Nitrogen-doped holey graphene nanoscrolls for high-energy and high-power supercapacitors
2021-05-14FengSuShunghoZhengFngynLiuXiongZhngFngyunSuZhongShuiWu
Feng Su,Shungho Zheng,Fngyn Liu,Xiong Zhng,Fngyun Su,Zhong-Shui Wu,*
a Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
b University of Chinese Academy of Sciences, Beijing 100049, China
c Institute of Electrical Engineering, Chinese Academy of Sciences, Beijing 100190, China
d CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
e Dalian National Laboratory for Clean Energy, Chinese Academy of Sciences, Dalian 116023, China
ABSTRACT Porous structure and heteroatom doping are two key parameters for significantly boosting the capacitive performance of graphene-based materials.Herein, we report a facile approach to prepare onedimensional (1D) nitrogen-doped holey graphene nanoscrolls (NHGNSs) through cold quenching treatment of two-dimensional graphene oxide sheets, followed by thermal annealing in the successive atmosphere of NH3and air.Benefiting from the synergy of the unique 1D tubular morphology,abundant nanoholes and nitrogen doping,the NHGNSs exhibit a high specific capacitance of 126 F/g at 1 A/g in ionic liquid electrolyte and excellent rate capability with 81% of the capacitance retained at 20 A/g.Furthermore, the fabricated symmetric supercapacitors based on NHGNSs achieve both high energy density of 53.5 Wh/kg at 875 W/kg and high power density of 17.5 kW/kg at 43.4 Wh/kg.The simple synthetic process and superior electrochemical performance suggest the great potential of NHGNSs for supercapacitor application.
Keywords:Graphene nanoscrolls Nitrogen doping Holey grapheme Supercapacitors Ionic liquid
Supercapacitors with the intriguing merits of fast charge delivery/storage,long lifespan,and excellent reliability have been demonstrated as a promising candidate for sustainable electrochemical energy storage, but the low energy density imposes substantial restrictions on their widespread application [1,2].Therefore, increasing energy density without sacrificing power density has become one of the main challenges at present.In principle, the enhancement of energy density for electric double layer capacitors(EDLCs)can be realized through developing highcapacitance electrode materials working in electrolytes with wide stable potential window[3].Owing to the unique combination of large specific surface area, high electrical conductivity, excellent mechanical properties and chemical stability, graphene has attracted extensive attention for electrochemical energy storage[4], and various graphene-based derivatives and composites have shown promise for high-performance supercapacitors [5–7].
Graphene nanoscrolls(GNSs)can be regarded as a new class of one-dimensional (1D) tubular graphene derivative obtained by continuous rolling of two-dimensional graphene sheets.In addition to inheriting the excellent properties of graphene, the unique 1D tubular structure of GNSs can avoid the restacking and agglomeration of graphene sheets during the fabrication process,and the relatively open structure also allows external molecules to enter the interlayer space [8].So far, the reported preparation approaches of GNSs using graphene or graphene oxide (GO) as precursors mainly include ultrasonication-induced self-assembly[9], interface forces-driven formation [10], templated assembly[11] and cold quenching method [8,12].
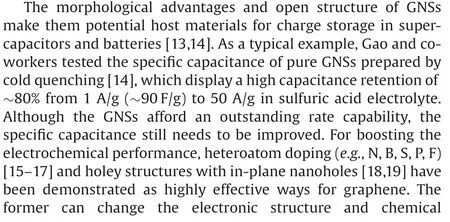

Herein, we report the facile preparation of nitrogen-doped holey GNSs(NHGNSs)derived from GONSs synthesized by the cold quenching method for high-energy and high-power supercapacitors in ionic liquid electrolyte.The NHGNSs were obtained by thermal annealing of the GONSs in NH3atmosphere for nitrogen doping and subsequent controlled air etching process for in-plane mesopore creation.Benefiting from the synergy of nitrogen doping and holey structure, the NHGNSs exhibit significantly enhanced capacitance and rate capability compared with pure GNSs and nitrogen doped GNSs (NGNSs).More importantly, the fabricated NHGNSs-based symmetric supercapacitors provide high energy density of 53.5 Wh/kg and maintain 43.4 Wh/kg at high power density of 17.5 kW/kg.
A schematic illustration for the stepwise preparation of NHGNSs is presented in Fig.1.First, the GONSs were synthesized by the cold quenching method in liquid nitrogen using hot aqueous dispersion of large-size GO sheets (Fig.S1a in Supporting information) as the precursor,followed by vacuum freeze-drying.Next, the GONSs were thermally annealed in NH3at 500for simultaneous nitrogen doping and reduction to synthesize the NGNSs.Successively,the NGNSs were heated in air for a moderate carbon corrosion reaction.To determine the reaction condition,thermalgravimetric analysis (TGA, Fig.S2 in Supporting information) of the NGNSs was first conducted, which indicated that the calcination temperature should be lower than 390to prevent excessive loss of carbon.Then, the reaction temperature was further optimized according to the weight retention after a heating duration of 5 h and controlled just below the threshold of dramatic weight loss to ensure efficient etching for in-plane hole formation.After the optimized air oxidation process at 360, the NHGNSs were obtained with a yield of 83%–86% through the reaction between the defective sites of NGNSs and oxygen, in which the graphitic carbon was mostly maintained[19].For comparison,the GONSs were also thermally annealed in N2at 800to prepare the GNSs.
The morphology of the as-prepared samples was characterized by scanning electron microscopy(SEM)and transmission electron microscopy (TEM).As shown in Fig.2 and Fig.S1 (Supporting information), the NHGNSs possess unique 1D tubular topology with diameters mainly ranging from 200 nm to 500 nm.It should be noted that a small amount of graphene sheets still exist in the final product.High-resolution TEM image (Fig.2c) confirms the mesopore formation on the wall of NHGNSs.The topological open structure and the existence of in-plane holes can provide abundant pathways for ion migration and increase the accessible surface and edge for ion adsorption [21].
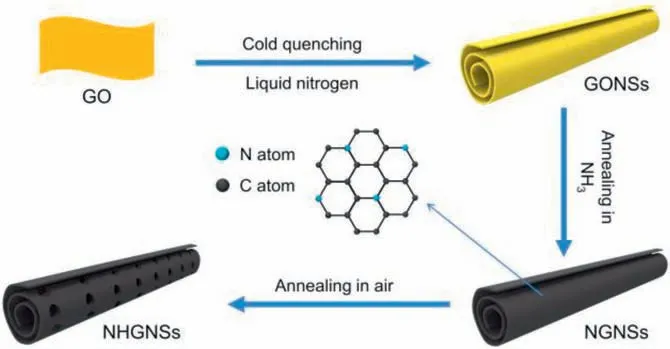
Fig.1.Schematic illustration of the preparation of NHGNSs.
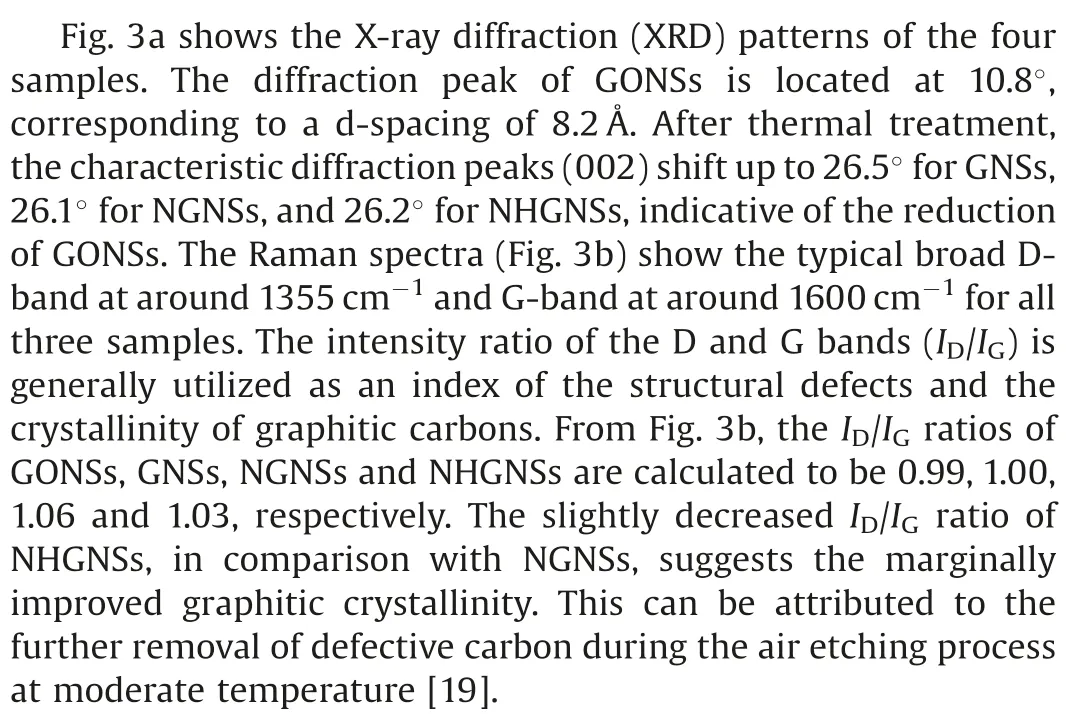
X-ray photoelectron spectroscopy(XPS)was further performed to probe the chemical composition of GONSs and NHGNSs.Fig.3c shows the full XPS spectra, in which characteristic peaks corresponding to C 1s and O 1s are observed for both samples.The C/O ratio of NHGNSs is about 7.1 (Table S1 in Supporting information), much higher than that of GONSs (2.0) due to the reduction by NH3at high temperature.Moreover, an additional N 1s peak appears for NHGNSs, demonstrative of the successful incorporation of nitrogen atoms.Notably, the nitrogen content of NHGNSs is as high as 9.0 at%by XPS analysis.The high-resolution N 1s spectrum of NHGNSs (Fig.3d) can be well fitted into three main peaks at 398.2,399.7 and 401.2 eV,arising from pyridinic N,pyrrolic N and graphitic N, respectively.According to the fitting results,the percentages of pyridinic N,pyrrolic N and graphitic N in NHGNSs are calculated to be 23.6,56.5,and 19.9 at%,respectively.The high doping level of nitrogen in graphene lattice may conduce to the enhancement of electrochemical performance.
To evaluate the capacitive performance of the above active materials,two-electrode symmetric cells were assembled using 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4) ionic liquid as electrolyte and measured within a high voltage of 3.5 V by cyclic voltammetry (CV), galvanostatic charge and discharge(GCD),and electrochemical impedance spectroscopy(EIS).The CV curves (Fig.4a and Fig.S3 in Supporting information) display quasi-rectangular shape without distinct redox peaks,which show little distortion even at an ultrahigh scan rate of 1 V/s,indicative of excellent electrical double-layer charge storage.This typical capacitive behavior is also demonstrated by the triangle-shaped GCD profiles (Fig.4b) measured at various current densities ranging from 1 A/g to 20 A/g(based on the mass of active material in a single electrode).According to the discharge segment of GCD curves,the NHGNSs deliver a high specific capacitance of 126 F/g at 1 A/g(Fig.4c).Even at a high current density of 20 A/g,the NHGNSs can still provide 102 F/g with a remarkable retention rate of 81%,demonstrative of outstanding rate capability.In a sharp contrast,the GNSs measured under the same conditions (Fig.S4 in Supporting information) exhibit a low specific capacitance of 60 F/g at 1 A/g with only 51%of the capacitance retained at 20 A/g.Besides, the specific capacitance of the NGNSs (Fig.S5 in Supporting information) is 98 F/g at 1 A/g, and a capacitance retention of 65% is obtained at 20 A/g.The great improvement of both specific capacitance and rate capability of NHGNSs(Figs.4c,d and Fig.S6 in Supporting information), in comparison with NGNs and GNSs, can be ascribed to the synergy of nitrogen doping and holey structure.The nitrogen doping is capable of modulating the surface chemistry and improving the electrode wettability [20].Meanwhile, the holey structure can facilitate ion transport and increase accessible surface area[21].The larger slope of NHGNSsbased supercapacitors in the low frequency region of EIS(Fig.S7 in Supporting information) further suggests the enhanced ion diffusion kinetics.In addition,the slightly higher ohmic resistance of NHGNSs-based supercapacitors may originate from the little oxygen doping in the air oxidation process.

Fig.2.(a) SEM and (b, c) TEM images of NHGNSs.

Fig.3.(a) XRD patterns and (b) Raman spectra of NHGNSs, NGNSs, GNSs, and GONSs.(c) Overall XPS spectra of NHGNSs and GONSs.(d) N 1s XPS spectrum of NHGNSs.
The energy densities and power densities of the symmetric supercapacitors based on NHGNSs, NGNSs and GNSs are calculated from the GCD profiles, and Ragone plot is presented in Fig.4e.The NHGNSs-based supercapacitors achieve a high energy density of 53.5 Wh/kg at an average power density of 875 W/kg and still retain 43.4 Wh/kg at a high average power density of 17.5 kW/kg based on the mass of NHGNSs in the two electrodes, which is much better than the GNSs-based supercapacitors (25.5 Wh/kg at 875 W/kg and 12.9 Wh/kg at 17.5 kW/kg)and NGNSs-based supercapacitors(41.9 Wh/kg at 875 W/kg and 27.2 Wh/kg at 17.5 kW/kg) and also superior to many reported graphene- and carbon-based supercapacitors 22–27].Furthermore,cycling stability was tested at a current density of 10 A/g, and the NHGNSs-based supercapacitors retain 91% of the initial capacitance with excellent coulombic efficiency of100%after 2000 cycles (Fig.4f).
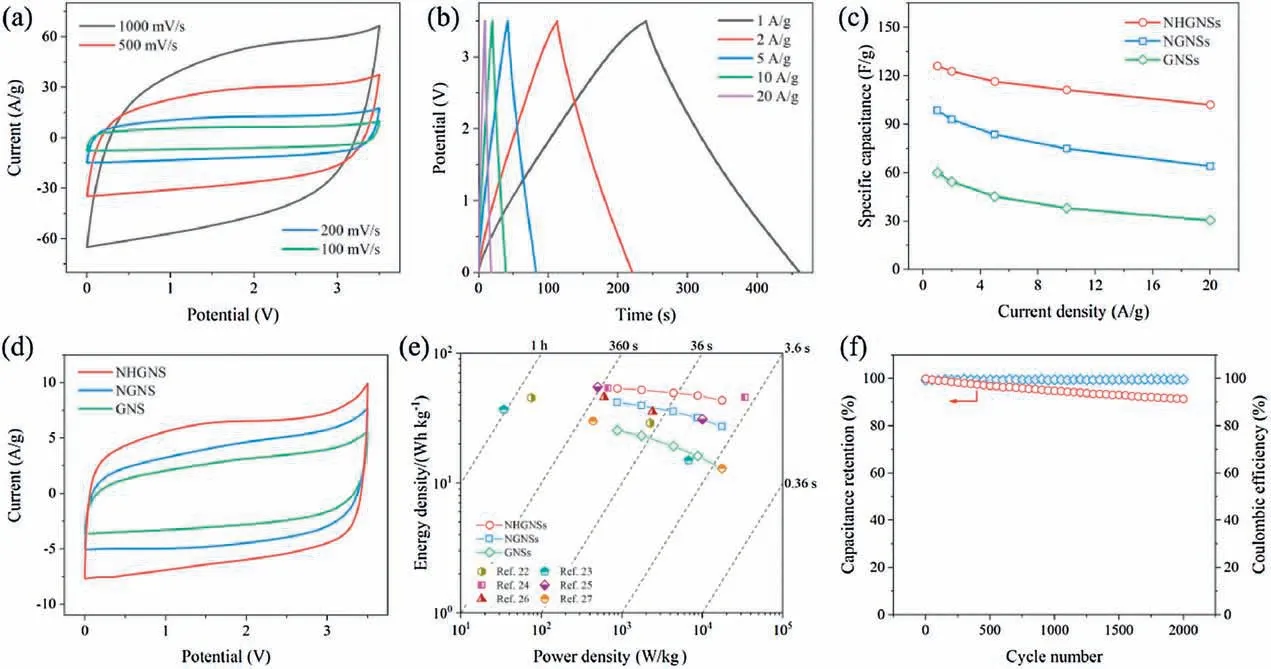
Fig.4.Electrochemical performance of NHGNSs,NGNSs and GNSs for supercapacitors in EMIMBF4with a voltage of 3.5 V.(a)CV curves of NHGNSs obtained at various scan rates ranging from 100 mV/s to 1000 mV/s.(b)GCD profiles of NHGNSs measured at different current densities ranging from 0.5 A/g to 10 A/g.(c)Specific capacitances of
In summary,we have reported the facile production of NHGNSs through cold quenching of GO sheets and subsequent thermal annealing of GONSs in the successive atmosphere of NH3and air.When serving as electrode material for supercapacitors with ionic liquid electrolyte, the NHGNSs deliver a high specific capacitance of 126 F/g and excellent rate capability with 81%of the capacitance maintained from 1 A/g to 20 A/g.This electrochemical performance is superior to those of GNSs and NGNSs, demonstrative of the critical roles of nitrogen doping and holey structure for boosting the capacitive performance.More importantly, the assembled NHGNSs-based supercapacitors can offer a high energy density of 53.5 Wh/kg and retain 43.4 Wh/kg even at a high power density of 17.5 kW/kg,outperforming many reported graphene-and carbonbased supercapacitors.Therefore, it can be concluded that the unique structure enables NHGNSs a promising electrode material for supercapacitors and even other electrochemical energy storage devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Nos.51872283, 21805273),National Key R&D Program of China (Nos.2016YBF0100100,2016YFA0200200), Liaoning BaiQianWan Talents Program, LiaoNing Revitalization Talents Program (No.XLYC1807153), Natural Science Foundation of Liaoning Province, Joint Research Fund Liaoning-Shenyang National Laboratory for Materials Science(No.20180510038), DICP (Nos.DICP ZZBS201708, DICP ZZBS201802),DICP&QIBEBT (No.DICP&QIBEBT UN201702), DNL Cooperation Fund,CAS(Nos.DNL180310,DNL180308,DNL201912,DNL201915).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.07.025.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
