Quinoline-based aggregation-induced delayed fluorescence materials for highly efficient non-doped organic light-emitting diodes
2021-05-14LingZhngYinFengWngMengLiQingYuGoChunFengChen
Ling Zhng,Yin-Feng Wng,Meng Li,Qing-Yu Go*,Chun-Feng Chen,*
a Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
b College of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, China
c University of Chinese Academy of Sciences, Beijing 100049, China
ABSTRACT Three new emitters, namely 10,10'-(quinoline-2,8-diyl)bis(10H-phenoxazine) (Fene),10,10'-(quinoline-2,8-diyl)bis(10H-phenothiazine) (Fens) and 10,10'-(quinoline-2,8-diyl)bis(9,9-dimethyl-9,10-dihydroacridine) (Yad), featuring quinoline as a new electron acceptor have been designed and conveniently synthesized.These emitters possessed small singlet–triplet splitting energy and twisted structures, which not only endowed them show thermally activated delayed fluorescence (TADF)properties but also afforded a remarkable aggregation-induced emission (AIE) feature.Moreover, they also showed aggregation-induced delayed fluorescence (AIDF) property and good photoluminescence(PL) property, which are the ideal emitters for non-doped organic light-emitting diodes (OLEDs).Furthermore, high-performance non-doped OLEDs based on Fene, Fens and Yad were achieved, and excellent maximum external quantum efficiencies(EQEmax)of 14.9%,13.1%and 17.4%,respectively,were obtained.It was also found that all devices exhibited relatively low turn-on voltages ranging from 3.0 V to 3.2 V probably due to their twisted conformation and the AIDF properties.These results demonstrated the quinoline-based emitters could have a promising application in non-doped OLEDs.
Keywords:Quinoline Aggregation-induced emission Thermally activated delayed fluorescence Non-doped organic light-emitting diode
Organic light-emitting diodes (OLEDs) have attracted much attention in academic and industrial communities owing to their low energy cost, high-quality color, light weight, rapid response and flexibility[1–4].However,the traditional fluorescent emitters for OLEDs still require improvement in terms of their devices cannot exceed the theoretical maximum exciton utilization of 25%,according to the principle of conservation of spin[5–7].Although phosphorescent OLEDs can fully utilize electrogenerated singlet and triplet excitons to provide high electroluminescence (EL)efficiency by utilizing precious-metal-based phosphorescent materials to increase spin-orbit coupling of singlet (S1) state and triplet (T1) state [8–11].These phosphorescent materials are expensive for containing transition metals such as iridium and platinum [12–14], which can raise the manufacturing cost and limit their practical applications [15].As the promising thirdgeneration organic luminescent material after the conventional fluorescent and phosphorescent materials, purely organic materials with thermally activated delayed fluorescence (TADF)have attracted considerable attention due to their high exciton utilization efficiency [16–20].
TADF emitters for OLEDs have made great progress so far[21–25].However, the development of high-performance OLED devices generally needed guest-host doping technique to alleviate concentration-caused emission quenching and exciton annihilation processes[26,27].The doping technique requires complicated control of the doping concentration[28]and the reproducibility of OLED device is cumbersome as well [29–32].Owing to their rigid structures and special properties, heterocyclic compounds have been widely utilized as acceptors for TADF materials, which included pyrimidine [33], heptazine [34], quinoxzline [35],phenanthroline [36], triazine [37,38], pyrazine [39,40], dibenzo[a,j]phenazine[41]and naphthyridine[42].Among different kinds of heterocycles, quinoline derivatives stand out as important molecules with a wide range of interesting pharmacological activity and unique physicochemical property.However, there have been no reports quinoline-based TADF emitters and their applications in OLEDs so far.Herein,we report three emitters Fene,Fens and Yad with quinoline as a new electron acceptor.These emitters have strong intramolecular interactions,which can cause stable excited state configuration and restrict aggregation-caused quenching (ACQ) effect.Consequently, the emitters exhibited aggregation-induced emission (AIE) and aggregation-induced delayed fluorescence (AIDF) properties, and highly efficient nondoped OLEDs based on Fene, Fens and Yad achieved high maximum EQEs of 14.9%,13.1% and 17.4%, respectively.
The synthetic routes of Fene, Fens and Yad were shown in Scheme 1.According to the literature procedure[43],8-bromo-2-chloroquinoline was prepared.Then, compounds Fene, Fens and Yad were conveniently synthesized in good yields by palladiumcatalyzed cross-coupling reactions of 8-bromo-2-chloroquinoline with 9,9-dimethyl-acridan, phenothiazine and phenoxazine, respectively.The target compounds were purified via column chromatography and temperature-gradient sublimation with high vacuum conditions.Their structures were confirmed by1H NMR,13C NMR, HRMS spectra and single-crystal X-ray structures.


Scheme 1.Synthesis of emitters Fene, Fens and Yad.
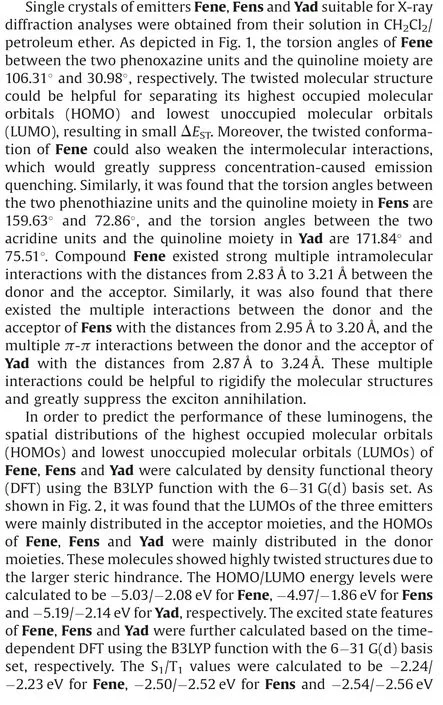
Fig.1.The torsion angles of (a) Fene, (b) Fens and (c) Yad.

Fig.2.The HOMO and LUMO of Fene, Fens and Yad obtained by the Gaussian 09 DFT B3LYP/6-31+G* level of theory.
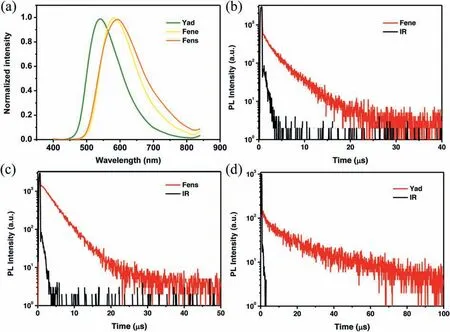
Fig.3.(a) Fluorescence spectra of Fene, Fens and Yad in neat films at room temperature; Transient PL spectra of (b) Fene, (c) Fens and (d) Yad in neat films at room temperature.

Fig.4.Steady-state excitation-emission mapping of (a) Fene, (b) Fens and (c) Yad in neat films at room temperature; the temperature-dependent transient photoluminescence spectra of (d) Fene, (e) Fens and (f) Yad in the neat films.

Thermogravimetric analyses (TGA) were also carried out to investigate the thermal stability of the emitters.As shown in Figs.S4–S6 (Supporting information), Fene, Fens and Yad exhibited good thermal stability with a 5% weight loss decomposition temperature(Td)of 360,320 and 314respectively.These results indicated that these luminogens possessed adequate thermal stability for the light-emitting devices.
Cyclic voltammetry (CV) was further used to investigate the electrochemical properties of the emitters.As shown in Fig.S7(Supporting information),according to the onsets of oxidation and reduction potentials in their CV curves,the HOMO/LUMO levels of Fene,Fens and Yad were determined to be-5.90/-3.04 eV,-5.63/-2.53 eV and -6.09/-3.29 eV, respectively.These favorable HOMO/LUMO levels matched well with the widely used hole transporting material of 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC)and electron-transporting layer of 1,3,5-tri(mpyrid-3-yl-phenyl)benzene (TmPyPB), which could facilitate the charge carrier injection and transportation from adjacent layer to the emissive layer.

Fig.5.PL spectra of(a)Fene,(b)Fens and(c)Yad in THF/water mixtures with different water fractions(fw);PL decay curves of(d)Fene,(e)Fens and(f)Yad in THF/water mixtures with different water fractions (fw) in air.

Fig.6.(a) Electroluminescence spectra of devices at ; external quantum efficiency versus luminance curves for the devices of(b)Fene,(c)Fens and(d)Yad.
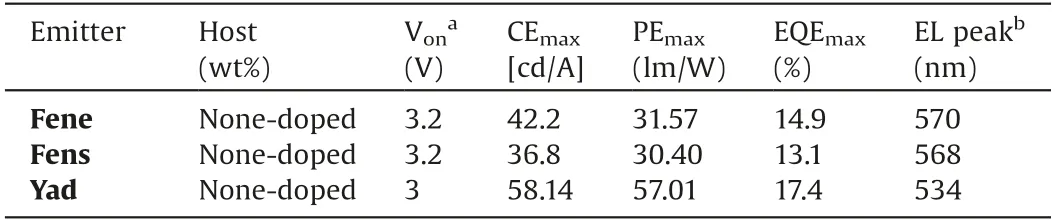
Table 1 Summary of EL data for the devices.
The PL spectra of emitters Fene, Fens and Yad in THF/water mixtures were measured to investigate their AIE properties.As shown in Fig.5, the PL spectra of Fene, Fens and Yad showed negligible emission in dilute THF solution.However, when the value of water fraction reached to 80%, relatively strong fluorescence emissions were observed.With the continuing increase of water fraction, the fluorescence intensities enhanced gradually.When the value of water fraction reached to 90%,the fluorescence intensities of Fene,Fens and Yad were much stronger than those in THF, suggesting that the typical AIE characteristics existed.The transient PL curves of Fene, Fens and Yad were also measured in THF under air atmosphere at room temperature.Interestingly,when the water fraction varied from 80% to 90% in THF-water mixtures, the lifetimes and proportion of DF gradually increased with the increase of water fractions under air atmosphere at room temperature.Rotations of the aromatic rings and vibrations of Fene, Fens and Yad resulted in the conformational changes and thus influenced the RISC processes with the loss of DF in solution under air atmosphere.Long-lived luminescence under air atmosphere indicated that the triplet excited states in aggregate states were protected from oxygen,consequently leading to the effective RISC and delayed fluorescence emission.The outstanding emission characteristics of these materials could make them to have the great potential application as emitters in non-doped OLEDs.Thus,as shown in Fig.6,the devices were fabricated with the structure of indium tin oxide (ITO)/(TAPC) (30 nm)/4,40,400-tri(N-carbazolyl)-triphenylamine (TCTA) (5 nm)/emission (15 nm)/(TmPyPB)(65 nm)/LiF (1 nm)/Al (100 nm), in which TAPC and TmPyPB were used as hole- and electron-transportation layers, respectively.Moreover,TCTA was inserted between TAPC and the emitting layer to reduce the hole-injection barrier and achieve step-by-step increments for each energy level.We further used LiF served as the electron-injection layer to eliminate the barrier caused by the interface electric double layer and reduce the Fermi level of the cathode.The non-doped OLEDs were fabricated by using codeposited films of emission layers.As shown in Table 1, the nondoped optimal devices based on Fene,Fens and Yad displayed the maximum EL emissions at 570, 568 and 534 nm, respectively.Moreover, the non-doped devices of Fene, Fens and Yad showed excellent performances with the EQEmaxof 14.9%,13.1%and 17.4%,respectively.It was also found that the turn-on voltages of the nondoped devices are all low ranging from 3.0 V to 3.2 V.
In summary, we have conveniently synthesized three emitters Fene, Fens and Yad with quinoline as the new electron acceptor,which exhibited both TADF and AIE properties.Smalland twisted structures made them show good photoluminescence and AIDF properties,which could be greatly benefit for highly efficient non-doped OLEDs.Consequently, non-doped OLEDs based on the emitters were fabricated, which achieved excellent EQEmaxof 14.9% and 13.1% with neat films of Fene and Fens, respectively.Moreover, the non-doped device based on the Yad neat film achieved the EQEmaxof 17.4% owing to the good photoluminescence and obvious AIDF property.Notably, all devices exhibited relatively low turn-on voltages ranging from 3 V to 3.2 V.This represents the first highly efficient TADF emitters based on the quinoline electron acceptor.The results presented herein also suggested that the quinoline-based emitters could have a potential application in non-doped OLEDs for displays and solid-state lighting.
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank the National Natural Science Foundation of China(Nos.91956119, 21871272, 21521002) for financial supports.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.07.041.
杂志排行
Chinese Chemical Letters的其它文章
- Quantitative assessment of rhodamine spectra
- Copper-cobalt-nickel oxide nanowire arrays on copper foams as self-standing anode materials for lithium ion batteries
- Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics
- An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density
- Assembly and packing models of [Ti6Co12] ring based on the titanium-capped cobalt clathrochelates
- A stable Co(II)-based metal-organic framework with dual-functional pyrazolate-carboxylate ligand: Construction and CO2selective adsorption and fixation
