the quality of bioimaging because of their strong PL, small size (<10 nm), non-toxicity, and biodegradability29,31,32,68,101. Furthermore, MQDs inherit biocompatibility and anti-bacterial efficiency from MXenes, which makes them a potential candidate for bioimaging when compare with most inorganic QDs100,107,108,117,118. The first reported Ti3C2MQDs were used for multicolor cellular imaging29. The MQDs exhibited excitation-dependent PL spectra in an aqueous solution with PLQYs of approximately 10%. After ingestion by RAW264.7 cells via endocytosis, emission of blue, green, and red colors was observed in the confocal images at excitation wavelengths of 405, 488, and 543 nm, respectively (Fig. 11(a)). Furthermore, the PL of MQDs was weaker in the nucleus region when compared with that in the cell membrane and cytoplasm, which indicates that the MQDs could readily enter the cell without penetrating through the nucleus to avoid genetic damage. The survival rates of RAW264.7 cells when incubated with MQDs for 48 h exceeded 90%,confirming the low cytotoxicity.
Cao et al.68prepared V2C MQDs and successfully functionalized them with polyethylene glycol and TAT peptide to perform bioimaging of MCF-7 and NHDF cells. To test the cell uptake and nucleus target of the MQDs, the cell membrane was dyed with DiO (green)and the exosome membranes were stained with DiI(red). The overlay of blue PL of V2C with membranes dyed with DiO and DiI (Fig. 11(d)) showed that the MQDs entered both MCF-7 and NHDF cells via the endocytic uptake pathway. This pathway is preferable for bioimaging because of the weak nucleus-targeting ability due to inefficient lysosome escape.
Cellular imaging of N- and P-functionalized Ti3C2MXene quantum dots (N,P-MQDs) was carried out by Quan et al.110The THP-1 macrophages were incubated with 25 μg·mL-1of N,P-MQDs for 24 h. The N,P-MQDs demonstrated high efficiency for the detection of Cu2+due to the inner filter effect. When the Cu2+ions were captured by the amino group of the N,P-MQDs, an absorbent complex was formed on the surface of the N,PMQDs. This resulted in significant quenching of the strong emission from the N,P-MQDs at 560 nm (Fig.11(b,c)). A decrease in the PL intensity was observed upon addition of up to 100 μM Cu2+of N,P-MQDs.

Fig. 11 | (a) Bright-field imaging of RAW264.7 cells and confocal imaging of RAW264.7 cells incubated with MQD-100 at 405 nm, 488 nm, and 543 nm excitation. (b),(c) Fluorescent imaging (Ex = 488 nm) of the THP-1 monocytes incubated (b) with N,P-MQDs (c) without N,P-MQDs (d)CLSM images of MCF-7 and NHDF cells after incubation of V2C-TAT@Ex or V2C-TAT@Ex-RGD (Scale bar: 40 μm). The figures were reproduced with permission from: (a) ref.29. Copyright 2017, John Wiley and Sons; (b),(c) ref.110. Copyright 2019, Royal Society of Chemistry; (d) ref.68.Copyright 2019, American Chemical Society.
Conclusion and perspective
The synthesis of MQDs has raised considerable interest because they not only retain the properties of MXene but also demonstrate light-emitting properties. Currently,studies on light-emitting MQDs have shown progress in terms of synthesis methods to fabricate multicolor-PLemitting MQDs, and the highest reported QY has been 28.12%. Despite its excellent properties, MXene does not possess PL emission, which limits its applications.However, MQDs overcome this limitation and have thus found applications in optoelectronic devices, PL-based sensors, and bioimaging. Regardless of the great progress, research on MQDs is still in its early stages, and the PL mechanism has not yet been fully comprehended.Comprehensive studies are required for better understanding considering the challenges for their potential applications.
Synthesis of nitride and carbon nitride MQDs
Currently, over 100 types of MAX phases have been reported. Among these, more than 30 kinds of MXenes have been experimentally obtained since 20117, and many more are expected to be reported according to theoretical predictions119. Even so, the types of MQDs that have been fabricated have been mostly derived from the carbide group of MXene. Only one nitride MQD has been reported to have a broad range of absorption from UV to IR wavelengths, but no PL observations have been reported64. In addition, there are no reports on carbon nitride MQDs.
With the current advances in research on 2D-derived QDs (2D-QDs), various methods can be applied to synthesize different types of MQDs from different groups of MXenes, including carbon, nitride, and carbon nitride. It is expected that with the large number of currently available MXenes, more interesting properties or improved properties could be observed, which would help to advance the research on MQDs.
Synthesis methods of light-emitting MQDs
There are two main approaches for synthesizing MQDs:top-down and bottom-up. Among these, only the topdown method has successfully yielded light-emitting MQDs32. We emphasize the need to explore more methods to further advance the research on MQDs. The reason is that the PL behavior of MQDs is highly dependent on the functionalization, parent precursors, and synthesis method.
By controlling these three variables and understanding the surface chemistry, it is possible to design MQDs with desirable properties. Currently, only Ti3C2, Nb2C,and V2C MQDs have been reported to show light-emitting properties. By varying the synthesis procedure,light-emitting MQDs with more interesting properties could be fabricated.
Optoelectronic applications
We focused on light-emitting MQDs in this review because MQDs have promising future prospects in optoelectronic applications. The increase in energy consumed every year is worrisome because the world still relies on fossil fuels as the main energy source, which is scarce and sometimes hazardous. Thus, finding a cheap and clean energy source that can provide high power conversion efficiency is in high demand. MQDs not only have shown potential as a material to be used in efficient light-emitting devices but also exhibit low toxicity, making them potential material for bio-applications.However, the QY of MQDs is low and needs improvement. Furthermore, the emission and absorption of MQDs are tuned mostly by functionalization, while the tunability of semiconductors can be simply achieved by changing the reaction temperature and/or time. Understanding the origin of the PL of MQDs is the main key to fully explore the potential of MQDs for optoelectronic applications.
MXene is known to be a suitable material for solar cells. Currently, researchers have used MXene as one of the electron transport layers in perovskite solar cells(PSCs) in order to increase the short-circuit current density (Jsc), open-circuit voltage (Voc), and fill factor(FF), resulting in a much higher power conversion efficiency (PCE) compared with that of PSCs without MXene layers119,120. The merits of fabricating 2D-QDs are that they retain the existing properties of its parent precursors, making it usable for the same applications as those of the 2D materials. As per our knowledge, there is no published work on using MQDs as active materials for solar cells.
Currently, there exist challenging issues related to device compatibility because most semiconductor QDs show a significantly low quantum efficiency when used for devices99. Research on MQDs is new compared with that on other QDs, especially for device applications.
Medical applications
For medical applications, the strongest advantage of MQDs over conventional inorganic semiconductor QDs is their low toxicity. MQDs have been used in the medical field, for example, in photothermal therapy for cancer68,immunomodulation, regenerative medicine, biosensors,biolabeling110and bioimaging29. However, most of the tests conducted were only for a short term. More vigorous clinical trials should be conducted to confirm that the MQDs do not incur any potential harmful health risks. Although MQDs are biodegradable and can be removed from biological systems through excretion, there is a limitation for applications that require long sustainability.
Acknowledgements
This work was supported by National Research Foundation of Korea(2019R1A2C1006586).
Competing interests
The authors declare no competing financial interests.
