Effect of Mie resonance on photocatalytic hydrogen evolution over dye-sensitized hollow C-TiO2nanoshells under visible light irradiation
2021-05-14XixiYoXiuliHuYingyingCuiJileiHungWenjunZhngXuhongWngDweiWng
Xixi Yo,Xiuli Hu,*,Yingying Cui,Jilei Hung,Wenjun Zhng,Xuhong Wng,Dwei Wng*
a School of Materials Engineering, Suzhou Key Laboratory of Functional Ceramic Materials, Changshu Institute of Technology, Changshu 215500, China
b Department of Environmental Science and Earth Sciences, Clemson University, Clemson, SC 29634, United States
ABSTRACT Light utilization is one of the key factors for the improvement of photocatalytic performance.Herein,we design C-TiO2hollow nanoshells with strong Mie resonance for enhanced photocatalytic hydrogen evolution in a dye-sensitized system under visible light irradiation (420 nm).By tuning the inner diameters of hollow nanoshells,the Mie resonance in hollow nanoshells is adjusted for better excitation of dye molecules,which thus greatly enhances the light utilization in visible light region.This work shows the potential of Mie resonance in nanoshells can be an alternative strategy to increase the light utilization for photocatalysis.
Keywords:Mie resonance Hollow nanoshells Dye sensitization Water splitting Photocatalysis
Photocatalysis is promising for various applications, including contaminant removal, hydrogen evolution, CO2reduction and nitrogen fixation[1–5].However,its practical application is largely limited by the low light utilization efficiency, especially the low efficiency in the visible light region in natural sunlight.Many efforts to improve the photocatalytic performance of semiconductors have been proposed, such as elemental doping,sensitization,and coupling with narrow band-gap semiconductors[6–11].Recently, Mie scattering in hollow microspheres has attracted extensive attention due to the enhancement of light interaction [12–14].Mie scattering is a physical phenomenon,which occurs when the size of the particles is comparable to the wavelength of the incident light.Especially, the Mie resonance(resonant Mie scattering) occurs when the particles have hollow nanoshell morphology because the hollow structure may significantly enhance the transport mean free path of incident light[13–15].This will allow the light travelling a round trip inside the shell to escape from the powder with minimum multiple scattering and to produce constructive interference with the light directly scattered from the microspheres.
The hollow microspheres have demonstrated to be an efficient scattering layer for dye-sensitized solar cells whereas the improved photovoltaic conversion efficiency was due to the Mie scattering [16–20].It was also reported that the Mie scattering of TiO2spheres was responsible for the superior photoactivity of H2evolution under UV light irradiation(l<387 nm)[21].However,it is still unclear whether the accurate tuning of scattering peak to match the absorption range of dyes or semiconductors benefits the photocatalysis greater.To address this issue, we design C-TiO2hollow nanoshells with strong Mie resonance for enhanced photocatalytic hydrogen evolution in an Eosin Y-sensitized system under visible light irradiation.The carbon species are embedded into the hollow nanoshells by the carbonization of organic species during the calcination process.The addition of carbon can further suppress the multiple scattering and enhance the Mie resonance intensity [12,22].Pt co-catalyst is deposited on the surface of CTiO2to promote charge separation through a photo-reduction method.The peaks of Mie resonance shift from ultraviolet to visible light when the inner diameters of C-TiO2hollow nanoshells varies from 160 nm to 210 nm.The 210 nm size C-TiO2hollow nanoshells with Eosin Y sensitization exhibits the highest photocatalytic hydrogen evolution rate (468.1 mmol) among all the studied sizes under visible light illumination(nm)due to the better excitation of Eosin Y dyes by Mie resonance in hollow nanoshells.The theoretical backscattering in hollow nanoshells is calculated based on Mie’s theory, and a possible mechanism considering charge transfer and light utilization is proposed for the photocatalytic process.

The optical absorption property was conducted on a UV–vis spectroscopy.As shown in Figs.2a and b,the 160(18)@C-TiO2and 210(18)@C-TiO2hollow nanoshells has a strong absorption in the whole UV–vis light region.The absorption in UV light region results from the band-to-band transition of anatase TiO2,while the absorption in visible light region originates from the carbon species.After Pt deposition, the absorption increases slightly[28,29].The Eosin Y dye shows the characteristic absorption peak at about 516 nm.The reflectance spectra of the hollow nanoshells were recorded on an optical microscope (Zeiss, Axioscope)equipped with a probe type Ocean Optics HR4000CG-UV–vis spectrometer in reflection mode(Fig.2c).In this work,we mainly focus on the research of photocatalytic hydrogen evolution under visible light irradiation(420 nm).No reflection peaks in visible light region are observed for 160(18)@C-TiO2, while 210(18)@CTiO2hollow nanoshells displays an obvious reflection peak centered at 436 nm.Thus, 160(18)@C-TiO2and 210(18)@C-TiO2hollow nanoshells appear to be grayish color and violet color,respectively( Figs.2e and f).The bright color of the C-TiO2hollow nanoshells is resulted from the Mie resonance, which can be confirmed by theoretical calculation based on Mie’s theory (see Supporting information for details)[13,30,31].As shown in Fig.2d,the simulated scattering peak is located at 362 nm for the smaller 160(18)@C-TiO2hollow nanoshells, while the peak has a red shift to 436 nm when the inner diameter increases from 160 nm to 210 nm.As widely known,anatase TiO2is a typical semiconductor with a band gap of3.2 eV, which can strongly absorb UV light below 380 nm.Therefore, the scattering peak below 380 nm cannot be observed clearly in the reflectance spectra of C-TiO2hollow nanoshells.The calculated scattering peak (436 nm) of 210(18)@C-TiO2hollow nanoshells is well consistent with the experimental reflectance spectrum, which corresponds to the violet color.The enhanced light scattering from Mie resonance in hollow nanoshells can be further absorbed by the neighbouring dye molecules when the light of Mie resonance can excite the sensitized dyes.It is thus reasonable to believe that effective use of solar energy can be achieved by rationally tuning the peak of Mie resonance.
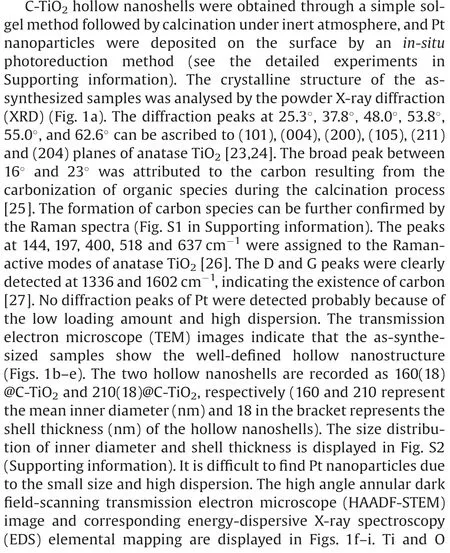
Fig.1.XRD patterns of C-TiO2and C-TiO2-Pt hollow nanoshells with different sizes(a).TEM images of 160(18)@C-TiO2(b),160(18)@C-TiO2-1%Pt(c),210(18)@C-TiO2(d)and 210(18)@C-TiO2-1%Pt(e).HAADF-STEM image of 210(18)@C-TiO2-1%Pt(f),and corresponding EDS elemental mapping of Ti(g),O(h)and Pt(i)from the region in(f).Scale bar in (f): 250 nm.

Fig.2.(a)UV–vis absorption spectra of 160(18)@C-TiO2,160(18)@C-TiO2-1%Pt and Eosin Y solution.(b)UV–vis absorption spectra of 210(18)@C-TiO2,210(18)@C-TiO2-1%Pt and Eosin Y solution.(c) Reflectance spectra of C-TiO2hollow nanoshells with different sizes.(d) Simulation of backscattering in C-TiO2hollow nanoshells.(e) Digital photograph of 160(18)@C-TiO2hollow nanoshells.(f) Digital photograph of 210(18)@C-TiO2hollow nanoshells.

Fig.3.(a) Photocatalytic H2evolution over different samples under visible light irradiation (420 nm).(b) Nitrogen adsorption-desorption isotherms and pore size distributions(inset)of C-TiO2-1%Pt hollow nanoshells.(c)Stability performance for H2evolution under visible light irradiation over Eosin Y-sensitized 210(18)@C-TiO2-1%Pt hollow nanoshells.
The effect of Mie resonance in hollow nanoshells on the photocatalytic activity is investigated by the photocatalytic hydrogen evolution in an Eosin Y-sensitized system with Pt as the cocatalyst and triethanolamine(TEOA)as the sacrificial donor under visible light irradiation(Fig.3a).The C-TiO2and C-TiO2-1%Pt hollow nanoshells show no activity for H2generation due to the wide band gap of anatase TiO2.However, the Eosin Y-sensitized C-TiO2-1%Pt hollow nanoshells presents the enhanced photocatalytic performance for water splitting due to the excitation of Eosin Y molecules and the electrons transfer from excited state Eosin Y to TiO2under visible light irradiation[32–34].Meanwhile,the Eosin Ysensitized 210(18)@C-TiO2-1%Pt exhibits a higher H2-evolution rate(468.1), which is 4.4 times as much as Eosin Ysensitized 160(18)@C-TiO2hollow nanoshells(105.5Theexperimentalparametersofphotocatalytichydrogengeneration are particularly identical over 160(18)@C-TiO2and 210(18)@C-TiO2,includingcatalystamount,Ptloadingamount,EosinYconcentration,and TEOA amount.The only difference between the two samples is their different inner diameter, and thus different Mie scattering.Moreover,we alsotake the specific surfaceareainto account,and the nitrogen adsorption-desorption isotherms with pore size distributions are displayed in Fig.3b.These two samples show the type Ⅳisotherms with H3 hysteresis loops at high relative pressure,indicating the existence of slitlike mesopores [35].The 160(18)@C-TiO2-1%Pt and 210(18)@C-TiO2-1%Pt hollow nanoshells have the specificsurfaceareaof144.5and121.1 m2/g,respectively,andbothof themshowthesimilarporesizedistributionfrom2 nmto10 nm.The photocatalytic activity of Eosin Y-sensitized 210(18)@C-TiO2-1%Pt is 5.2timesashighasthatofEosinY-sensitized160(18)@C-TiO2-1%Ptif the photocatalytic H2-generation rate is calculated by per unit surface area.Therefore,the enhanced photocatalytic H2production of Eosin Y-sensitized 210(18)@C-TiO2-1%Pt is because its Mie scattering peak (436 nm) can excite the Eosin Y molecules more compared to 160(18)@C-TiO2-1%Pt under visible light irradiation.The apparent quantum efficiency(AQE)for hydrogen evolution over 210(18)@C-TiO2-1%Pt with Eosin Y sensitization reaches 22.5%.In addition,it is found that the H2-evolution rate gradually decreases with increasing reaction time, possibly due to the degradation of sensitized dyes (Fig.3a) [36].The UV–vis absorption spectra are measured to determine the change of Eosin Y dyes (Fig.S3 in Supporting information).The Eosin Y dyes are gradually degraded in thephotocatalyticprocessofhydrogenevolution,andtheabsorption peak shifts to shorter wavelength, resulting in the decreased photocatalytic activity with increasing reaction time.Furthermore,we investigate the stability of hydrogen evolution over 210(18)@CTiO2-1%Pt with Eosin Y sensitization under visible light irradiation420 nm).Aftereach run,the catalyst is collected bycentrifuging from the reaction mixture and re-dispersed in the fresh 10% TEOA aqueous solution with Eosin Y dyes and then evacuated in Labsolar-6A photocatalytic system(Beijing Perfectlight Technology Co.,Ltd., China).As shown in Fig.3c, the photocatalytic performance remained stable after a few cycles, indicating the good stability of 210(18)@C-TiO2system.
In order to further explore the effect of shell size on the photocatalytic performance, larger size C-TiO2hollow nanoshells are studied for photocatalytic water splitting.With further increasing the shell inner diameter to 240 nm with a thickness of 18 nm, cyan-colored C-TiO2hollow nanoshells can be obtained(Fig.S4 in Supporting information).The sample of 240(18)@C-TiO2hollow nanoshells shows a broad reflection peak centered at about 480 nm, which is consistent with the calculated backscattering peak based on Mie’s theory(Fig.S5 in Supporting information).The broad reflection peak may be caused by the polydispersion of size distribution because it is practically difficult to get completely monodispersed hollow nanoshells with the exactly same diameter and shell thickness especially when the diameter increased.The 240(18)@C-TiO2-1%Pt shows a photocatalytic H2evolution rate ofin the Eosin Y sensitized system under visible light irradiation (Fig.S6a in Supporting information), which is slightly lower than Eosin Y sensitized 210(18)@C-TiO2-1%Pt,but is much higher than Eosin Y sensitized 160(18)@C-TiO2-1%Pt.The 240(18)@C-TiO2-1%Pt also show the type Ⅳnitrogen adsorptiondesorption isotherm with a specific surface area of 112.2 m2/g(Fig.S6b in Supporting information).If the specific surface area is considered as one of the effect factors, similar photocatalytic activity order is obtained (Eosin Y sensitized 210(18)@C-TiO2-1%Pt > 240(18)@C-TiO2-1%Pt > 160(18)@C-TiO2-1%Pt).
Based on the above experimental results and discussion, a possible mechanism was proposed for the photocatalytic process in terms of charge separation,Mie resonance,and light utilization(Fig.4).Under visible light irradiation,the Eosin Y is excited to the excited state Eosin Y* by transferring the electrons in highest occupied molecular orbital to lowest unoccupied molecular orbital[37,38].Then,the Eosin Y* injects the electrons to the conduction band of anatase TiO2because the reduction potential of Eosin Y(-0.8 V vs.NHE) is more negative than the conduction band of anatase TiO2(-0.2 V vs.NHE)[39,40].After that,the electrons will rapidly migrate to the Pt nanoparticles for H2production due to the high work function and low overpotential of Pt [36,38].In this work, the scattered light in hollow nanoshells can be greatly enhanced by the phenomenon of Mie resonance (constructive interference of Light 1 and Light 2 in Fig.4.Light 1 is the directly scattered light from the surface, and Light 2 is the escaped light after travelling around in the hollow nanoshells), and the peak of Mie resonance can be tuned by optimizing the shell diameter,thickness or the relative refractive index [12–14].Herein, no scattering peak is observed for 160(18)@C-TiO2above 400 nm,and an obvious scattering peak at 436 nm is detected for 210(18)@CTiO2hollow nanoshells.The scattered light at 436 nm can be further utilized to excite the Eosin Y molecules and generate more excited electrons, which thus greatly improves the utilization efficiency of incident light and enhances the photocatalytic performance for hydrogen production.The enhanced scattering light from 420 nm to 516 nm in 240(18)@C-TiO2is still feasible for the excitation of Eosin Y molecules, resulting in efficient light utilization and improved photocatalytic performance.However,the photo energy of resonant Mie scattering in 210(18)@C-TiO2is higher than that in 240(18)@C-TiO2.Thus, the 210 nm size C-TiO2hollow nanoshells with Eosin Y sensitization exhibits the highest photocatalytic H2evolution rate among all the studied sizes.It is difficult to obtain C-TiO2hollow nanoshells with narrow scattering peak at about 516 nm for the accurate matching with the characteristic absorption peak of Eosin Y due to the polydispersion of size distribution.In future,more efficient ways may be explored for the synthesis of monodipersed hollow nanoshells of semiconductor-based materials with independent scattering peak in visible light region.In some sense, this work well demonstrates the Mie resonance in hollow nanoshells is efficient for the improved light utilization by tuning the scattering peaks.
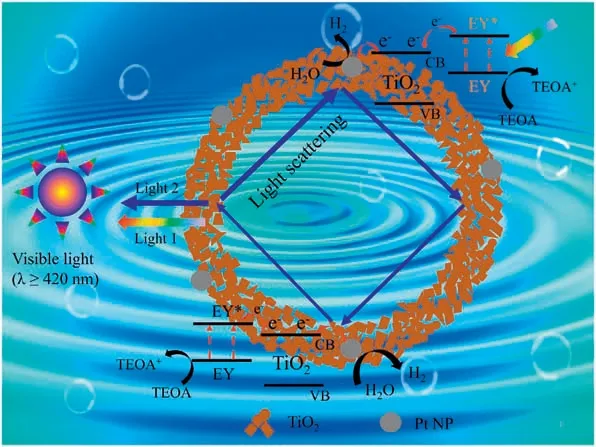
Fig.4.Proposed mechanism for photocatalytic H2evolution over Eosin Y-sensitized C-TiO2hollow nanoshells with Mie resonance.
In summary,C-TiO2hollow nanoshells with Mie resonance have been explored for photocatalytic H2evolution in the Eosin Y sensitized system under visible light irradiation.When the Mie resonance in hollow nanoshells can excite Eosin Y dyes, the enhanced scattering light can be further utilized for the photoexcitation of Eosin Y molecules and thus more electrons are produced for water reduction.This work demonstrates rational tuning of Mie resonance in hollow nanoshells can be a promising strategy for efficient utilization of incident light, and thus improved photocatalytic performance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support for this project was provided by the National Natural Science Foundation of China (Nos.51702023, 51702022)and Natural Science Research of Jiangsu Higher Education Institutions of China (No.17KJB430001).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.05.013.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
