Efficacy of intravitreal injection of Aflibercept vs Ranibizumab in the treatment of diabetic retinopathy
2021-05-10ChangHuang1GuoGuoYi2MinFu
Chang Huang1, Guo-Guo Yi2, Min Fu
1The Second Clinical School, Southern Medical University, Guangzhou 510080, Guangdong Province, China2Department of Ophthalmology, the Six Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China3Department of Ophthalmology, Zhujiang Hospital, Southern Medical University, Guangzhou 510220, Guangdong Province, China
Abstract
INTRODUCTION
Due to the rapid changes in lifestyle, there is great concern that diabetes could become an epidemic[1]. Diabetic retinopathy (DR) are some of the main causes of blindness in the developed countries, which characteristic of microaneurysm, thickening of basement membrane and cell loss, these could eventually lead to blindness[2]. Thus far the most commonly used treatment option for DR is laser therapy[3]. However, photocoagulation has several limitations: one adverse impact is that laser treatment may affect peripheral vision and consequently cause a substantial decrease in night vision[4]. Laser can delay the progress of the disease, can’t improve your vision, and a negative impact on peripheral vision, therefore must develop new treatments and drugs. It should be noted that VEGF plays an important role in its pathogenesis[5]. That is, long-term vasodilation can lead to changes in microaneurysms and vascular structures, which may cause luminal stenosis, haemodynamic changes, and the formation of neovascularization. Additionally, VEGF plays an important role in stimulating neovascularization[6]. Bleeding from new blood vessels can destroy the integrity of the vitreous, causing dissociative retinal detachment and impairing vision[7]. VEGF expression is triggered by hypoxia, and in proliferative diabetic retinopathy (PDR) which is expressed in vitreous and preretinal new vessels[8]. Therefore, it is necessary to effectively inhibit VEGF. Scientists are working to develop drugs that inhibit VEGF. In clinical trials, ranizumab and aflixipu were successively marketed.
Ranibizumab (Lucentis, Genentech/Roche) is designed to treat DR by manipulating the structure of a full-length monoclonal antibody (mAb) A.4.6.1 directed against human VEGF-A[9]. The fragment antigen binding (FAb) fragment of A.4.6.1 is referred to as Fab-12[10]. Fab-12 has been widely used in DR, DME, retinal vein occlusion (RVO) and AMD[11]. To some extent, this therapeutic has a few limitations in ophthalmic treatment[12]. In addition, Fab-12 has systematic drawbacks in some clinical studies, such as hypertension, proteinuria, inhibition of bone growth and infertility[13]. Aflibercept is a fusion protein formed by the recombination of the extracellular region of human VEGF receptor-1 and 2, which includes the human immunoglobulin Fc segment[14]. Intravitreous Aflibercept can improve vison in eyes with DME or DR[15], but there are fewer reports on Aflibercept than Ranibizumab. There are no concrete reports of endophthalmitis, or events suggestive of endophthalmitis[16]. Clinical studies have shown that the two drugs have different anti-VEGF mechanisms and have significant efficacy in patients with DR[17]. The purpose of this paper is to compare the clinical efficacy of ranibizumab and Aflibercept according to BCVA, VA and CMT, and to provide evidence-based basis for individualized treatment of DR.
SUBJECTSANDMETHODS
DataSourceandSearchStrategyThe Meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[18]. MEDLINE, Web of Science, PubMed, Cochrane, Nature Series, ScienceDirect, and ESI Databases were searched for articles published until May 2019 combining the following terms [(“Ziv-Aflibercept” or “Aflibercept” and “DR” or “Diabetes retinopathy” or “Diabetic retinopathy”) and “Ranibizumab” and “Randomized”]. No language restrictions were applied.
StudySelectionThe study included 1240 patients (age 38 to 58) with type 2 diabetes. They all came from different countries, including Egypt, Japan, England and the United States. They were published between two and three years ago. The best corrected visual acuity (BCVA), visual acuity (VA) and central macular thickness (CMT) were analyzed.
DataExtractionMeasurement information, year of publication, number of treated and control eye patients, age, sex, country, and type of diabetes were collected from each study and entered into RevMan 5.3. Extractive results included efficacy after treatment with either afrisib or ranizumab. There are three aspects of comparison, BCVA, VA and CMT.
QualityAssessmentAn assessment scale was designed with 11 items based on the Newcastle Ottawa Scale (NOS)[19]. “Yes” or “no” or “not clear” the answer should be “yes” or “no” or “not clear”, and if the answer is “yes”, then there will be a score of “1”; Otherwise, the item will score “0”. Huang C evaluates the quality of the included studies, and studies with scores above 8 are considered high quality studies.
DataSynthesisandAnalysisRelative risks (RRs) of the effect of randomized treatments were calculated using the metan routine (STATA Statacorp, version 14.0) to account for the probability of events occurring in the treatment group versus the control group[20]. Relative risks (RRs) and 95% confidence intervals (CIs) for each outcome were calculated separately for each trial, with grouped data using the intention-to-treat principle[21]. The combined RRs are log-transformed and weighted by the inverse variance. Estimates of population effects were calculated using a random effects model. The hypothesis of homogeneity of different treatment effects was tested by Q-statistic, and further quantified by I2 statistic. Q-statisticP<0.05 defined significant heterogeneity. I2 indicates insignificant heterogeneity between 0% and 40%, moderate heterogeneity between 30% and 60%, significant heterogeneity between 50% and 90%, and significant heterogeneity between 75% and 100%[22]. The significance level for all outcome and heterogeneity analyses was set atP≤0.05.
SensitivityAnalysisIn order to investigate the therapeutic effect of afiricip on patients and whether there was a difference compared with ranizumab, we performed a Meta-analysis by stratified trials with the intracavinal injection of afiricip and the comparison drug (ranizumab). We input one event for each study group with a zero trial result for sensitivity analysis to avoid any distortion due to the difference in size between the treatment and control groups.
PublicationBiasTo assess potential publication bias, funnel plots were developed and weighted linear regression was used, with the natural log of the odds ratio as the dependent variable and the reciprocal of the total sample size as the independent variable. This approach is an improved MacAskill test that gives a more balanced Type I error rate in the tail probability region than other publication bias tests[23]. The significance level for the publication bias analysis was set atP<0.05.
RESULTS
StudyCharacteristicsandQualityAssessmentOf the 780 articles identified in the preliminary study, 328 were retrieved for more detailed evaluation and 10 randomized trials were included in the analysis. Patients over 18 years of age with DR were included in the study (Figure 1).
Table 1 shows the detailed characteristics of these studies. There are 6 papers with high marks. That includes 1240 people from Egypt, Japan, the United States and Britain. It should be noted that there were 7 trials including IVA and IVR. In addition, other trials selected only IVA or IVR during the course. The scale used for quality assessment is shown in Figure 2 and the results are shown in Table 1.
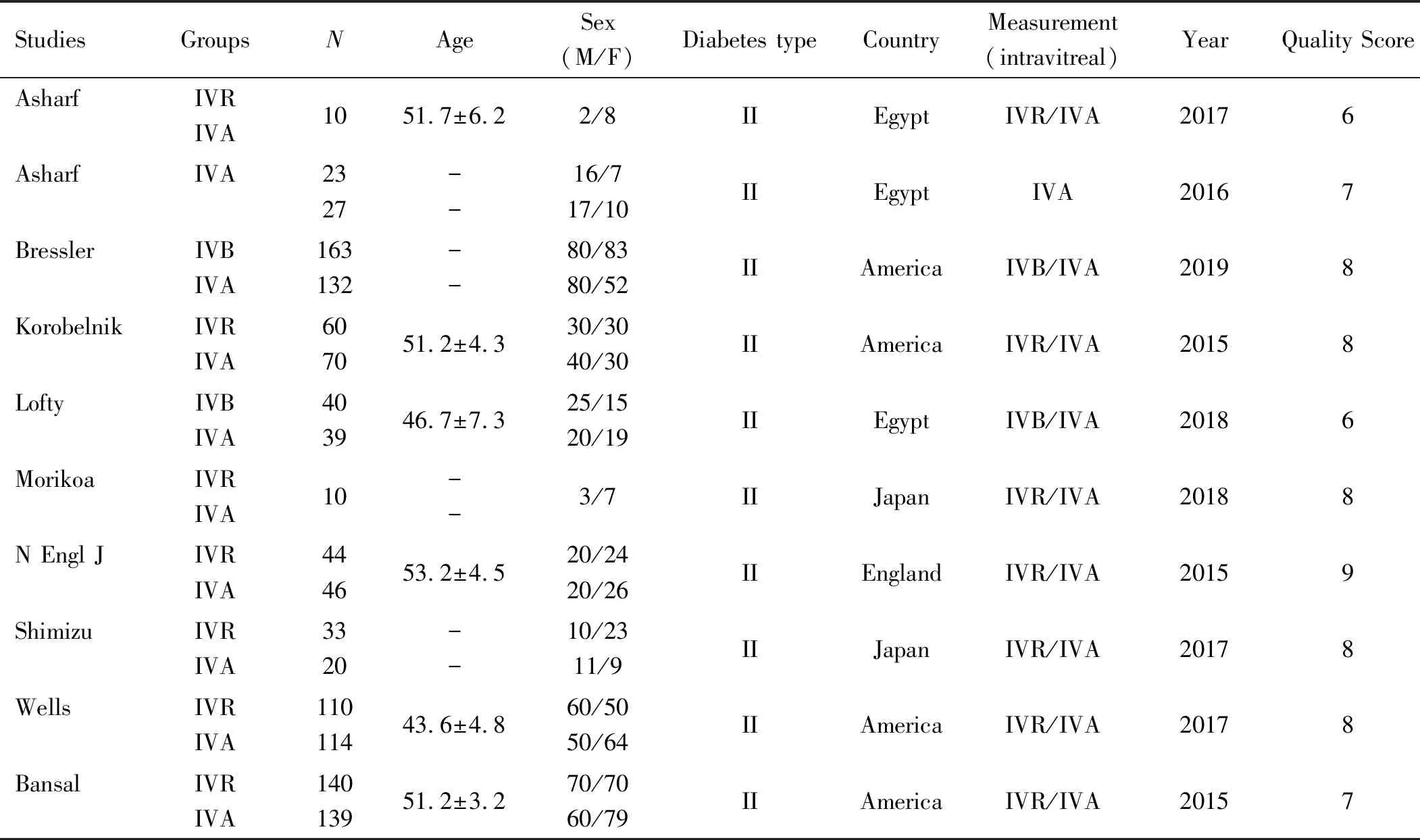
Table 1 Characteristics of eligible studies

Figure 2 Quality assessment scale.

Figure 3 Funnel plot for comparison between Aflibercept and Ranibizumab in BCVA, VA and CMT.

Figure 4 Forest plot summarizing the comparison between Aflibercept and Ranibizumab in BCVA Significance test for estimate P<0.00001, Bars indicate the 95% CI.

Figure 5 Forest plot summarizing the comparison between Aflibercept and Ranibizumab in VA Significance test for estimate P<0.00001, Bars indicate the 95% CI.
OutcomesAnalysis
BestcorrectedvisualacuitySix studies reported the BCVA of patients after receiving treatments. The heterogeneity test result of the combined effect amount is (P=0.0003,I2=78%), and the random effect model analysis is shown in Figure 3. The Meta-analysis result was [MD=0.05, 95%CI(0.03, 0.08),P=0.0003]. The BCVA of patients in the Ranibizumab treatment group was higher than that of the Aflibercept group, and the difference was significant. The detailed results are depicted in Figure 4.
VisualacuityAdditionally, 4 studies reported patient VA after treatment administration. The heterogeneity test result of the combined effect amount was (P=0.00001,I2=94%), and the random effect model analysis is shown in Figure 3. The Meta-analysis result was [MD=5.98, 95%CI(4.70, 7.25),P=0.00001]. The VA of patients in the Ranibizumab treatment group was higher than that of those in the Aflibercept group, and the difference was significant. The detailed results are depicted in Figure 5.
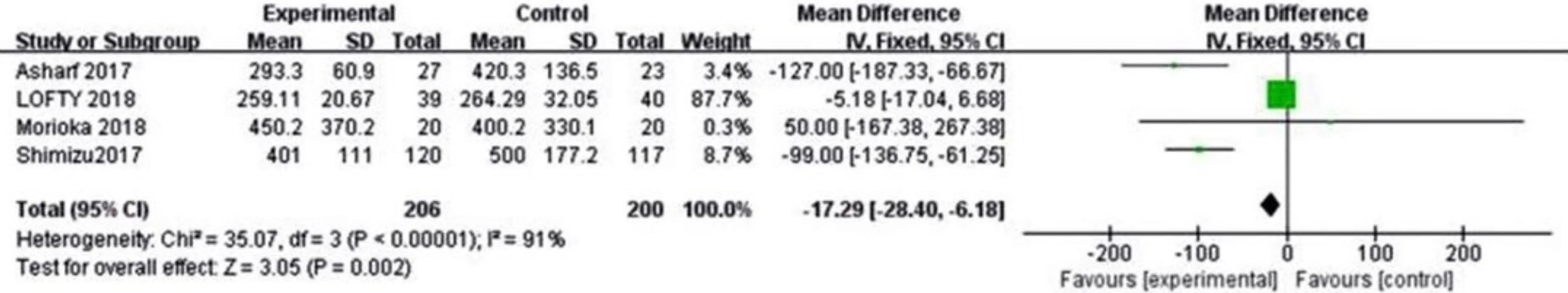
Figure 6 Forest plot summarizing the comparison between Aflibercept and Ranibizumab in CMT Significance test for estimate
CentralmacularthicknessFour studies reported the CMT of patients after receiving treatments. The heterogeneity test result of the combined effect amount was (P=0.00001,I2=91%), and the random effect model analysis is shown in Figure 3. The Meta-analysis result was [MD= -17.29, 95%CI(-28.40, -6.18),P=0.00001]. The VA of patients in the Aflibercept treatment group was higher than that of those in the Ranibizumab group, and the difference was significant. The detailed results are depicted in Figure 6.
SensitivityandpublicationbiasanalysisSensitivity analysis was conducted for each indicator, and each included study was excluded respectively to determine the results of Meta-analysis. The results are reported using a random-effects model, which allows for more conservative estimates because the results of fixed and random-effects models are similar. Heterogeneity tests were used to assess heterogeneity between studies. We produced forest plots to assess multivariate adjusted relative risk and the corresponding 95%CI. Using regression analysis, we assessed whether IVR and IVA were associated with certain prognostic variables at the study level. We used Cochrane Q-statistic (we consideredP<0.05 to indicate statistically significant heterogeneity) andI2statistics to assess the heterogeneity of relative risk across studies. Figure 7 depicts the detailed results.
DISCUSSION

DR is the manifestation of organ damage in DM[24]. Laser or anti-VEGF drugs are popular for alleviating DR[25]. Intravitreal injection of anti-VEGF drugs has been shown to be more effective than laser photocoagulation of diabetic macula edema (DME), which was the standard treatment in the 1980s[26]. Moreover, using laser therapy alone may also lead to some complications or shortcomings. For instance, nausea, eye swelling, eye pain, tearing, and elevated intraocular pressure may occur during the procedure[27]. Because VEGF plays an important role in the development of DR, anti-VEGF drugs have been gradually applied in clinical practice and achieved good results. Against this background, Aflibercept and Ranibizumab were realized in a new era. Both drugs can treat DR by inhibiting VEGF. Ranibizumab (Lucentis, Genentech/Roche) is A high-affinity antigen associated with A monoclonal antibody fragment that neutralizes all bioactive forms of VEGF-A[28]. Ranibizumab has been widely used in the treatment of DME, DR and RVO[29]. In addition, the use of 0.5 mg Ranibizumab mayFigure 7 Publication of bias risk maps per document.
increase the incidence of cataracts[30]. Aflibercept is a newly-applied clinical drug that has recently been introduced to the market. Compared to previously marketed Ranibizumab, Aflibercept binding affinity for VEGF is substantially greater, and a mathematical model predicted that Aflibercept might have a substantially longer duration of action in the eye[31]. Aflibercept plays a role in localized treatment through intravitreal injection. After intravitreal injection, part of the intraocular and endogenous VEGF binds to inactive Aflibercept, which is called the VEGF complex. In addition, the other part of Aflibercept is absorbed into the body circulation[32]. Aflibercept is suitable for DR, DME, AMD and CRVO[33]. The most common adverse reaction of Aflibercept is eye pain, cataract, vitreous detachment, and increased intraocular pressure. The process of intravitreal injection may lead to endophthalmitis, so the whole procedure should observe aseptic rules[34]. BCVA, VA and CMT have been applied to evaluate the efficacy of anti-VEGF drugs[35]. The BCVA and VA after treatment in both groups were higher than those before treatment, which can indicate an improvement in vision[36]. In contrast, CMT after treatment was lower than that before treatment, which may be monitored to determine the efficacy over time[37].
To summarize our Meta-analysis, Aflibercept and Ranibizumab are both beneficial to treat DR patients. Meta-regression analysis showed that both Aflibercept and Ranibizumab had improvements in BCVA [MD=0.05, 95%CI(0.03, 0.08),P=0.0003], VA [MD=5.98, 95%CI(4.70, 7.25),P=0.00001], and CMT [MD=-17.29, 95%CI(-28.40, -6.18),P=0.00001]. Subgroup analysis confirmed that Aflibercept had a markedly better effect on CMT than did Ranibizumab (P<0.00001), while Ranibizumab resulted in a wonderful improvement in BCVA (P<0.0001) and VA (P<0.00001). Therefore, the choice of ranizumab or aflisip should be evaluated separately based on the patient’s baseline condition,i.e. BCVA and VA or CMT is of greater concern. In addition, patients’ age, gender, type of diabetes in patients with diabetes, and the overall study design may affect the results of the Meta-regression. Finally, for practical reasons, qualified studies only cover those written in English, which can lead to bias. The publication bias funnel plot is very important and has a slight asymmetry, suggesting publication bias. Therefore, it is necessary to avoid publication bias from the experimental design stage. Meta-analysis showed that sample size had an effect on heterogeneity. Further research is needed to analyze this finding.
In this Meta-analysis, we screened the literatures strictly according to the inclusion criteria, and finally included 10 articles. The research literature is an open study, and some studies do not describe in terms of allocation concealment, so there may be execution bias. Another limitation is that the conclusions of this study need to be rigorously designed, large-sample, double-blind RCTs to verify.
In summary, a large sample study confirmed that the use of Aflibercept or Ranizumab in patients with diabetic retinopathy or dimethyl ether was associated with a significant reduction in CMT. In particular, Aflibercept was superior to Ranizumab in CMT. In addition, the efficacy of Ranizumab in BCVA and VA is better than that of Aflibercept, further demonstrating the efficacy of anti-VEGF drugs requires larger sample size, longer studies, so as to help physicians and patients better manage DR.
