Integration of Fe2O3-based photoanode and atomically dispersed cobalt cathode for efficient photoelectrochemical NH3synthesis
2021-05-14WeikngWngShengboZhngYnynLiuLiRongZhengGuozhongWngYunxiZhngHiminZhngHuijunZho
Weikng Wng,Shengbo Zhng,Ynyn Liu,Li-Rong Zheng,Guozhong Wng,Yunxi Zhng,Himin Zhng,*,Huijun Zho,d,*
a Key Laboratory of Materials Physics, Centre for Environmental and Energy Nanomaterials, Anhui Key Laboratory of Nanomaterials and Nanotechnology, CAS Center for Excellence in Nanoscience, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China
b University of Science and Technology of China, Hefei 230026, China
c Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China
d Centre for Clean Environment and Energy, Griffith University, Gold Coast Campus, QLD 4222, Australia
ABSTRACT Realizing nitrogen reduction reaction(NRR)to synthesis NH3under mild conditions has gained extensive attention as a promising alternative way to the energy- and emission-intensive Haber–Bosch process.Among varieties of potential strategies, photoelectrochemical (PEC) NRR exhibits many advantages including utilization of solar energy, water (H2O) as the hydrogen source and ambient operation conditions.Herein,we have designed a solar-driven PEC-NRR system integrating high-efficiency Fe2O3-based photoanode and atomically dispersed cobalt(Co)cathode for ambient NH3synthesis.Using such solar-driven PEC-NRR system, high-efficiency Fe2O3-based photoanode is responsible for H2O/OH-oxidation,and meanwhile the generated photoelectrons transfer to the single-atom Co cathode for the N2reduction to NH3.As a result,this system can afford an NH3yield rate of 1021.5mg mgCo-1h-1and a faradic efficiency of 11.9% at an applied potential bias of 1.2 V (versus reversible hydrogen electrode) on photoanode in 0.2 mol/L NaOH electrolyte under simulated sunlight irradiation.
Keywords:PEC-NRR Co-SAC CoPi/Ti-Fe2O3NH3synthesis Photoelectrochemisty
As an essential life building block and important carrier of hydrogen energy, ammonia (NH3) is currently synthesized by the industrial scale Haber-Bosch process [1].However, this over a century-old Haber-Bosch process requires high temperature(400–500) and pressure (100–350 atm), consumes tremendous energy and natural gas(as the hydrogen source),and concurrently results in greenhouse gas(e.g.,CO2)emission[2,3].Therefore,it is highly desirable to develop alternative routes for nitrogen (N2)reduction to NH3under ambient temperature and pressure conditions.In recent years, photocatalytic, electrocatalytic and photoelectrochemical (PEC) techniques have been intensively investigated for the N2reduction reaction (NRR) at ambient conditions, demonstrating great potentials to replace the traditional Haber-Bosch process [4,5].Nonetheless, it is critically important for developing high-efficiency catalysts, capable of adsorption and activation of intrinsically inert N2molecules, as well as reasonable configuration of the catalysts in these systems for high-efficiency NH3synthesis.
As we know,it has been widely accepted that the photocatalytic oxidation and reduction half-reactions occur at the same photocatalyst particle for a particulate suspension photocatalysis system, resulting in a rapid recombination of photogenerated carriers and thus obviously decreasing photocatalytic efficiency[6,7].This issue existed in the photocatalytic system can be well solved by using the PEC technique, in where a suitable potential bias is applied to a photocatalyst immobilized on a conducting substrate to allow the combination of electrochemical technique with photocatalysis and greatly minimize the charge recombination, thereby significantly increasing the photocatalytic efficiency[8,9].Owing to these advantages, the PEC technique has been successfully employed to water splitting to generate H2, CO2reduction, organic compound oxidation, environmental detection and so on [8–10].Recently, several research groups have demonstrated that N2reduction to NH3by the PEC technique is experimentally feasible, exhibiting high NH3yield rate and selectivity [11,12].Hamers et al.reported that the illuminated hydrogen-terminated diamond under ultraviolet (UV) light in a dual-compartment H-cell can result in electron emission to produce solvated electrons in water,thus inducing NRR at ambient temperature and pressure [13].MacFarlane and co-workers synthesized an Au nanoparticles modified p-type Si photoelectrode, achieving an NH3yield rate of 6.0mgwithout an applied potential bias by photoelectrochemical N2reduction under 2 sun illumination and 7 atm pressure [14].In addition, the development of high-efficiency photocathodes in PEC technique,such as the aerophilic-hydrophilic heterostructured Si-based electrode and R-BiOI photocathode, has become another feasible means for high active and selective N2reduction to NH3.In their studies, the synthesized Si-based and R-BiOI photocathodes have the bifunctionality of solar-light absorption and N2adsorption/activation [12,15].Therefore, reasonable design of the photocathodes’ composition and structure is critically important for high-efficiency PEC N2reduction,but great challenging.Comparatively, varieties of high-efficiency photoanodes have been widely developed and fabricated for PEC applications[16].Indeed,most of recent reported photoanodes in PEC applications are mainly focused on the oxidation half-reactions to overcome the slow reaction kinetics in water oxidation or organic oxidation[9,17,18].As for the PEC N2reduction to NH3, a high-efficiency cathodic catalyst is essential in the PEC system.Therefore,the integration of a high-performance photoanode and an efficient cathodic NRR catalyst in a PEC system can extend its N2reduction application.However, there is no related report in literatures so far.
Herein, we report the conversion N2into NH3by solar-driven PEC technique, composed of high-efficiency cobalt phosphate(CoPi)modified Ti-doped Fe2O3(CoPi/Ti-Fe2O3)nanoarrays photoanode and cobalt single-atom catalyst (Co-SAC) constructed cathode.As shown in Scheme S1 (Supporting information), the fabricated CoPi/Ti-Fe2O3photoanode under a suitable potential bias and solar light irradiation is responsible for the oxidation halfreaction in alkaline media,namely,oxidation of H2O/OH–to release O2, and meanwhile the photogenerated electrons under suitable potential bias transfer to the Co-SAC cathode to attack the adsorbed N2on the Co-SAC, thus yielding NH3.As a result, such solar-driven PEC-NRR system can achieve an NH3yield rate of 1021.5and faradic efficiency (FE) of 11.9% at an applied bias of 1.2 V versus reversible hydrogen electrode(vs.RHE)on photoanode in 0.2 mol/L NaOH electrolyte.The high NH3synthesis performance using such solar-driven PEC-NRR system can be attributed to the developed high-efficiency CoPi/Ti-Fe2O3photoanode and Co-SAC cathode to overcome the slow reaction kinetics of anode H2O oxidation,provide abundant photoelectrons and N(O)-coordinated Co active sites for N2adsorption/activation,leading to an efficient conversion of N2to NH3.
In this work, we employed a facile hydrothermal method to fabricate FeOOH nanoarrays on FTO conductive glass[19],followed by chemical vapor deposition(CVD)of TiCl4and thermal treatment to obtain Ti doped Fe2O3nanoarrays(denoted as Ti-Fe2O3)[20].For comparison,the pure Fe2O3nanoarrays on FTO substrate(denoted as Fe2O3) were also fabricated by thermal treatment of FeOOH nanoarrays.Subsequently, CoPi modified Ti-Fe2O3(CoPi/Ti-Fe2O3)nanoarrays photoanode was fabricated by a photoassisted deposition method [19].In this study, the aim of Ti doping and CoPi modification in Fe2O3nanoarrays is to promote the charge conductivity and water oxidation efficiency of photoanode,respectively, thus enhancing the PEC efficiency of photoanode[17,21,22].
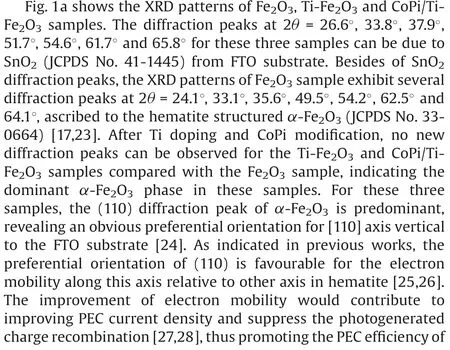
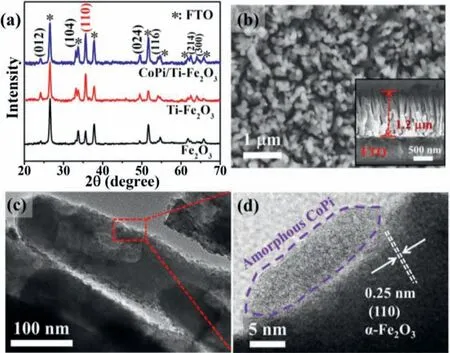
Fig.1.(a) XRD patterns of Fe2O3-based photoanodes.(b) Vertical-view and cross-sectional SEM, (c) TEM and (d) HRTEM images of CoPi/Ti-Fe2O3photoanode.
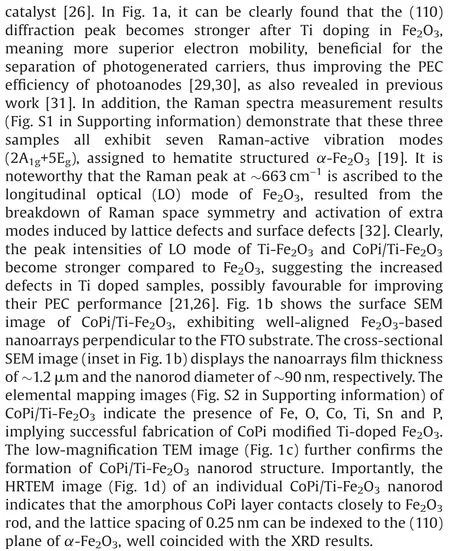

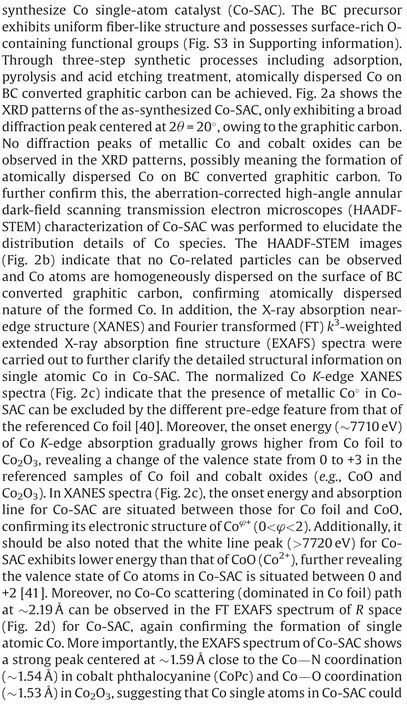
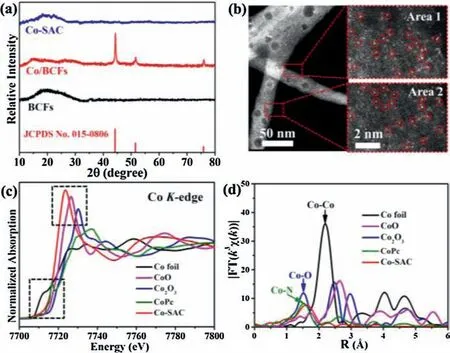
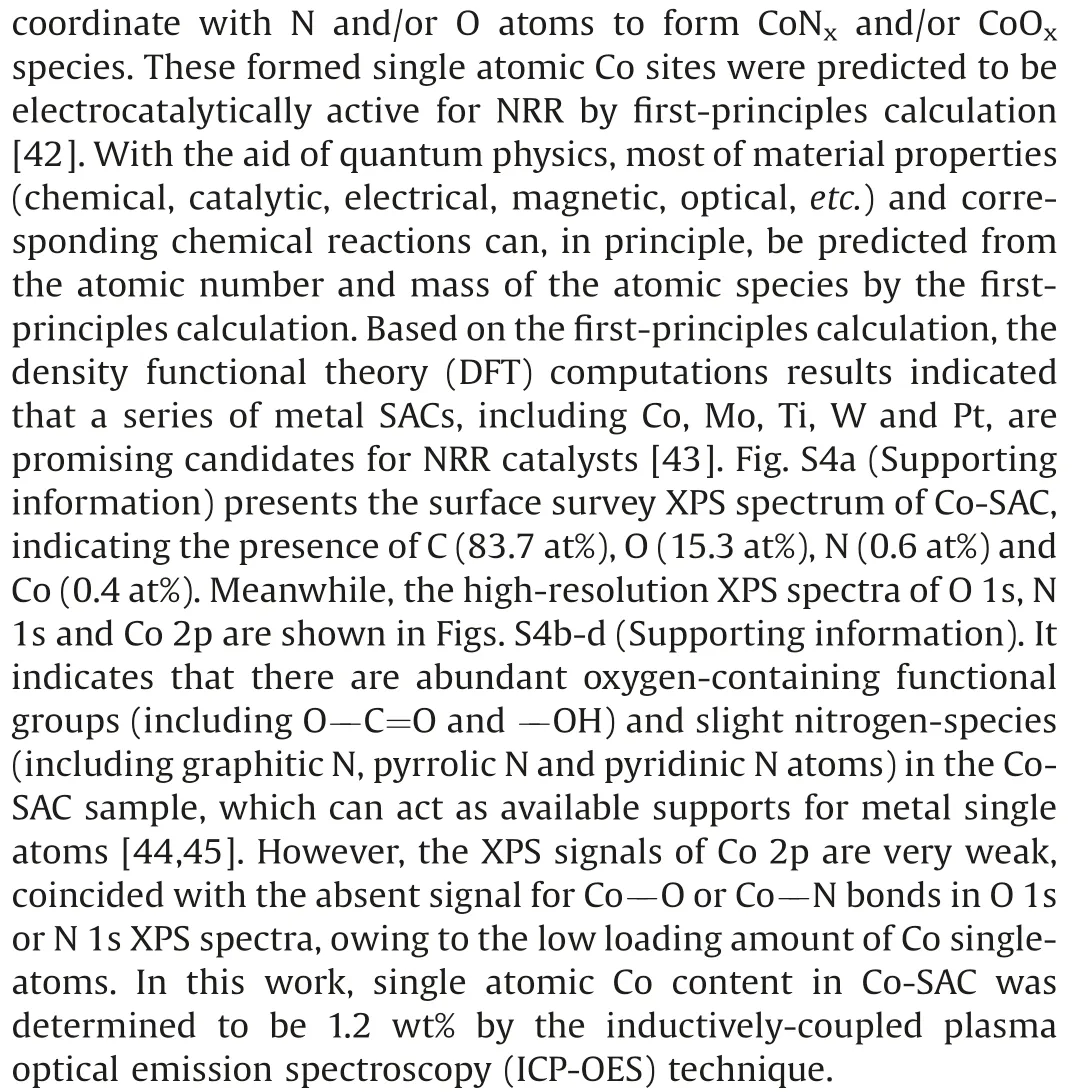
Fig.2.(a)XRD patterns of BCFs,Co/BCFs and Co-SAC.(b)HAADF-STEM image and selected-area magnified HAADF-STEM images with bright SA-Co marked in red circles of Co-SAC.(c) Normalized Co K-edge XANES spectra and (d) FT k3-weighted EXAFS spectra of Co-SAC and reference samples.
In a PEC-NRR system integrated of CoPi/Ti-Fe2O3photoanode and Co-SAC cathode, the NRR performance of Co-SAC is highly dependent on the performance of CoPi/Ti-Fe2O3photoanode.Therefore, we firstly investigated the optical properties of the assynthesized CoPi/Ti-Fe2O3photoanode.Fig.3a shows the UV–vis diffuse reflection spectra (DRS) of Fe2O3, Ti-Fe2O3and CoPi/Ti-Fe2O3samples.The calculated bandgap is 2.09 eV, 2.05 eV and 2.05 eV for Fe2O3, Ti-Fe2O3and CoPi/Ti- Fe2O3based on the DRS,respectively (Fig.3b).Obviously, Ti doping in Fe2O3results in narrower bandgap than that of pristine Fe2O3, meaning its better visible-light harvesting capacity [17,46].Moreover, the CoPi modification on Ti-Fe2O3has not significant influence on its bandgap,still exhibiting superior visible light absorption property for CoPi/Ti-Fe2O3, favourable for high PEC performance.Fig.3c shows the incident photon-to-current efficiency (IPCE) curves of Fe2O3, Ti-Fe2O3and CoPi/Ti-Fe2O3measured at 1.23 V (vs.RHE in 0.2 mol/L NaOH solution)under simulated sunlight irradiation(AM 1.5 G, 100 mW/cm2).The results reveal that CoPi/Ti-Fe2O3possesses the highest IPCE value of35% at a wavelength of 370 nm,and its IPCE is over ~10%in wider wavelength range from 300 nm to 500 nm, indicating that Ti doping and CoPi modification can afford high PEC performance of Fe2O3photoelectrode [25].
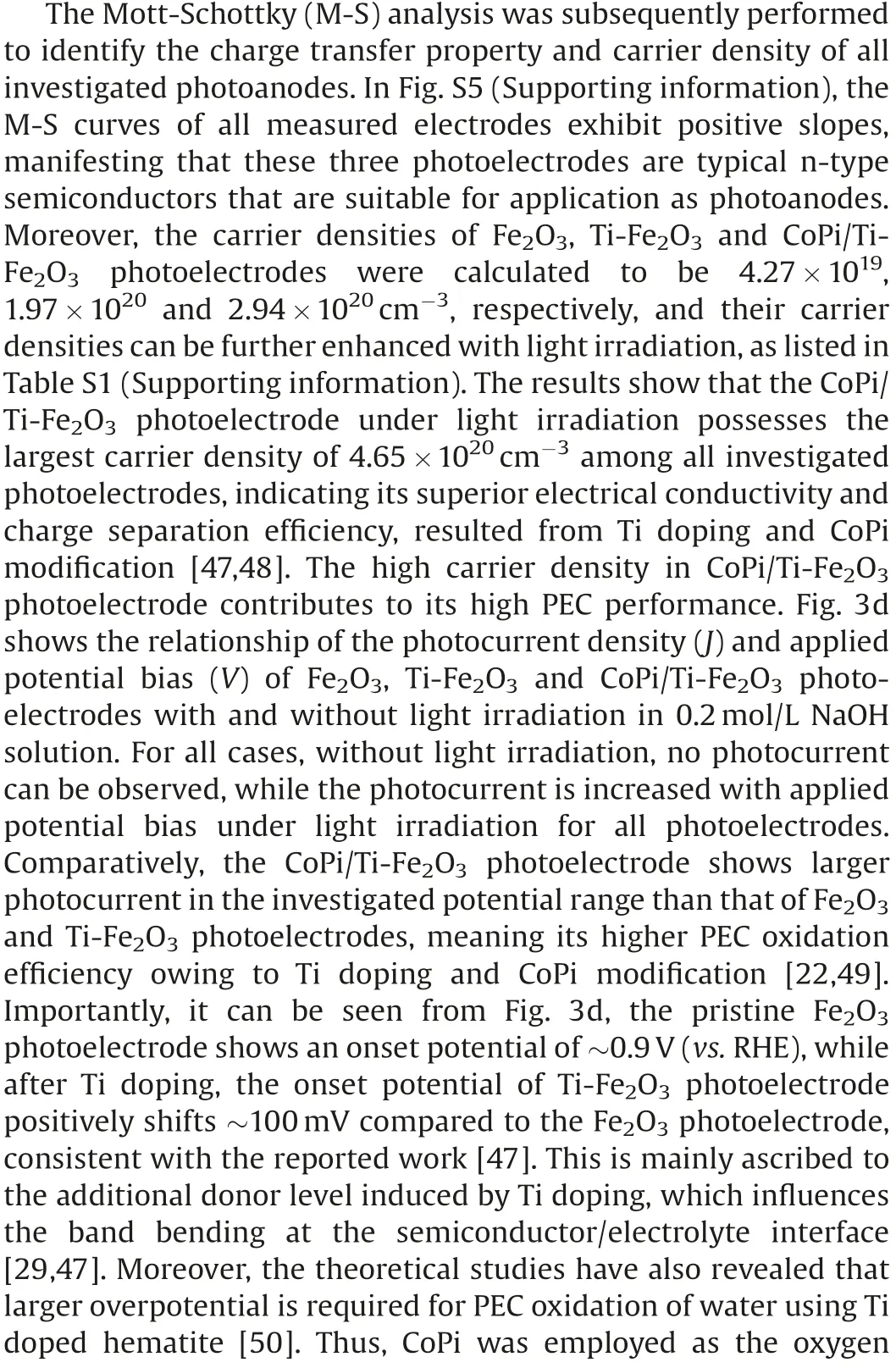
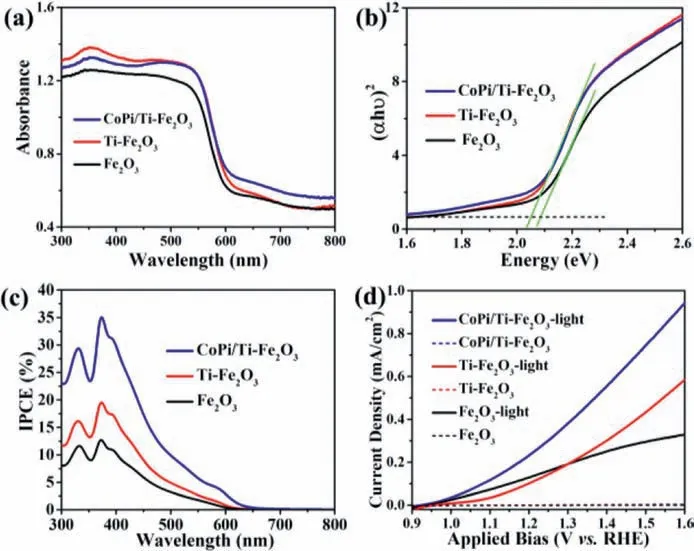
Fig.3.(a) UV–vis DRS, (b) Bandgap determination via Tauc plots, (c) IPCE (measured under 1.23 V vs.RHE) and (d) LSV under light irradiation (5 mV/s) of Fe2O3-based photoanodes.

Subsequently, we evaluated the PEC NRR performance using CoPi/Ti-Fe2O3photoanode and Co-SAC cathode integrated in a photoelectrochemical cell (Scheme S1).Fig.S6 (Supporting information) shows the photograph of photoelectrochemical cell composed of CoPi/Ti-Fe2O3photoanode and Co-SAC cathode.Prior to all measurements,14N2(or15N2) feeding gas was pre-treated using 0.01 mol/L H2SO4solution and distilled water to eliminate any environmental NH3interferences [52].Then, the used N2tail gas passing through the cathodic compartment was absorbed by two series of 0.01 mol/L H2SO4solution to avoid the loss of NH3analyzed by the indophenol blue method (Fig.S7 in Supporting information) [53].Using this photoelectrochemical system, the yielded NH3from PEC NRR on Co-SAC cathode was quantitatively preliminary experimental results demonstrate that the NH3product yielded can be detected in the samples obtained from the cathodic compartment and tail gas absorption solution.Therefore, the NH3yield is the collective amount of the NH3produced from the cathodic compartment and tail gas absorption solution in this work.Fig.4a shows the dependence of the NH3yield rate and faradaic efficiency(FE)on different applied potential bias employed on the CoPi/Ti-Fe2O3photoanode in 0.2 mol/L NaOH electrolyte under AM 1.5 G simulated solar light irradiation of 1 h(light intensity of 100 mW/cm2).The corresponding photocurrent density curves are shown in Fig.4b.The results demonstrate that the largest NH3yield rate can achieved to be 1021.5mg mgCo-1h-1(12.26mgcat.-1h-1) with the highest FE of 11.9% on Co-SAC cathode at an applied potential bias of 1.2 V (vs.RHE) on photoanode, which is comparable to recently reported singleatomic NRR catalysts (Table S2 in Supporting information).With further increasing potential bias, the photocurrent density is obviously enhanced(Fig.4b),but the NH3yield rate and FE are both decreased, mainly attributed to the competitive hydrogen evolution reaction (HER) concurrently happened on the Co-SAC cathode [39,53].For comparison, we also performed the experiment at open-circuit potential (OCP) condition under AM 1.5 G simulated solar-light irradiation of 1 h, the yielded NH3is almost undetectable (Fig.4a and Fig.S8 in Supporting information).The above result suggests that the photoelectrocatalytic approach by employing a potential bias on the CoPi/Ti-Fe2O3can dramatically enhance its photogenerated charge transfer efficiency [54], thus improving the NRR performance on the Co-SAC.Additionally, we also compared the PEC NRR performance on the Co-SAC cathode using Fe2O3and Ti-Fe2O3photoanode at 1.2 V(vs.RHE)in 0.2 mol/L NaOH solution under AM 1.5 G simulated solar light irradiation of 1 h.As shown in Fig.S9 (Supporting information), the NH3yield rate is 371.4 and 422.4mg mgCo-1h-1with FE of 4.5% and 11.1% on the Co-SAC cathode using the Fe2O3and Ti-Fe2O3photoanode,respectively,obviously lower than that(1021.5mg mgCo-1h-1with FE of 11.9%) on the Co-SAC cathode using the CoPi/Ti-Fe2O3photoanode, indicating that the CoPi/Ti-Fe2O3photoanode possesses higher PEC performance.To confirm the yielded NH3resulted from the Co-SAC cathode in this solar-driven CoPi/Ti-Fe2O3photoanode involved PEC system, several control experiments were also conducted in this work.As shown in Fig.S8, the yielded NH3is ignorable when the experiments were carried out in N2-saturated 0.2 mol/L NaOH solution without Co-SAC catalyst at a potential bias of 1.2 V (vs.RHE) on photoanode with light irradiation (denoted as blank) and with Co-SAC catalyst and light irradiation but under open-circuit condition (denoted as opencircuit).In addition,when the experiments were carried out in Arsaturated 0.2 mol/L NaOH solution with Co-SAC catalyst at a potential bias of 1.2 V (vs.RHE) on photoanode (denoted as Arsaturated electrolyte), the measurable NH3is also ignorable.The above control experimental results indicate that the yielded NH3is from the NRR on Co-SAC cathode in the solar-driven CoPi/Ti-Fe2O3photoanode involved PEC system without any noticeable environmental interference.To further confirm this, the isotopic labeling experiments were subsequently conducted using14N2and15N2as the feeding gases in 0.2 mol/L NaOH solution at an applied potential bias of 1.2 V (vs.RHE) on photoanode for 1 h PEC-NRR period[53].Then we analyzed qualitatively and quantitatively the1H nuclear magnetic resonance(NMR)spectra of the samples[55].Based on the NMR spectra of14NH4+and15NH4+standards, the corresponding calibration curves are shown in Fig.S10(Supporting information).The experimental results (Fig.4c) show that the yielded concentration of14NH4+and15NH4+calculated by1H NMR is 1.21 and 1.33mg/mL, respectively, nearly identical with the determined values (1.26mg/mL for14NH4+and 1.38mg/mL for15NH4+) by the indophenol blue method.The almost identical14NH4+and15NH4+concentrations determined by both methods categorically demonstrate that the yielded NH3is indeed originated from the Co-SAC catalyzed NRR in the solar-driven CoPi/Ti-Fe2O3photoanode involved PEC system.
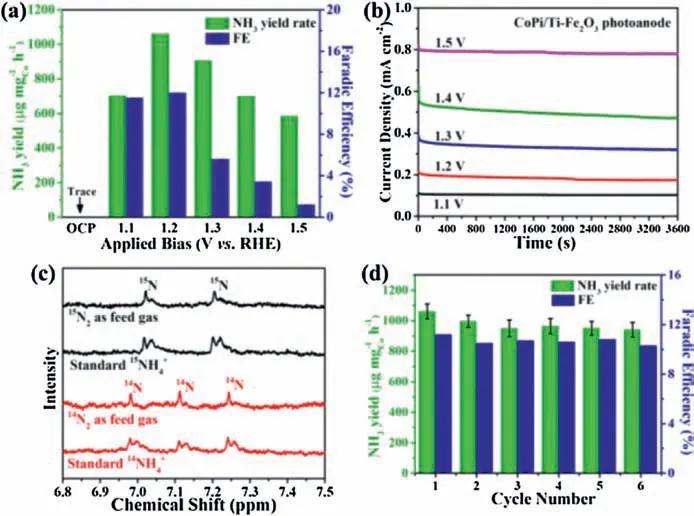
Fig.4.(a)PEC-NRR performance on Co-SAC with different applied bias on CoPi/Ti-Fe2O3photoanode and(b)corresponding operating photo-current density(J)vs.time(t).(c)1H NMR spectra of the yielded 14NH4+and 15NH4+from14N2and 15N2feed gases,and standards(all standard concentration of 10mg/mL);(d)NH3yield rate and FE stability on Co-SAC with CoPi/Ti-Fe2O3NAs photoanode (applied bias of 1.2 V vs.RHE in 0.2 mol/L NaOH).
The recycling stability of the PEC-NRR system is tested as shown in Fig.4d.Employing an applied potential bias of 1.2 V(vs.RHE)on CoPi/Ti-Fe2O3photoanode with light irradiation,the NH3yield rate and FE on Co-SAC cathode in 0.2 mol/L NaOH solution can be well maintained after 6 consecutive cycles.After 6 cycles of measurements, the collected cathode electrocatalyst (labeled as Co-SACused)was characterized by the XRD and HAADF-STEM techniques.The XRD characterization results (Fig.S11a in Supporting information) reveal that only one broad peak centered at 2u =20ascribed to the graphitic carbon can be detected and no characteristic peaks derived from metallic Co nanoparticles are detectable, similar to that of pristine Co-SAC catalyst (Fig.2a).Moreover, the HAADF-STEM image (Fig.S11b in Supporting information) of the Co-SAC-used still shows atomically dispersed nature of Co on the BC converted graphitic carbon.The above results indicate that the Co-SAC possesses high stability,favourable for high-efficiency PEC-NRR to NH3.
In summary, we developed a photoelectrochemical N2reduction system integrated of CoPi/Ti-Fe2O3photoanode and Co-SAC cathode.Utilizing such PEC-NRR system,the NH3yield rate on Co-SAC cathode can be achieved to be 1021.5with a faradic efficiency of 11.9% at an applied potential bias of 1.2 V (vs.RHE) in 0.2 mol/L NaOH electrolyte under simulated solar irradiation.The high PEC-NRR to NH3performance can be resulted from high-efficient CoPi/Ti-Fe2O3photoanode providing abundant photoelectrons and Co-SAC catalyst supplying electrocatalytically active Co-N(O)xsites for N2adsorption and activation.The findings in this work demonstrate the feasibility of integrating highefficiency photoanode and efficient NRR catalyst cathode for solardriven PEC-NRR to NH3application.Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.51872292 and 51672277) and the CAS/SAFEA International Partnership Program for Creative Research Teams of Chinese Academy of Sciences, China.The authors would like to thank the 1W1B station for XAFS measurements in the Beijing Synchrotron Radiation Facility (BSRF).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.013.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
